Effects of Sulfamethoxazole on Fertilization and Embryo Development in the Arbacia lixula Sea Urchin
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Gamete Collection
2.3. Experimental Plan
- -
- The simultaneous exposure (SE) of the gametes, which were simultaneously combined in multiwell plates with the experimental solutions. The effects were observed on the first embryonic division after 4 h. The percentages of unfertilized eggs and the regular divisions were evaluated;
- -
- The pre-exposure (PE), which was achieved by pre-treating the eggs and spermatozoa separately for 2 h before fertilization. The percentages of unfertilized eggs and the regular divisions were evaluated; and
- -
- The post-fertilization exposure (PFE), in which previously fertilized eggs were subsequently exposed to differing drug concentrations for 2 h. The effects were observed starting at 4 h post-exposure. The percentages of irregular, regular, and delayed divisions were evaluated.
2.4. Statistical Analysis
3. Results
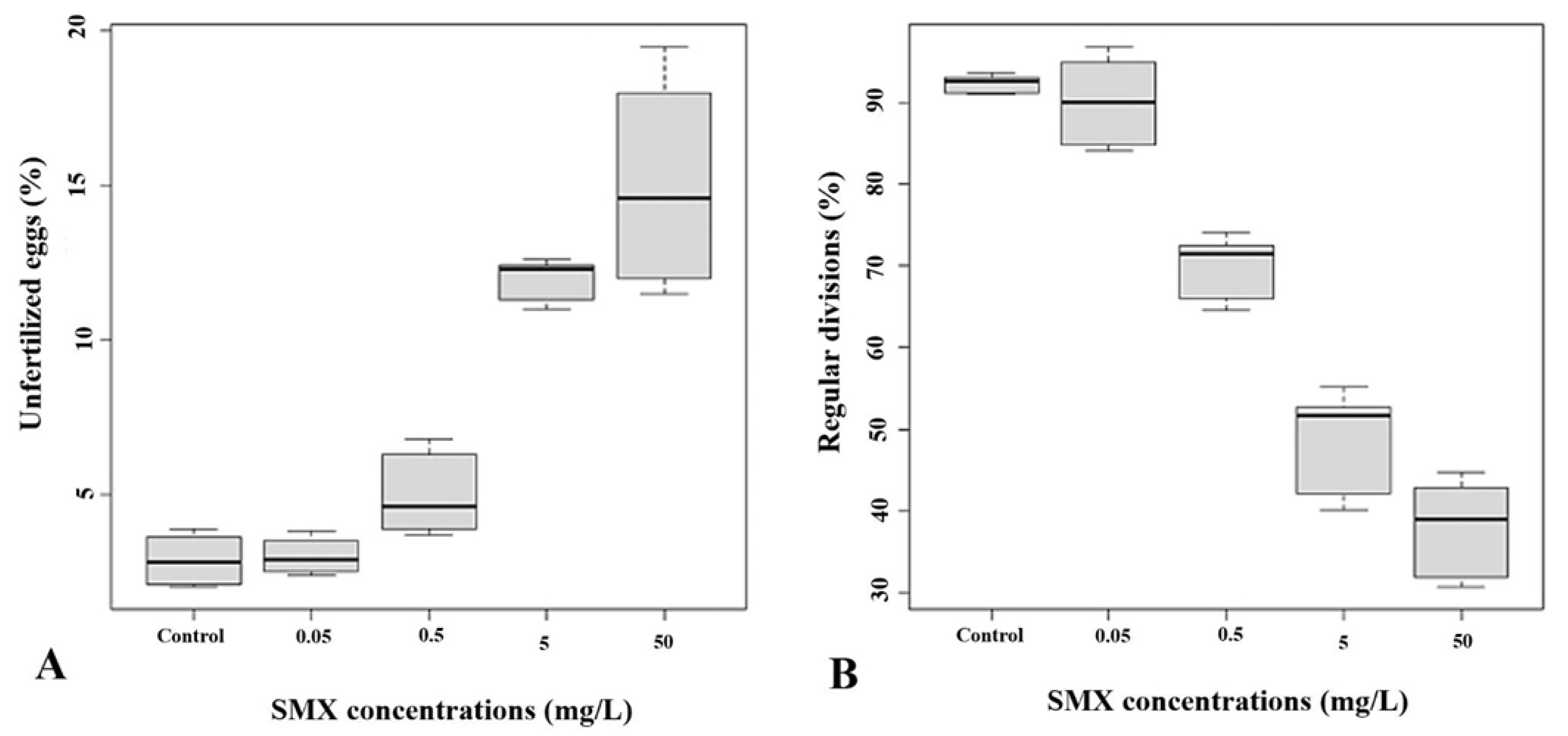
3.1. Simultaneous Exposure Effects
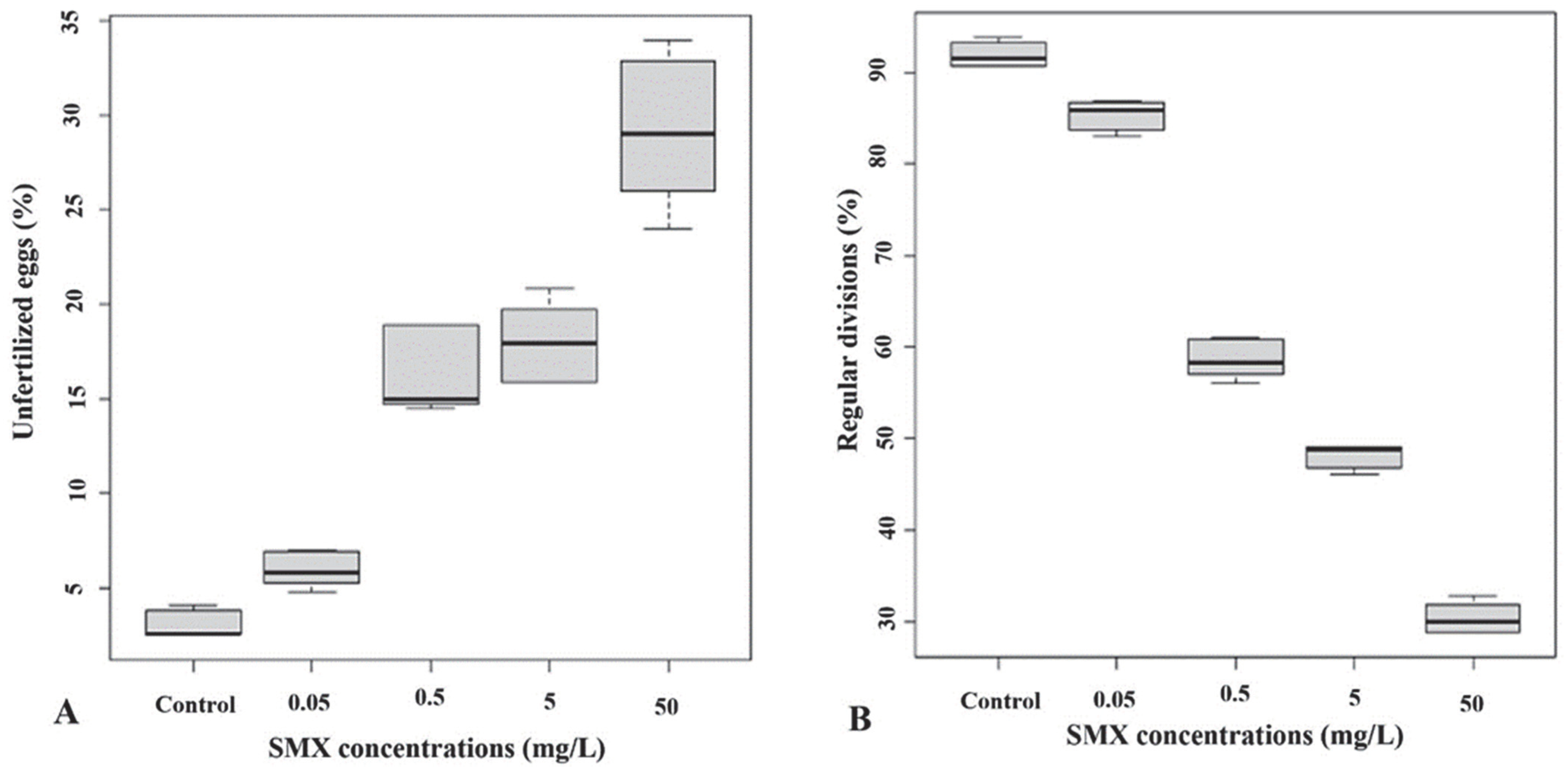
3.2. Preventive Exposure Effects
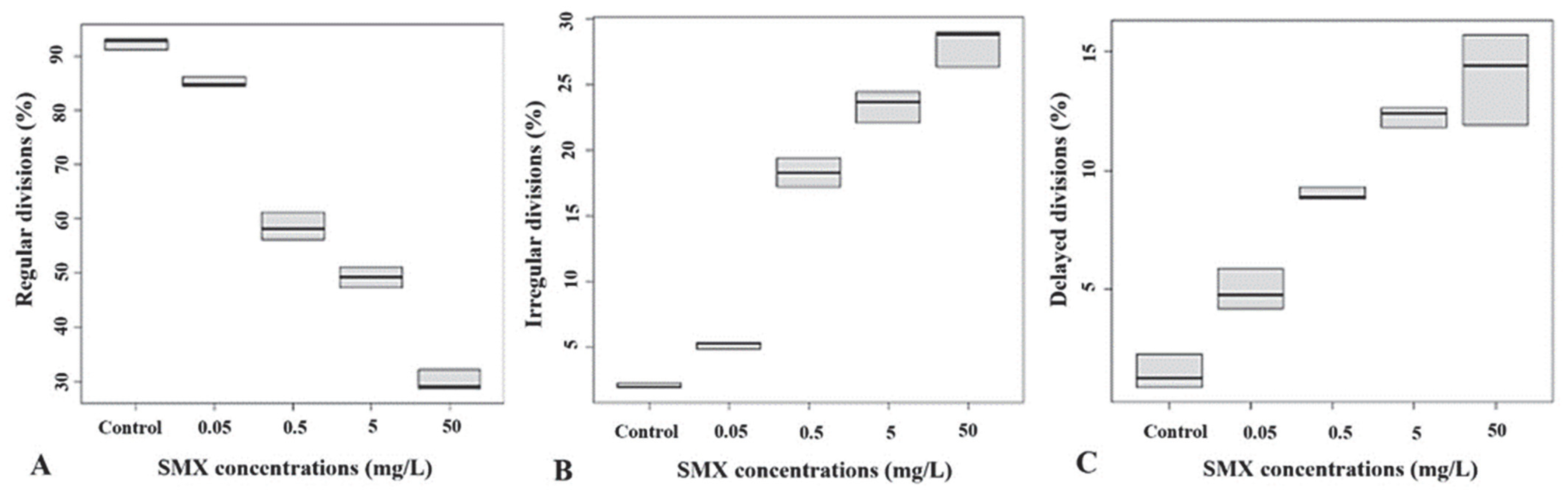
3.3. Post-Fertilization Exposure Effects
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patel, N.; Khan, M.D.; Shahane, S.; Rai, D.; Chauhan, D.; Kant, C.; Chaudhary, V.K. Emerging Pollutants in Aquatic Environment: Source, Effect, and Challenges in Biomonitoring and Bioremediation—A Review. Pollution 2020, 6, 99–113. [Google Scholar] [CrossRef]
- Mauro, M.; Pérez-Arjona, I.; Perez, E.J.B.; Ceraulo, M.; Bou-Cabo, M.; Benson, T.; Espinosa, V.; Beltrame, F.; Mazzola, S.; Vazzana, M.; et al. The effect of low frequency noise on the behaviour of juvenile Sparus aurata. J. Acoust. Soc. Am. 2020, 147, 3795–3807. [Google Scholar] [CrossRef] [PubMed]
- Mauro, M.; Lazzara, V.; Arizza, V.; Luparello, C.; Ferrantelli, V.; Cammilleri, G.; Inguglia, L.; Vazzana, M. Human Drug Pollution in the Aquatic System: The Biochemical Responses of Danio rerio Adults. Biology 2021, 10, 1064. [Google Scholar] [CrossRef] [PubMed]
- Luparello, C.; Mauro, M.; Lazzara, V.; Vazzana, M. Collective Locomotion of Human Cells, Wound Healing and Their Control by Extracts and Isolated Compounds from Marine Invertebrates. Molecules 2020, 25, E2471. [Google Scholar] [CrossRef] [PubMed]
- Luparello, C.; Mauro, M.; Arizza, V.; Vazzana, M. Histone Deacetylase Inhibitors from Marine Invertebrates. Biology 2020, 9, E429. [Google Scholar] [CrossRef] [PubMed]
- Inguglia, L.; Chiaramonte, M.; Di Stefano, V.; Schillaci, D.; Cammilleri, G.; Pantano, L.; Mauro, M.; Vazzana, M.; Ferrantelli, V.; Nicolosi, R.; et al. Salmo salar fish waste oil: Fatty acids composition and antibacterial activity. PeerJ 2020, 8, e9299. [Google Scholar] [CrossRef]
- Mauro, M.; Queiroz, V.; Arizza, V.; Campobello, D.; Custódio, M.R.; Chiaramonte, M.; Vazzana, M. Humoral responses during wound healing in Holothuria tubulosa (Gmelin, 1788). Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2021, 253, 110550. [Google Scholar] [CrossRef]
- Vizzini, A.; Bonura, A.; La Paglia, L.; Fiannaca, A.; La Rosa, M.; Urso, A.; Mauro, M.; Vazzana, M.; Arizza, V. Transcriptomic Analyses Reveal 2 and 4 Family Members of Cytochromes P450 (CYP) Involved in LPS Inflammatory Response in Pharynx of Ciona robusta. Int. J. Mol. Sci. 2021, 22, 11141. [Google Scholar] [CrossRef]
- Branchet, P.; Arpin-Pont, L.; Piram, A.; Boissery, P.; Wong-Wah-Chung, P.; Doumenq, P. Pharmaceuticals in the marine environment: What are the present challenges in their monitoring? Sci. Total Environ. 2021, 766, 142644. [Google Scholar] [CrossRef]
- Faleye, A.C.; Adegoke, A.A.; Ramluckan, K.; Bux, F.; Stenström, T.A. Identification of antibiotics in wastewater: Current state of extraction protocol and future perspectives. J. Water Health 2017, 15, 982–1003. [Google Scholar] [CrossRef]
- Madikizela, L.M.; Tavengwa, N.T.; Chimuka, L. Status of pharmaceuticals in African water bodies: Occurrence, removal and analytical methods. J. Environ. Manag. 2017, 193, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-Y.; Wang, Q.-H.; Hao, R.-J.; Liao, Y.-S.; Du, X.-D.; Deng, Y.-W. Effects of replacing microalgae with an artificial diet on pearl production traits and mineralization-related gene expression in pearl oyster Pinctada fucata martensii. Aquac. Res. 2017, 48, 5331–5337. [Google Scholar] [CrossRef]
- Zhong, Y.; Chen, Z.-F.; Dai, X.; Liu, S.-S.; Zheng, G.; Zhu, X.; Liu, S.; Yin, Y.; Liu, G.; Cai, Z. Investigation of the interaction between the fate of antibiotics in aquafarms and their level in the environment. J. Environ. Manag. 2018, 207, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Aydin, S.; Aydin, M.E.; Ulvi, A.; Kilic, H. Antibiotics in hospital effluents: Occurrence, contribution to urban wastewater, removal in a wastewater treatment plant, and environmental risk assessment. Environ. Sci. Pollut. Res. 2019, 26, 544–558. [Google Scholar] [CrossRef]
- Le-Minh, N.; Khan, S.J.; Drewes, J.E.; Stuetz, R.M. Fate of antibiotics during municipal water recycling treatment processes. Water Res. 2010, 44, 4295–4323. [Google Scholar] [CrossRef]
- Gracia-Lor, E.; Sancho, J.V.; Serrano, R.; Hernández, F. Occurrence and removal of pharmaceuticals in wastewater treatment plants at the Spanish Mediterranean area of Valencia. Chemosphere 2012, 87, 453–462. [Google Scholar] [CrossRef]
- Carvalho, I.T.; Santos, L. Antibiotics in the aquatic environments: A review of the European scenario. Environ. Int. 2016, 94, 736–757. [Google Scholar] [CrossRef]
- Ekpeghere, K.I.; Sim, W.-J.; Lee, H.-J.; Oh, J.-E. Occurrence and distribution of carbamazepine, nicotine, estrogenic compounds, and their transformation products in wastewater from various treatment plants and the aquatic environment. Sci. Total Environ. 2018, 640–641, 1015–1023. [Google Scholar] [CrossRef]
- Assress, H.A.; Nyoni, H.; Mamba, B.B.; Msagati, T.A. Occurrence and risk assessment of azole antifungal drugs in water and wastewater. Ecotoxicol. Environ. Saf. 2020, 187, 109868. [Google Scholar] [CrossRef]
- Mearns, A.J.; Reish, D.J.; Oshida, P.S.; Ginn, T.; Rempel-Hester, M.A.; Arthur, C.; Rutherford, N.; Pryor, R. Effects of Pollution on Marine Organisms. Water Environ. Res. 2015, 87, 1718–1816. [Google Scholar] [CrossRef]
- Fabbri, E.; Franzellitti, S. Human pharmaceuticals in the marine environment: Focus on exposure and biological effects in animal species: Fate and Effects of Pharmaceuticals in Marine Environments. Environ. Toxicol. Chem. 2015, 35, 799–812. [Google Scholar] [CrossRef] [PubMed]
- Mezzelani, M.; Gorbi, S.; Da Ros, Z.; Fattorini, D.; D’Errico, G.; Milan, M.; Bargelloni, L.; Regoli, F. Ecotoxicological potential of non-steroidal anti-inflammatory drugs (NSAIDs) in marine organisms: Bioavailability, biomarkers and natural occurrence in Mytilus galloprovincialis. Mar. Environ. Res. 2016, 121, 31–39. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, L.L.D.; Antunes, S.C.; Gonçalves, F.; Rocha, O.; Nunes, B. Acute and chronic ecotoxicological effects of four pharmaceuticals drugs on cladoceran Daphnia magna. Drug Chem. Toxicol. 2016, 39, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.R.; Tacão, M.; Machado, A.L.; Golovko, O.; Zlabek, V.; Domingues, I.; Henriques, I. Long-term effects of oxytetracycline exposure in zebrafish: A multi-level perspective. Chemosphere 2019, 222, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Giménez, V.; Nunes, B. Effects of commonly used therapeutic drugs, paracetamol, and acetylsalicylic acid, on key physiological traits of the sea snail Gibbula umbilicalis. Environ. Sci. Pollut. Res. 2019, 26, 21858–21870. [Google Scholar] [CrossRef] [PubMed]
- Di Paola, D.; Iaria, C.; Capparucci, F.; Cordaro, M.; Crupi, R.; Siracusa, R.; D’Amico, R.; Fusco, R.; Impellizzeri, D.; Cuzzocrea, S.; et al. Aflatoxin B1 Toxicity in Zebrafish Larva (Danio rerio): Protective Role of Hericium erinaceus. Toxins 2021, 13, 710. [Google Scholar] [CrossRef] [PubMed]
- Di Paola, D.; Capparucci, F.; Abbate, J.M.; Cordaro, M.; Crupi, R.; Siracusa, R.; D’Amico, R.; Fusco, R.; Genovese, T.; Impellizzeri, D.; et al. Environmental Risk Assessment of Oxaliplatin Exposure on Early Life Stages of Zebrafish (Danio rerio). Toxics 2022, 10, 81. [Google Scholar] [CrossRef]
- Bonnefille, B.; Gomez, E.; Alali, M.; Rosain, D.; Fenet, H.; Courant, F. Metabolomics assessment of the effects of diclofenac exposure on Mytilus galloprovincialis: Potential effects on osmoregulation and reproduction. Sci. Total Environ. 2018, 613–614, 611–618. [Google Scholar] [CrossRef]
- Sukul, P.; Spiteller, M. Sulfonamides in the environment as veterinary drugs. Rev. Environ. Contam. Toxicol. 2006, 187, 67–101. [Google Scholar]
- Apaydın, S.; Török, M. Sulfonamide derivatives as multi-target agents for complex diseases. Bioorganic Med. Chem. Lett. 2019, 29, 2042–2050. [Google Scholar] [CrossRef]
- Wishart, D.S.; Feunang, Y.D.; Guo, A.C.; Lo, E.J.; Marcu, A.; Grant, J.R.; Sajed, T.; Johnson, D.; Li, C.; Sayeeda, Z.; et al. DrugBank 5.0: A Major Update to the DrugBank Database for 2018. Nucleic Acids Res. 2018, 46, D1074–D1082. [Google Scholar] [CrossRef] [PubMed]
- Blair, B.; Nikolaus, A.; Hedman, C.; Klaper, R.; Grundl, T. Evaluating the degradation, sorption, and negative mass balances of pharmaceuticals and personal care products during wastewater treatment. Chemosphere 2015, 134, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mozaz, S.; Vaz-Moreira, I.; Varela Della Giustina, S.; Llorca, M.; Barceló, D.; Schubert, S.; Berendonk, T.U.; Michael-Kordatou, I.; Fatta-Kassinos, D.; Martinez, J.L.; et al. Antibiotic residues in final effluents of European wastewater treatment plants and their impact on the aquatic environment. Environ. Int. 2020, 140, 105733. [Google Scholar] [CrossRef]
- Limbu, S.M.; Zhou, L.; Sun, S.-X.; Zhang, M.-L.; Du, Z.-Y. Chronic exposure to low environmental concentrations and legal aquaculture doses of antibiotics cause systemic adverse effects in Nile tilapia and provoke differential human health risk. Environ. Int. 2018, 115, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Xu, X.; Wei, X.; Wan, J.; Zhang, Y. Biomarker Effects in Carassius auratus Exposure to Ofloxacin, Sulfamethoxazole and Ibuprofen. Int. J. Environ. Res. Public Health 2019, 16, 1628. [Google Scholar] [CrossRef] [PubMed]
- Serra-Compte, A.; Álvarez-Muñoz, D.; Solé, M.; Cáceres, N.; Barceló, D.; Rodríguez-Mozaz, S. Comprehensive study of sulfamethoxazole effects in marine mussels: Bioconcentration, enzymatic activities and metabolomics. Environ. Res. 2019, 173, 12–22. [Google Scholar] [CrossRef]
- Drzymała, J.; Kalka, J. Ecotoxic interactions between pharmaceuticals in mixtures: Diclofenac and sulfamethoxazole. Chemosphere 2020, 259, 127407. [Google Scholar] [CrossRef]
- Duan, W.; Cui, H.; Jia, X.; Huang, X. Occurrence and ecotoxicity of sulfonamides in the aquatic environment: A review. Sci. Total Environ. 2022, 820, 153178. [Google Scholar] [CrossRef]
- Thiebault, T. Sulfamethoxazole/Trimethoprim ratio as a new marker in raw wastewaters: A critical review. Sci. Total Environ. 2020, 715, 136916. [Google Scholar] [CrossRef]
- Lazzara, V.; Arizza, V.; Luparello, C.; Mauro, M.; Vazzana, M. Bright Spots in the Darkness of Cancer: A Review of Starfishes-Derived Compounds and Their Anti-Tumor Action. Mar. Drugs 2019, 17, 617. [Google Scholar] [CrossRef]
- Mauro, M.; Lazzara, V.; Punginelli, D.; Arizza, V.; Vazzana, M. Antitumoral compounds from vertebrate sister group: A review of Mediterranean ascidians. Dev. Comp. Immunol. 2020, 108, 103669. [Google Scholar] [CrossRef] [PubMed]
- Punginelli, D.; Schillaci, D.; Mauro, M.; Deidun, A.; Barone, G.; Arizza, V.; Vazzana, M. The potential of antimicrobial peptides isolated from freshwater crayfish species in new drug development: A review. Dev. Comp. Immunol. 2021, 126, 104258. [Google Scholar] [CrossRef] [PubMed]
- Marques, J.; Vilanova, E.; Mourão, P.A.S.; Fernàndez-Busquets, X. Marine organism sulfated polysaccharides exhibiting significant antimalarial activity and inhibition of red blood cell invasion by Plasmodium. Sci. Rep. 2016, 6, 24368. [Google Scholar] [CrossRef] [PubMed]
- Parisi, M.G.; Mauro, M.; Sarà, G.; Cammarata, M. Temperature increases, hypoxia, and changes in food availability affect immunological biomarkers in the marine mussel Mytilus galloprovincialis. J. Comp. Physiol. B 2017, 187, 1117–1126. [Google Scholar] [CrossRef]
- Pinto, J.; Costa, M.; Leite, C.; Borges, C.; Coppola, F.; Henriques, B.; Monteiro, R.; Russo, T.; Di Cosmo, A.; Soares, A.M.; et al. Ecotoxicological effects of lanthanum in Mytilus galloprovincialis: Biochemical and histopathological impacts. Aquat. Toxicol. 2019, 211, 181–192. [Google Scholar] [CrossRef]
- Świacka, K.; Maculewicz, J.; Smolarz, K.; Szaniawska, A.; Caban, M. Mytilidae as model organisms in the marine ecotoxicology of pharmaceuticals—A review. Environ. Pollut. 2019, 254, 113082. [Google Scholar] [CrossRef]
- Vazzana, M.; Mauro, M.; Ceraulo, M.; Dioguardi, M.; Papale, E.; Mazzola, S.; Arizza, V.; Beltrame, F.; Inguglia, L.; Buscaino, G. Underwater high frequency noise: Biological responses in sea urchin Arbacia lixula (Linnaeus, 1758). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2020, 242, 110650. [Google Scholar] [CrossRef]
- Vazzana, M.; Ceraulo, M.; Mauro, M.; Papale, E.; Dioguardi, M.; Mazzola, S.; Arizza, V.; Chiaramonte, M.; Buscaino, G. Effects of acoustic stimulation on biochemical parameters in the digestive gland of Mediterranean mussel Mytilus galloprovincialis (Lamarck, 1819). J. Acoust. Soc. Am. 2020, 147, 2414–2422. [Google Scholar] [CrossRef]
- Morroni, L.; Rakaj, A.; Grosso, L.; Fianchini, A.; Pellegrini, D.; Regoli, F. Sea cucumber Holothuria polii (Delle Chiaje, 1823) as new model for embryo bioassays in ecotoxicological studies. Chemosphere 2020, 240, 124819. [Google Scholar] [CrossRef]
- Böttger, S.A.; McClintock, J.B. The effects of organic and inorganic phosphates on fertilization and early development in the sea urchin Lytechinus variegatus (Echinodermata: Echinoidea). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2001, 129, 307–315. [Google Scholar] [CrossRef]
- Arslan, O.C.; Parlak, H. Embryotoxic effects of nonylphenol and octylphenol in sea urchin Arbacia lixula. Ecotoxicology 2007, 16, 439–444. [Google Scholar] [CrossRef] [PubMed]
- OECD. Test No. 211: Daphnia magna Reproduction Test, OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 2012. [Google Scholar] [CrossRef]
- His, E.; Heyvang, I.; Geffard, O.; de Montaudouin, X. A comparison between oyster (Crassostrea gigas) and sea urchin (Paracentrotus lividus) larval bioassays for toxicological studies. Water Res. 1999, 33, 1706–1718. [Google Scholar] [CrossRef]
- Marin, M.G.; Moschino, V.; Cima, F.; Celli, C. Embryotoxicity of butyltin compounds to the sea urchin Paracentrotus lividus. Mar. Environ. Res. 2000, 50, 231–235. [Google Scholar] [CrossRef]
- Volpi Ghirardini, A.; Arizzi Novelli, A.; Tagliapietra, D. Sediment toxicity assessment in the Lagoon of Venice (Italy) using Paracentrotus lividus (Echinodermata: Echinoidea) fertilization and embryo bioassays. Environ. Int. 2005, 31, 1065–1077. [Google Scholar] [CrossRef] [PubMed]
- Dacie, J.V.; Lewis, S.M. Practical Haematology, 12th ed.; Bain, B.J., Bates, I., Laffan, M.A., Lewis, S.M., Eds.; Elsevier: Philadelphia, PA, USA, 2017. [Google Scholar]
- Heberer, T. Occurrence, fate, and removal of pharmaceutical residues in the aquatic environment: A review of recent research data. Toxicol. Lett. 2002, 131, 5–17. [Google Scholar] [CrossRef]
- Aulsebrook, L.C.; Bertram, M.G.; Martin, J.M.; Aulsebrook, A.E.; Brodin, T.; Evans, J.P.; Hall, M.D.; O’Bryan, M.K.; Pask, A.J.; Tyler, C.R.; et al. Reproduction in a polluted world: Implications for wildlife. Reproduction 2020, 160, R13–R23. [Google Scholar] [CrossRef]
- Lewis, C.; Ford, A.T. Infertility in male aquatic invertebrates: A review. Aquat. Toxicol. 2012, 120–121, 79–89. [Google Scholar] [CrossRef]
- Ribeiro, S.; Torres, T.; Martins, R.; Santos, M.M. Toxicity screening of Diclofenac, Propranolol, Sertraline and Simvastatin using Danio rerio and Paracentrotus lividus embryo bioassays. Ecotoxicol. Environ. Saf. 2015, 114, 67–74. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Pan, B.; Wang, C.; Bao, S.; Nie, X. Toxic effects of diclofenac on life history parameters and the expression of detoxification-related genes in Daphnia magna. Aquat. Toxicol. 2017, 183, 104–113. [Google Scholar] [CrossRef]
- Tyumina, E.A.; Bazhutin, G.A.; Cartagena Gómez, A.D.P.; Ivshina, I.B. Nonsteroidal Anti-inflammatory Drugs as Emerging Contaminants. Microbiology 2020, 89, 148–163. [Google Scholar] [CrossRef]
- Mohd Zanuri, N.B.; Bentley, M.G.; Caldwell, G.S. Assessing the impact of diclofenac, ibuprofen and sildenafil citrate (Viagra®) on the fertilisation biology of broadcast spawning marine invertebrates. Mar. Environ. Res. 2017, 127, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Pesando, D.; Robert, S.; Huitorel, P.; Gutknecht, E.; Pereira, L.; Girard, J.-P.; Ciapa, B. Effects of methoxychlor, dieldrin and lindane on sea urchin fertilization and early development. Aquat. Toxicol. 2004, 66, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Lewis, C.; Galloway, T. Reproductive Consequences of Paternal Genotoxin Exposure in Marine Invertebrates. Environ. Sci. Technol. 2009, 43, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Mello, L.C.; da Fonseca, T.G.; de Souza, A.D.M. Ecotoxicological assessment of chemotherapeutic agents using toxicity tests with embryos of Mellita quinquiesperforata. Mar. Pollut. Bull. 2020, 159, 111493. [Google Scholar] [CrossRef]
- Van Kesteren, C.; Beijnen, J.H.; Schellens, J.H.M. E7070: A novel synthetic sulfonamide targeting the cell cycle progression for the treatment of cancer. Anti-Cancer Drugs 2002, 13, 989–997. [Google Scholar] [CrossRef]
- Yi, R.; Ohno, Y.; Tian, Z.; Guo, S.; Chen, W.; Ma, X.; Win, N.N.; Li, Q.; Vahed, M.; Saito, K.; et al. CCL113, a novel sulfonamide, induces selective mitotic arrest and apoptosis in HeLa and HepG2 cells. Oncol. Rep. 2020, 44, 2770–2782. [Google Scholar] [CrossRef]
- Nunes, B.; Antunes, S.C.; Gomes, R.; Campos, J.C.; Braga, M.R.; Ramos, A.S.; Correia, A.T. Acute Effects of Tetracycline Exposure in the Freshwater Fish Gambusia holbrooki: Antioxidant Effects, Neurotoxicity and Histological Alterations. Arch. Environ. Contam. Toxicol. 2015, 68, 371–381. [Google Scholar] [CrossRef]
- Liu, J.; Wei, T.; Wu, X.; Zhong, H.; Qiu, W.; Zheng, Y. Early exposure to environmental levels of sulfamethoxazole triggers immune and inflammatory response of healthy zebrafish larvae. Sci. Total Environ. 2020, 703, 134724. [Google Scholar] [CrossRef]
- Medina, H.S.G. The effect of diamino diphenyl sulfone on the embryonic development of eggs from the sea urchin (Lytechinus variegatus). Comp. Biochem. Physiol. Part C Comp. Pharmacol. 1986, 83, 295–306. [Google Scholar] [CrossRef]
- Ragusa, M.A.; Costa, S.; Cuttitta, A.; Gianguzza, F.; Nicosia, A. Coexposure to sulfamethoxazole and cadmium impairs development and attenuates transcriptional response in sea urchin embryo. Chemosphere 2017, 180, 275–284. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazzara, V.; Mauro, M.; Celi, M.; Cammilleri, G.; Vizzini, A.; Luparello, C.; Bellini, P.; Ferrantelli, V.; Vazzana, M. Effects of Sulfamethoxazole on Fertilization and Embryo Development in the Arbacia lixula Sea Urchin. Animals 2022, 12, 2483. https://doi.org/10.3390/ani12182483
Lazzara V, Mauro M, Celi M, Cammilleri G, Vizzini A, Luparello C, Bellini P, Ferrantelli V, Vazzana M. Effects of Sulfamethoxazole on Fertilization and Embryo Development in the Arbacia lixula Sea Urchin. Animals. 2022; 12(18):2483. https://doi.org/10.3390/ani12182483
Chicago/Turabian StyleLazzara, Valentina, Manuela Mauro, Monica Celi, Gaetano Cammilleri, Aiti Vizzini, Claudio Luparello, Paola Bellini, Vincenzo Ferrantelli, and Mirella Vazzana. 2022. "Effects of Sulfamethoxazole on Fertilization and Embryo Development in the Arbacia lixula Sea Urchin" Animals 12, no. 18: 2483. https://doi.org/10.3390/ani12182483
APA StyleLazzara, V., Mauro, M., Celi, M., Cammilleri, G., Vizzini, A., Luparello, C., Bellini, P., Ferrantelli, V., & Vazzana, M. (2022). Effects of Sulfamethoxazole on Fertilization and Embryo Development in the Arbacia lixula Sea Urchin. Animals, 12(18), 2483. https://doi.org/10.3390/ani12182483







