Methods of Radiographic Measurements of Heart and Left Atrial Size in Dogs with and without Myxomatous Mitral Valve Disease: Intra- and Interobserver Agreement and Practicability of Different Methods
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Radiography
2.3. Observers
2.4. Radiographic Measurements
2.5. Observer Grading Assessment
2.6. Statistical Analysis
3. Results
3.1. Dogs
3.2. Radiographic Measurements—Group Comparison
3.3. Radiographic Stage Assignment—Comparison of the Different Observers
3.4. Comparison between Different Experienced Observers—Difficulty Grading
3.5. Comparison of Different Radiographic Measurements and Landmarks
3.6. Interobserver Agreement—Radiographic Measuring
3.7. Intraobserver Agreement—Radiographic Measuring
4. Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martin, M.W.S.; Stafford Johnson, M.J.; Celona, B. Canine dilated cardiomyopathy: A retrospective study of signalment, presentation and clinical findings in 369 cases. J. Small Anim. Pract. 2009, 50, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Borgarelli, M.; Haggstrom, J. Canine degenerative myxomatous mitral valve disease: Natural history, clinical presentation and therapy. Vet. Clin. N. Am. Small Anim. Pract. 2010, 40, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Keene, B.W.; Atkins, C.E.; Bonagura, J.D.; Fox, P.R.; Haggstrom, J.; Fuentes, V.L.; Oyama, M.A.; Rush, J.E.; Stepien, R.; Uechi, M. ACVIM consensus guidelines for the diagnosis and treatment of myxomatous mitral valve disease in dogs. J. Vet. Intern. Med. 2019, 33, 1127–1140. [Google Scholar] [CrossRef] [PubMed]
- Kresken, J.G.; Wendt, R.; Molder, P. Praxis der Kardiologie, 2nd ed.; Georg Thieme Verlag: Stuttgart, Germany, 2019; pp. 38–39, 56–138. [Google Scholar]
- Meomartino, L.; Greco, A.; Di Giancamillo, M.; Brunetti, A.; Gnudi, G. Imaging techniques in Veterinary Medicine. Part I: Radiography and Ultrasonography. Eur. J. Radiol. Open 2021, 8, 100382. [Google Scholar] [CrossRef]
- Wesselowski, S.; Gordon, S.G.; Meddaugh, N.; Saunders, A.B.; Haggstrom, J.; Cusack, K.; Janacek, B.; Matthews, D. Prediction of clinically important acquired cardiac disease without an echocardiogram in large breed dogs using a combination of clinical, radiographic and electrocardiographic variables. J. Vet. Cardiol. 2022, 40, 126–141. [Google Scholar] [CrossRef]
- Hansson, K.; Haggstrom, J.; Kvart, C.; Lord, P. Reader performance in radiographic diagnosis of signs of mitral regurgitation in cavalier King Charles spaniels. J. Small Anim. Pract. 2009, 50 (Suppl. S1), 44–53. [Google Scholar] [CrossRef]
- Guglielmini, C.; Diana, A. Thoracic radiography in the cat: Identification of cardiomegaly and congestive heart failure. J. Vet. Cardiol. 2015, 17 (Suppl. S1), S87–S101. [Google Scholar] [CrossRef]
- Mostafa, A.A.; Berry, C.R. Radiographic assessment of the cardiac silhouette in clinically normal large- and small-breed dogs. Am. J. Vet. Res. 2017, 78, 168–177. [Google Scholar] [CrossRef]
- Duler, L.; LeBlanc, N.L.; Cooley, S.; Nemanic, S.; Scollan, K.F. Interreader agreement of radiographic left atrial enlargement in dogs and comparison to echocardiographic left atrial assessment. J. Vet. Cardiol. 2018, 20, 319–329. [Google Scholar] [CrossRef]
- Killich, K. Kleintierkardiologie, 1st ed.; Georg Thieme Verlag: Stuttgart, Germany, 2018; pp. 93–108. [Google Scholar]
- Atkins, C.; Bonagura, J.; Ettinger, S.; Fox, P.; Gordon, S.; Haggstrom, J.; Hamlin, R.; Keene, B.; Fuentes, V.L.; Stepien, R. Guidelines for the Diagnosis and Treatment of Canine Chronic Valvular Heart Disease. J. Vet. Intern. Med. 2009, 23, 1142–1150. [Google Scholar] [CrossRef]
- Chetboul, V.; Tissier, R. Echocardiographic assessment of canine degenerative mitral valve disease. J. Vet. Cardiol. 2012, 14, 127–148. [Google Scholar] [CrossRef]
- Kim, S.; Seo, K.W.; Song, K.-H. An Assessment of Vertebral Left Atrial Size in Relation to the Progress of Myxomatous Mitral Valve Disease in Dogs. J. Vet. Clin. 2020, 37, 9–14. [Google Scholar] [CrossRef]
- Lam, C.; Gavaghan, B.J.; Meyers, F.E. Radiographic quantification of left atrial size in dogs with myxomatous mitral valve disease. J. Vet. Intern. Med. 2021, 35, 747–754. [Google Scholar] [CrossRef]
- Vezzosi, T.; Puccinelli, C.; Citi, S.; Tognetti, R. Two radiographic methods for assessing left atrial enlargement and cardiac remodeling in dogs with myxomatous mitral valve disease. J. Vet. Cardiol. 2021, 34, 55–63. [Google Scholar] [CrossRef]
- Levicar, C.; Granados-Soler, J.L.; Freise, F.; Raue, J.F.; Nolte, I.; Bach, J.-P. Comparison of different radiographic scores with associated echocardiographic measurements and prediction of heart enlargement in dogs with and without myxomatous mitral valve disease. J. Vet. Cardiol. 2022; in press. [Google Scholar] [CrossRef]
- Boswood, A.; Haggstrom, J.; Gordon, S.G.; Wess, G.; Stepien, R.L.; Oyama, M.A.; Keene, B.W.; Bonagura, J.; MacDonald, K.A.; Patteson, M.; et al. Effect of Pimobendan in Dogs with Preclinical Myxomatous Mitral Valve Disease and Cardiomegaly: The EPIC Study-A Randomized Clinical Trial. J. Vet. Intern. Med. 2016, 30, 1765–1779. [Google Scholar] [CrossRef]
- Hansson, K.; Haggstrom, J.; Kvart, C.; Lord, P. Interobserver variability of vertebral heart size measurements in dogs with normal and enlarged hearts. Vet. Radiol. Ultrasound 2005, 46, 122–130. [Google Scholar] [CrossRef]
- Buchanan, J.W.; Bücheler, J. Vertebral scale system to measure canine heart size in radiographs. J. Am. Vet. Med. Assoc. 1995, 206, 194–199. [Google Scholar]
- Le Roux, A.; Rademacher, N.; Saelinger, C.; Rodriguez, D.; Pariaut, R.; Gaschen, L. Value of tracheal bifurcation angle measurement as a radiographic sign of left atrial enlargement in dogs. Vet. Radiol. Ultrasound 2012, 53, 28–33. [Google Scholar] [CrossRef]
- Torad, F.A.; Hassan, E.A. Two-dimensional cardiothoracic ratio for evaluation of cardiac size in German shepherd dogs. J. Vet. Cardiol. 2014, 16, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Malcolm, E.L.; Visser, L.C.; Phillips, K.L.; Johnson, L.R. Diagnostic value of vertebral left atrial size as determined from thoracic radiographs for assessment of left atrial size in dogs with myxomatous mitral valve disease. J. Am. Vet. Med. Assoc. 2018, 253, 1038–1045. [Google Scholar] [CrossRef] [PubMed]
- Sanchez Salguero, X.; Prandi, D.; Llabres-Diaz, F.; Manzanilla, E.G.; Bussadori, C. A radiographic measurement of left atrial size in dogs. Ir. Vet. J. 2018, 71, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sanchez Salguero, X.; Prandi, D.; Llabrés-Díaz, F.; Manzanilla, E.G.; Badiella, L.; Bussadori, C. Heart to spine measurements to detect left atrial enlargement in dogs with mitral insufficiency. Ir. Vet. J. 2019, 72, 14. [Google Scholar] [CrossRef] [PubMed]
- Stepien, R.L.; Rak, M.B.; Blume, L.M. Use of radiographic measurements to diagnose stage B2 preclinical myxomatous mitral valve disease in dogs. J. Am. Vet. Med. Assoc. 2020, 256, 1129–1136. [Google Scholar] [CrossRef]
- Kraetschmer, S.; Ludwig, K.; Meneses, F.; Nolte, I.; Simon, D. Vertebral heart scale in the beagle dog. J. Small Anim. Pract. 2008, 49, 240–243. [Google Scholar] [CrossRef]
- Jepsen-Grant, K.; Pollard, R.E.; Johnson, L.R. Vertebral heart scores in eight dog breeds. Vet. Radiol. Ultrasound 2013, 54, 3–8. [Google Scholar] [CrossRef]
- Vezzosi, T.; Puccinelli, C.; Tognetti, R.; Pelligra, T.; Citi, S. Radiographic vertebral left atrial size: A reference interval study in healthy adult dogs. Vet. Radiol. Ultrasound 2020, 61, 507–511. [Google Scholar] [CrossRef]
- Poad, M.H.; Manzi, T.J.; Oyama, M.A.; Gelzer, A.R. Utility of radiographic measurements to predict echocardiographic left heart enlargement in dogs with preclinical myxomatous mitral valve disease. J. Vet. Intern. Med. 2020, 34, 1728–1733. [Google Scholar] [CrossRef]
- Barr, F.; Birch, S. Soft tissues. In BSAVA Manual of Canine and Feline Musculoskeletal Imaging; Kirberger, R.M., McEvoy, F.J., Eds.; British Small Animal Veterinary Association: Gloucester, UK, 2016; p. 66. [Google Scholar]
- Kirchner, J.; Gadek, D.; Goltz, J.P.; Doroch-Gadek, A.; Stückradt, S.; Liermann, D.; Kickuth, R. Standard versus inverted digital luminescence radiography in detecting pulmonary nodules: A ROC analysis. Eur. J. Radiol. 2013, 82, 1799–1803. [Google Scholar] [CrossRef]
- Cheng, C.-J.; Mandour, A.S.; Yoshida, T.; Watari, T.; Tanaka, R.; Matsuura, K. Changes in renin-angiotensin-aldosterone system during cardiac remodeling after mitral valvuloplasty in dogs. J. Vet. Intern. Med. 2022, 36, 397–405. [Google Scholar] [CrossRef]
- Nakayama, H.; Nakayama, T.; Hamlin, R.L. Correlation of cardiac enlargement as assessed by vertebral heart size and echocardiographic and electrocardiographic findings in dogs with evolving cardiomegaly due to rapid ventricular pacing. J. Vet. Intern. Med. 2001, 15, 217–221. [Google Scholar] [CrossRef]
- Saida, Y.; Tanaka, R.; Yamane, Y.; Suzuki, K.; Maruyama, R.; Koie, H.; Matsumoto, T.; Asano, R. Relationships between Vertebral Heart Size (VHS) and Echocardiographic Parameters in Dogs with Mitral Regurgitation. Adv. Anim. Cardiol. 2006, 39, 55–63. [Google Scholar] [CrossRef]
- Bagardi, M.; Manfredi, M.; Zani, D.D.; Brambilla, P.G.; Locatelli, C. Interobserver variability of radiographic methods for the evaluation of left atrial size in dogs. Vet. Radiol. Ultrasound 2021, 62, 162–174. [Google Scholar] [CrossRef]
- Baisan, R.A.; Vulpe, V. Vertebral heart size and vertebral left atrial size reference ranges in healthy Maltese dogs. Vet. Radiol. Ultrasound 2021, 63, 1–5. [Google Scholar] [CrossRef]
- Hansson, K.; Haggstrom, J.; Kvart, C.; Lord, P. Left atrial to aortic root indices using two-dimensional and M-mode echocardiography in cavalier King Charles spaniels with and without left atrial enlargement. Vet. Radiol. Ultrasound 2002, 43, 568–575. [Google Scholar] [CrossRef]
- Cornell, C.C.; Kittleson, M.D.; Della Torre, P.; Haggstrom, J.; Lombard, C.W.; Pedersen, H.D.; Vollmar, A.; Wey, A. Allometric scaling of M-mode cardiac measurements in normal adult dogs. J. Vet. Intern. Med. 2004, 18, 311–321. [Google Scholar] [CrossRef]
- Taylor, C.J.; Simon, B.T.; Stanley, B.J.; Lai, G.P.; Thieman Mankin, K.M. Norwich terriers possess a greater vertebral heart scale than the canine reference value. Vet. Radiol. Ultrasound 2020, 61, 10–15. [Google Scholar] [CrossRef]
- Lamb, C.; Wikeley, H.; Boswood, A.; Pfeiffer, D. Use of breed-specific ranges for vertebral heart scale in dogs as an aid to radiographic diagnosis of cardiac disease. Vet. Rec. 2001, 148, 707–711. [Google Scholar] [CrossRef]
- Borgarelli, M.; Crosara, S.; Lamb, K.; Savarino, P.; La Rosa, G.; Tarducci, A.; Haggstrom, J. Survival characteristics and prognostic variables of dogs with preclinical chronic degenerative mitral valve disease attributable to myxomatous degeneration. J. Vet. Intern. Med. 2012, 26, 69–75. [Google Scholar] [CrossRef]
- Lord, P.; Hansson, K.; Kvart, C.; Haggstrom, J. Rate of change of heart size before congestive heart failure in dogs with mitral regurgitation. J. Small Anim. Pract. 2010, 51, 210–218. [Google Scholar] [CrossRef]
- Lord, P.F.; Hansson, K.; Carnabuci, C.; Kvart, C.; Haggstrom, J. Radiographic heart size and its rate of increase as tests for onset of congestive heart failure in Cavalier King Charles Spaniels with mitral valve regurgitation. J. Vet. Intern. Med. 2011, 25, 1312–1319. [Google Scholar] [CrossRef] [PubMed]
- Andrei, B.; Birsan, O.; Vulpe, V. The diagnostic value of cardio-thoracic ratio in detecting heart size changes in dog. Rev. Rom. Med. Vet. 2016, 26, 5–9. [Google Scholar]
- Diana, A.; Guglielmini, C.; Pivetta, M.; Sanacore, A.; Di Tommaso, M.; Lord, P.F.; Cipone, M. Radiographic features of cardiogenic pulmonary edema in dogs with mitral regurgitation: 61 cases (1998–2007). J. Am. Vet. Med. Assoc. 2009, 235, 1058–1063. [Google Scholar] [CrossRef] [PubMed]
- Goette, A.; Kalman, J.M.; Aguinaga, L.; Akar, J.; Cabrera, J.A.; Chen, S.A.; Chugh, S.S.; Corradi, D.; D’Avila, A.; Dobrev, D.; et al. EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: Definition, characterization, and clinical implication. Europace 2016, 18, 1455–1490. [Google Scholar] [CrossRef]
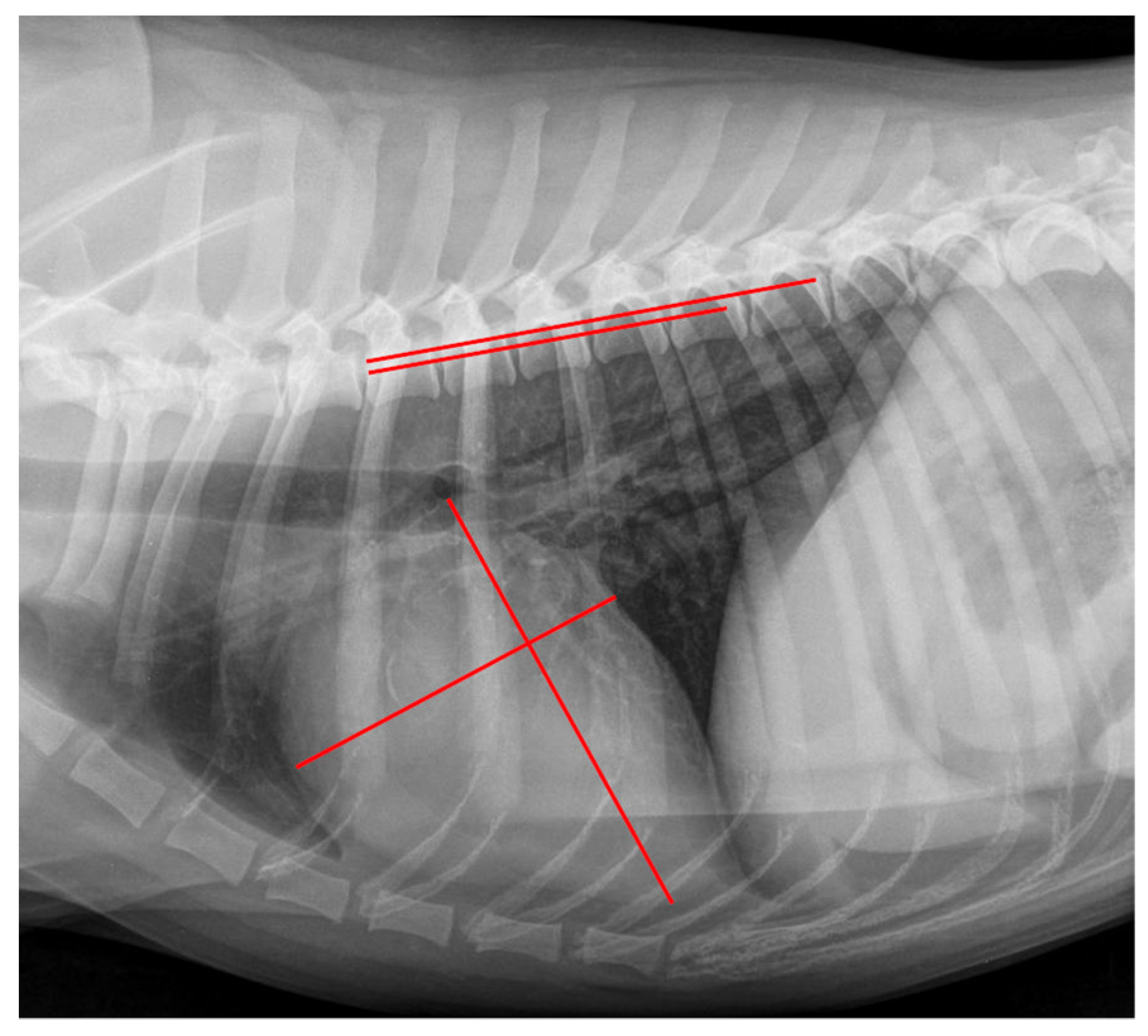
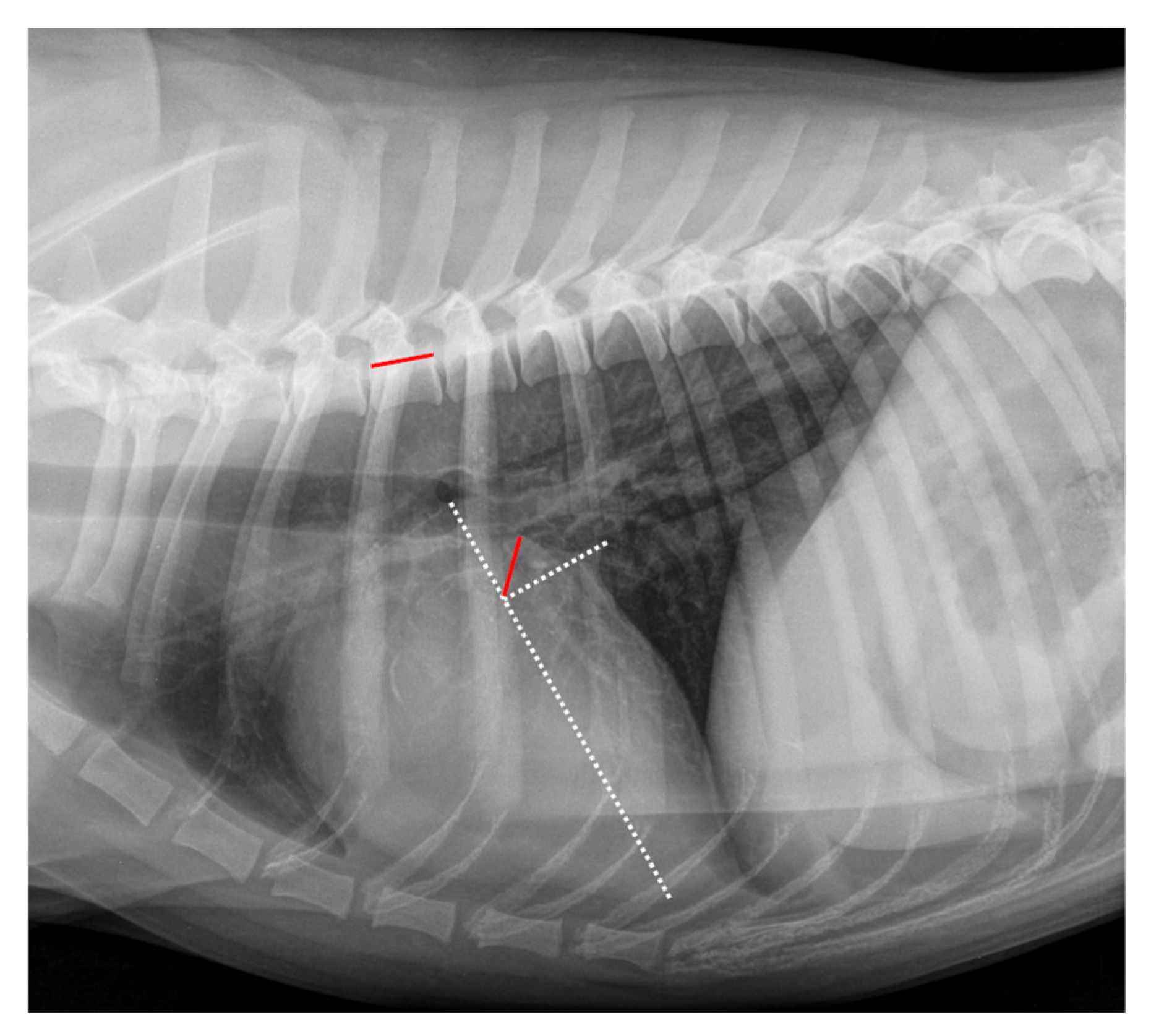
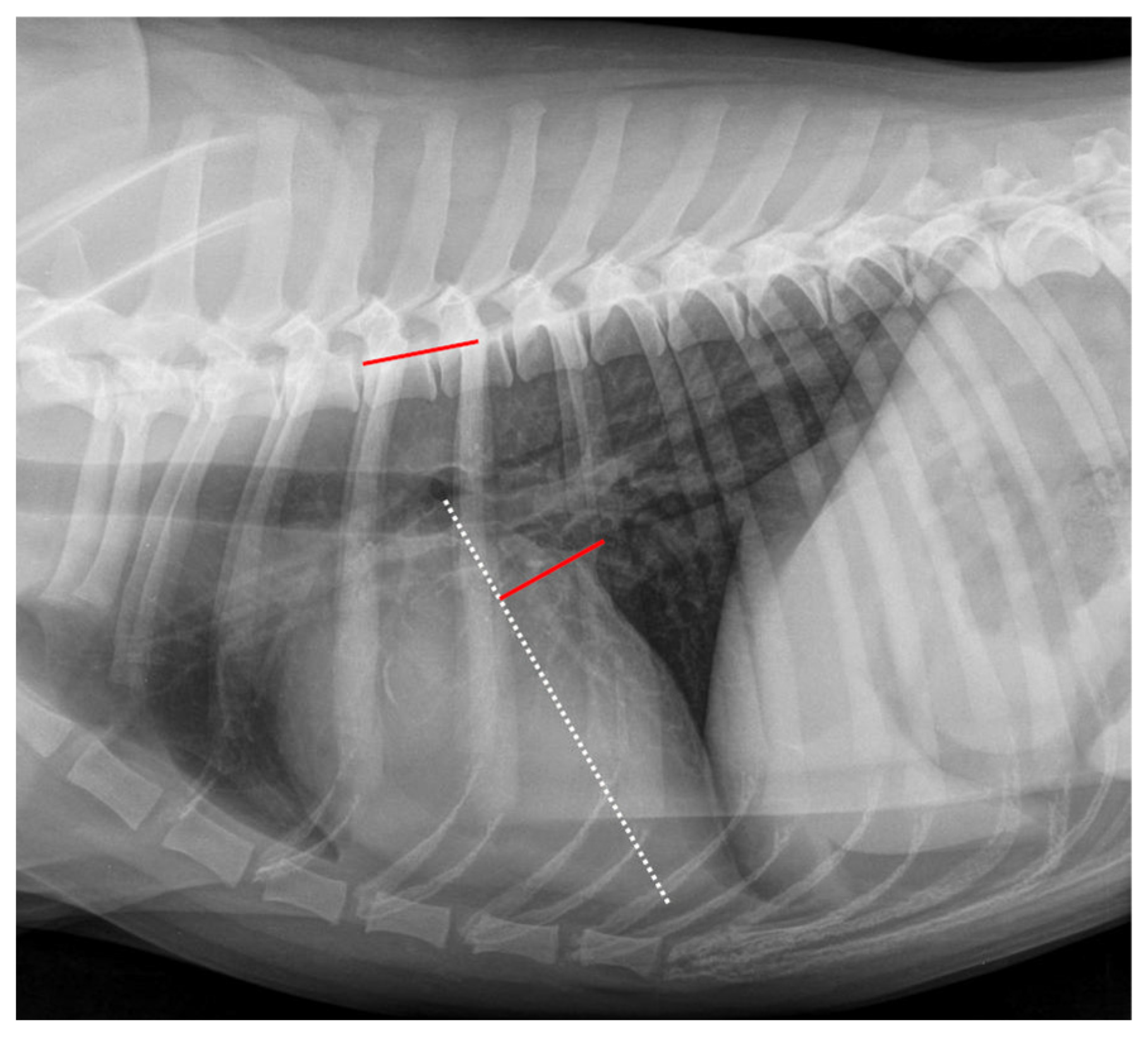
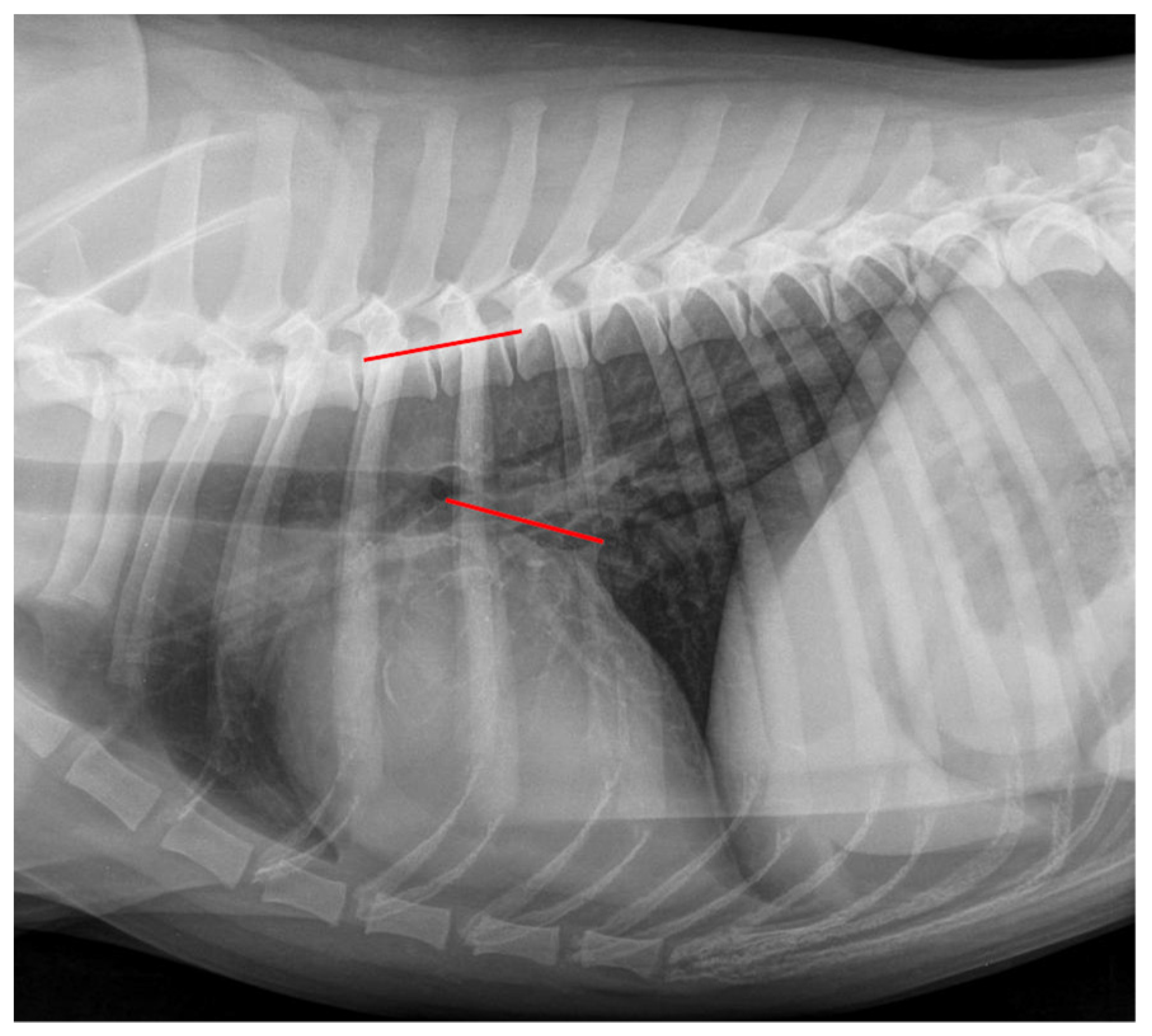
| Grade | Meaning |
|---|---|
| 1 | The observer was confident in determining the exact position of all points necessary for the radiographic measurement. |
| 2 | The observer was uncertain regarding the exact position of one of the measured points, with an expected low impact on the final result of the radiographic measurement. |
| 3 | The observer was uncertain regarding the exact position of two or more points with an expected low impact on the final result of the radiographic measurement. |
| 4 | The observer was uncertain regarding the exact position of one of the measured points with an expected high impact on the final result of the radiographic measurement. |
| 5 | The observer was uncertain regarding the exact position of two or more points with an expected high impact on the final result of the radiographic measurement. |
| CG | Stage B1 | Stage B2 | Stage C | ||
|---|---|---|---|---|---|
| VHS | All Observers | 10.7 ± 0.8 | 10.5 ± 0.6 * | 11.3 ± 0.8 † | 12.2 ± 1.1 ‡ |
| OB1 | 10.7 ± 0.9 | 10.5 ± 0.6 * | 11.3 ± 0.8 † | 12.1 ± 1.1 ‡ | |
| OB2 | 10.8 ± 0.8 | 10.6 ± 0.6 * | 11.4 ± 0.8 † | 12.3 ± 1.1 ‡ | |
| OB3 | 10.7 ± 0.8 | 10.4 ± 0.5 * | 11.3 ± 0.7 † | 12.2 ± 1.1 ‡ | |
| RLAD | All Observers | 1.8 ± 0.3 | 1.8 ± 0.3 * | 2.1 ± 0.4 † | 2.7 ± 0.5 ‡ |
| OB1 | 1.8 ± 0.2 | 1.9 ± 0.3 * | 2.1 ± 0.4 | 2.6 ± 0.5 ‡ | |
| OB2 | 1.9 ± 0.3 | 1.9 ± 0.3 * | 2.2 ± 0.4 † | 2.7 ± 0.6 ‡ | |
| OB3 | 1.7 ± 0.3 | 1.7 ± 0.3 | 2.1 ± 0.5 † | 2.7 ± 0.5 ‡ | |
| LAWidth | All Observers | 1.7 ± 0.3 | 1.7 ± 0.3 * | 2.0 ± 0.3 † | 2.2 ± 0.3 ‡ |
| OB1 | 1.6 ± 0.3 | 1.7 ± 0.3 * | 2.0 ± 0.3 † | 2.2 ± 0.3 ‡ | |
| OB2 | 1.7 ± 0.3 | 1.7 ± 0.3 * | 2.0 ± 0.3 † | 2.2 ± 0.3 ‡ | |
| OB3 | 1.7 ± 0.3 | 1.6 ± 0.3 * | 2.0 ± 0.3 † | 2.3 ± 0.4 ‡ | |
| VLAS | All Observers | 2.1 ± 0.3 | 2.1 ± 0.3 * | 2.5 ± 0.4 † | 2.9 ± 0.5 ‡ |
| OB1 | 2.0 ± 0.3 | 2.2 ± 0.3 * | 2.5 ± 0.4 † | 2.9 ± 0.4 ‡ | |
| OB2 | 2.2 ± 0.4 | 2.1 ± 0.3 * | 2.5 ± 0.4 † | 3.0 ± 0.5 ‡ | |
| OB3 | 2.1 ± 0.3 | 2.1 ± 0.3 * | 2.5 ± 0.4 † | 3.0 ± 0.5 ‡ |
| Conventional Radiographs | Inverted Radiographs | ||||||
|---|---|---|---|---|---|---|---|
| Radiographic measurement | Cut-off values | OB1 | OB2 | OB3 * | OB1 | OB2 | OB3 |
| VHS | 11.0 * | 0.840 | 0.833 | 0.847 | 0.833 | 0.827 | 0.853 * |
| RLAD | 2.0 * | 0.767 | 0.747 | 0.807 | 0.793 | 0.767 | 0.820 * |
| LAWidth | 1.8 * | 0.793 | 0.793 | 0.813 | 0.753 | 0.800 | 0.847 * |
| VLAS | 2.3 * | 0.787 | 0.793 | 0.820 | 0.793 | 0.780 | 0.833 * |
| Grades | CG | Stage B1 | Stage B2 | Stage C | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OB1 | OB2 | OB3 | OB1 | OB2 | OB3 | OB1 | OB2 | OB3 | OB1 | OB2 | OB3 | ||
| VHS | 1 | 17 | 21 | 40 | 22 | 25 | 42 | 23 | 24 | 45 | 13 | 16 | 40 |
| 2 | 23 | 18 | 5 | 19 | 14 | 6 | 19 | 17 | 3 | 24 | 19 | 5 | |
| 3 | 4 | - | 3 | 4 | 2 | 1 | 5 | 1 | 1 | 4 | 1 | 2 | |
| 4 | 2 | 1 | 1 | 3 | 1 | - | 2 | - | 1 | 3 | 1 | 2 | |
| 5 | 4 | 10 | 1 | 2 | 8 | 1 | 1 | 8 | - | 6 | 13 | 1 | |
| RLAD | 1 | 2 | 3 | 3 | 3 | 4 | 6 | 7 | 3 | 10 | 2 | 3 | 21 |
| 2 | 7 | 8 | 14 | 11 | 13 | 14 | 14 | 10 | 15 | 18 | 13 | 13 | |
| 3 | 11 | 3 | 2 | 13 | 3 | 5 | 12 | 2 | 1 | 10 | 3 | 3 | |
| 4 | 7 | 7 | 17 | 7 | 6 | 15 | 7 | 9 | 19 | 6 | 1 | 7 | |
| 5 | 23 | 29 | 14 | 16 | 24 | 10 | 10 | 26 | 5 | 14 | 30 | 6 | |
| LAWidth | 1 | 12 | 17 | 34 | 16 | 20 | 35 | 22 | 19 | 43 | 8 | 15 | 39 |
| 2 | 20 | 16 | 10 | 20 | 11 | 11 | 19 | 18 | 3 | 29 | 21 | 4 | |
| 3 | 6 | 1 | 3 | 5 | 5 | - | 5 | 1 | 2 | 2 | - | 2 | |
| 4 | 6 | - | 1 | 4 | 3 | 3 | 2 | 2 | 2 | 5 | 3 | 5 | |
| 5 | 6 | 16 | 2 | 5 | 11 | 1 | 2 | 10 | - | 6 | 11 | - | |
| VLAS | 1 | 27 | 23 | 35 | 26 | 23 | 36 | 35 | 29 | 44 | 31 | 30 | 44 |
| 2 | 11 | 17 | 11 | 15 | 19 | 10 | 10 | 13 | 3 | 10 | 10 | - | |
| 3 | - | 1 | 1 | 1 | - | - | 1 | - | 1 | - | - | 2 | |
| 4 | 12 | 4 | 2 | 7 | 5 | 3 | 4 | 2 | 2 | 7 | 7 | 4 | |
| 5 | - | 5 | 1 | 1 | 3 | 1 | - | 6 | - | 2 | 3 | - | |
| Evaluation Criteria | All Groups n = 600 (%) | CG n = 150 (%) | Stage B1 n = 150 (%) | Stage B2 n = 150 (%) | Stage C n = 150 (%) |
|---|---|---|---|---|---|
| Well identifiable radiographs | |||||
| VHS | 328 (54.7) | 78 (52) | 89 (59.3) | 92 (61.3) | 69 (46) |
| RLAD | 67 (11.2) | 8 (5.3) | 13 (8.7) | 20 (13.3) | 26 (17.3) |
| LAWidth | 281 (46.8) | 63 (42) | 71 (47.3) | 85 (56.7) | 62 (41.3) |
| VLAS | 383 (63.8) | 85 (56.7) | 85 (56.7) | 108 (72) | 105 (70) |
| radiographic landmarks | |||||
| carina tracheae *,**,†, ‡ | 79 (13.2) | 27 (18) | 18 (12) | 18 (12) | 16 (10.7) |
| dorsal margin of left atrium ** | 480 (80) | 135 (90) | 128 (85.3) | 120 (80) | 97 (64.7) |
| dorsal vena cava caudalis **,†, ‡ | 168 (25) | 46 (30.7) | 53 (35.3) | 32 (21.3) | 37 (24.7) |
| ventral vena cava caudalis * | 76 (12.7) | 15 (10) | 18 (12) | 15 (10) | 28 (18.7) |
| apex cordis *,**,† | 199 (33.2) | 53 (35.3) | 40 (26.7) | 41 (27.3) | 65 (43.3) |
| cranial heart side * | 16 (2.7) | 2 (1.3) | 5 (3.3) | 3 (2) | 6 (4) |
| Evaluation Criteria | CG | Stage B1 | Stage B2 | Stage C | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OB1 | OB2 | OB3 | OB1 | OB2 | OB3 | OB1 | OB2 | OB3 | OB1 | OB2 | OB3 | |
| Well identifiable radiographs | ||||||||||||
| VHS | 17 | 21 | 40 | 22 | 25 | 42 | 23 | 24 | 45 | 12 | 16 | 41 |
| RLAD | 2 | 3 | 3 | 3 | 4 | 6 | 7 | 3 | 10 | 2 | 3 | 21 |
| LAWidth | 2 | 17 | 34 | 16 | 20 | 35 | 22 | 20 | 43 | 8 | 15 | 39 |
| VLAS | 27 | 23 | 35 | 26 | 23 | 36 | 35 | 29 | 44 | 31 | 30 | 40 |
| radiographic landmarks | ||||||||||||
| carina tracheae *,**,†, ‡ | 7 | 12 | 8 | 6 | 7 | 5 | 6 | 8 | 4 | 5 | 8 | 3 |
| dorsal margin of left atrium ** | 44 | 45 | 46 | 41 | 43 | 44 | 36 | 45 | 39 | 30 | 42 | 25 |
| dorsal vena cava caudalis **,†, ‡ | 16 | 21 | 9 | 20 | 23 | 10 | 10 | 19 | 3 | 16 | 16 | 5 |
| ventral vena cava caudalis * | 6 | 7 | 2 | 7 | 7 | 4 | 6 | 9 | 0 | 11 | 13 | 4 |
| apex cordis *,**,† | 27 | 22 | 4 | 19 | 19 | 2 | 20 | 19 | 2 | 32 | 27 | 6 |
| cranial heart side * | 1 | 1 | 0 | 2 | 2 | 1 | 2 | 1 | 0 | 1 | 4 | 1 |
| VHS | RLAD | LAWidth | VLAS | |
|---|---|---|---|---|
| OB1 | 0.985 (0.981–0.989) | 0.929 (0.908–0.946) | 0.910 (0.883–0.931) | 0.944 (0.928–0.958) |
| OB2 | 0.978 (0.971–0.983) | 0.920 (0.896–0.938) | 0.926 (0.903–0.943) | 0.929 (0.904–0.943) |
| OB3 | 0.983 (0.978–0.987) | 0.943 (0.926–0.987) | 0.924 (0.901–0.941) | 0.964 (0.953–0.972) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Levicar, C.; Nolte, I.; Granados-Soler, J.L.; Freise, F.; Raue, J.F.; Bach, J.-P. Methods of Radiographic Measurements of Heart and Left Atrial Size in Dogs with and without Myxomatous Mitral Valve Disease: Intra- and Interobserver Agreement and Practicability of Different Methods. Animals 2022, 12, 2531. https://doi.org/10.3390/ani12192531
Levicar C, Nolte I, Granados-Soler JL, Freise F, Raue JF, Bach J-P. Methods of Radiographic Measurements of Heart and Left Atrial Size in Dogs with and without Myxomatous Mitral Valve Disease: Intra- and Interobserver Agreement and Practicability of Different Methods. Animals. 2022; 12(19):2531. https://doi.org/10.3390/ani12192531
Chicago/Turabian StyleLevicar, Charanthorn, Ingo Nolte, José Luis Granados-Soler, Fritjof Freise, Jonathan Friedemann Raue, and Jan-Peter Bach. 2022. "Methods of Radiographic Measurements of Heart and Left Atrial Size in Dogs with and without Myxomatous Mitral Valve Disease: Intra- and Interobserver Agreement and Practicability of Different Methods" Animals 12, no. 19: 2531. https://doi.org/10.3390/ani12192531
APA StyleLevicar, C., Nolte, I., Granados-Soler, J. L., Freise, F., Raue, J. F., & Bach, J.-P. (2022). Methods of Radiographic Measurements of Heart and Left Atrial Size in Dogs with and without Myxomatous Mitral Valve Disease: Intra- and Interobserver Agreement and Practicability of Different Methods. Animals, 12(19), 2531. https://doi.org/10.3390/ani12192531






