Aggressive, Submissive, and Affiliative Behavior in Sanctuary Chimpanzees (Pan Troglodytes) During Social Integration
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Facilities
Enclosures Utilized during Social Integrations
2.2. Subjects
2.3. Methods
2.3.1. Data Collection
2.3.2. Data Transcription
2.3.3. Data Analysis
3. Results
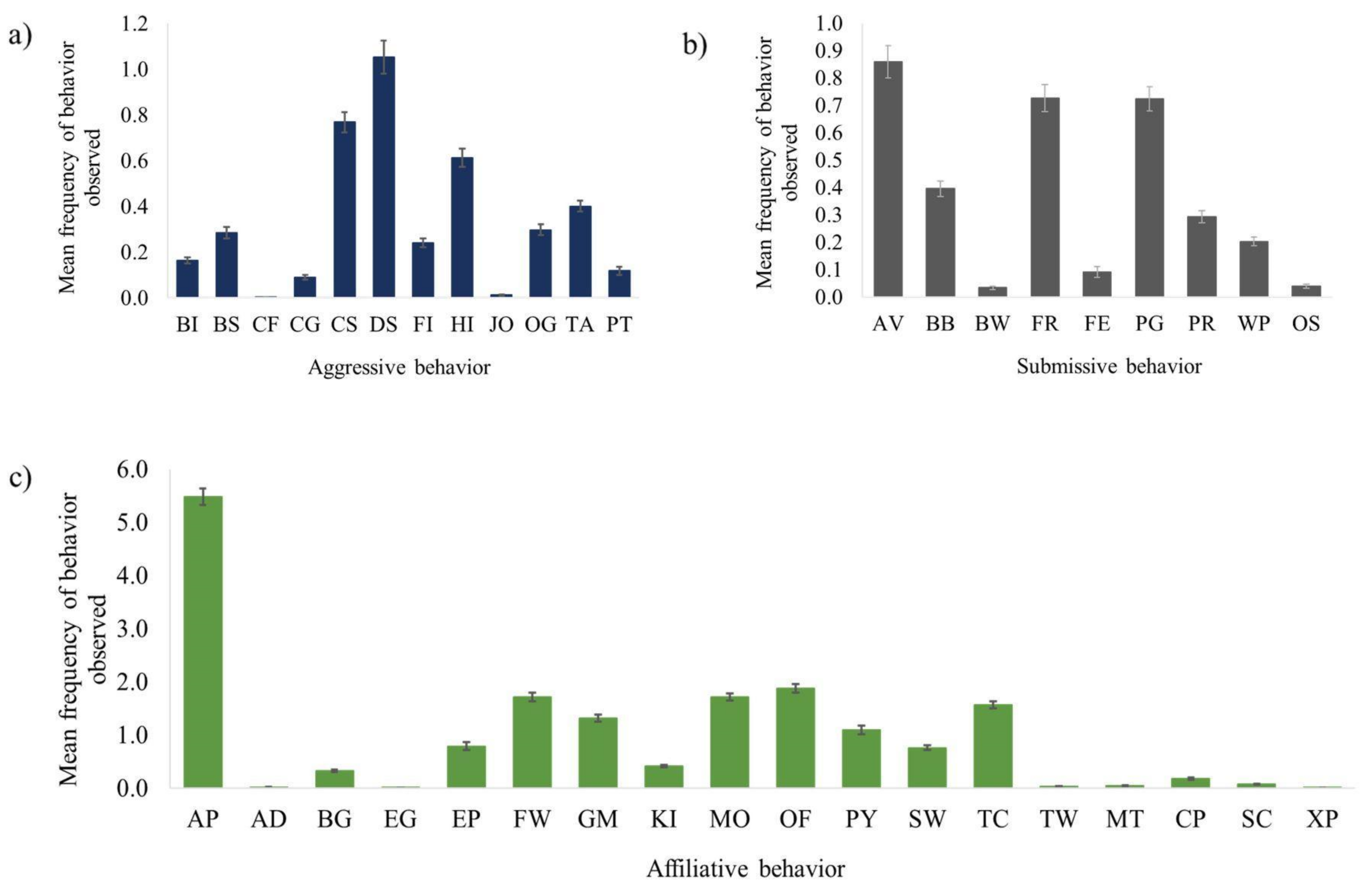
3.1. Aggressive Behavior
3.2. Submissive Behavior
3.3. Affiliative Behavior
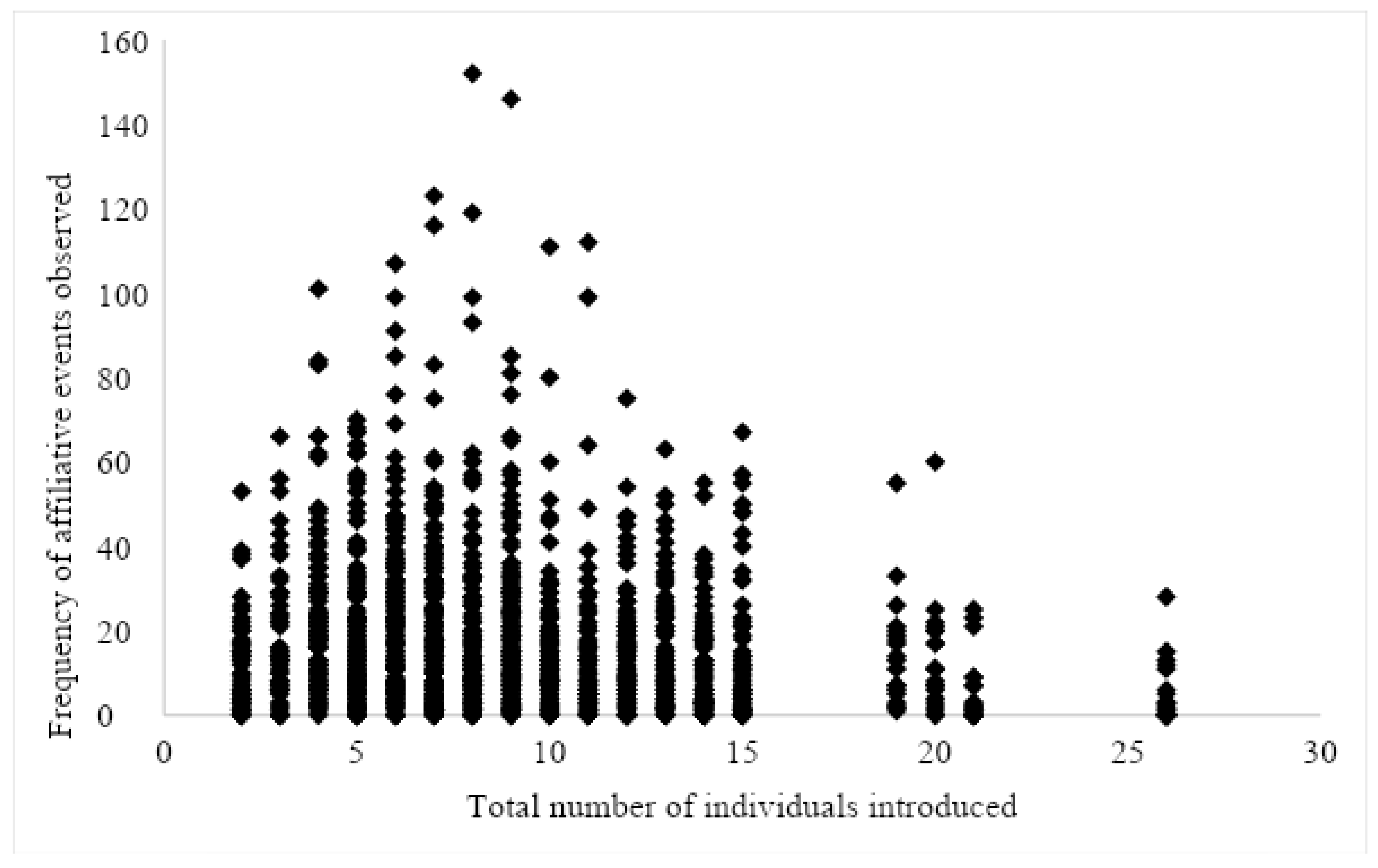
3.4. Location, Group Size, and Age Differences
4. Discussion
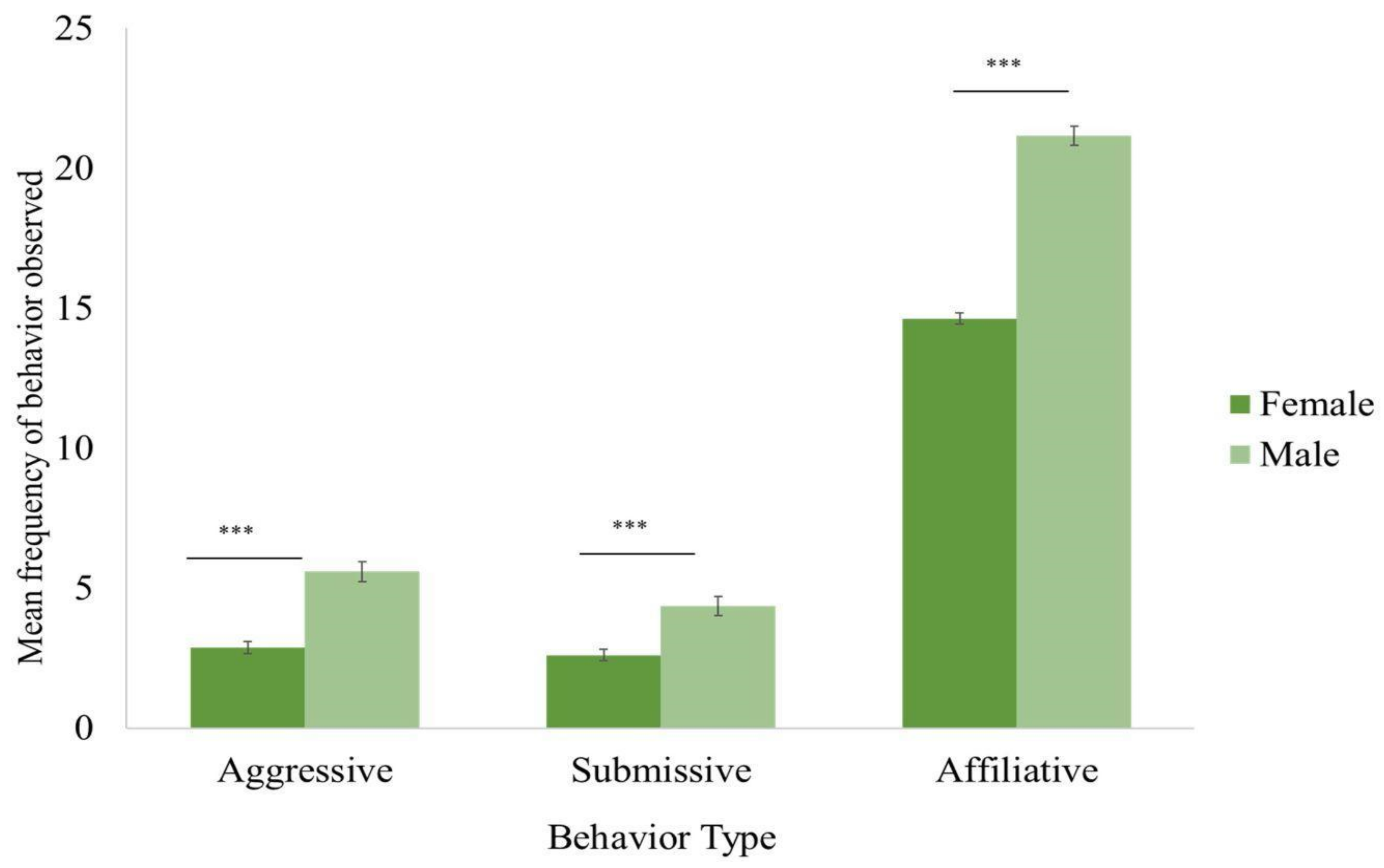
4.1. Sex Differences
4.1.1. Aggressive Behavior
4.1.2. Submissive Behavior
4.1.3. Affiliative Behavior
4.2. Rearing and Birth Status Differences
4.3. Location (Closed vs. Open), Group Size, and Age Differences
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| AFFILIATIVE BEHAVIOR | ||
|---|---|---|
| TWO LETTER CODE | BEHAVIOR | DEFINITION |
| AD | Aid | Assist another in an aggressive encounter. |
| AP | Approach | Move toward another chimpanzee intentionally with focus on that animal. |
| EB | Embrace | Two animals place arms around each other. |
| EF | Enlist to follow | Using facial expressions or physical contact, one chimpanzee encourages another chimpanzee to follow or travel to another location. Usually includes waiting and watching the second chimpanzee to get up and move toward or with the inviting party. |
| EG | Enlist to Groom | Inviting another to groom by presenting body part or other gesturing. |
| EP | Enlist to Play | Inviting another to participate in relaxed, mutually affiliative contact or chase using behavioral gestures or facial cues such as a play face. |
| FW | Follow | Walk after or directly behind another, maintaining close proximity without contact. |
| GM | Groom | Using fingers/lips to remove dead skin, dirt, or insects from another chimp. |
| GO | Groom other | Using fingers/lips to remove dead skin, dirt, or insects from another chimp with multiple individuals participating at the same time. |
| KI | Kiss | Open mouth contact with another individual’s mouth. |
| LA | Laugh | Soft pants that may become jerky pant grunts, usually with play face during a play session. |
| MO | Mouth | Open, relaxed jaw is placed on any part of the body other than the mouth as a friendly reassuring gesture. |
| MT | Mount | Embrace another with both arms from behind. Often seen during reunions. |
| OF | Other Affiliative | Positive interactions that are not defined. |
| PA | Pant | Breathy pants delivered in rapid rhythmic succession. Sound is similar to fast rhythmic breathing. Mouth relaxed; body may shake. Directed at another individual sometimes with bobbing of head. May accompany kissing or embracing. |
| PH | Pant Hoot | Series of long calls that build in intensity. May be a subordinate greeting or vocalization heard during times of high excitement. May also precede a male display. |
| PM | Play with multiple | Engaging in positive, relaxed interactions, sometimes with laughter, with multiple conspecifics at the same time. |
| PY | Play | Engaging in relaxed, positive interactions that are incompletely functional, spontaneous (not forced), pleasurable, and repetitive (not stereotypical) with conspecific(s). This may include relaxed chase, rough and tumble play, wrestling, tickling, etc. |
| RH | Reach Out | An individual extends their arm and presents a flat palm towards other individual. This may look like a chimpanzee trying to shake another’s hand or may be involved in greetings or asking for reassurance. |
| SM | Smell | Put nose close to other individual and sniff. Alternatively, an animal may touch another animal and then sniff his or her finger or hand. |
| SW | Sit with | Sit with other individual in physical contact. |
| TC | Touch | Physical contact with another in a friendly manner. |
| SEXUAL BEHAVIOR (INCLUDED UNDER AFFILIATIVE HERE) | ||
| CP | Copulate | Mating between male and estrous female. |
| IN | Inspect | Exploring genital area of another individual with hands. |
| MA | Masturbate | Self-stimulation of genitals; may be with an object. |
| OX | Other Sexual | Includes females presenting and mounting other females or males grabbing another male’s genitals. |
| SC | Solicit Copulation | Male presents erect penis to female, or otherwise gestures by stomping his feet or using vocalizations to entice a female to engage in copulation. Estrous female presents or gestures by crouching and fear grimacing to invite copulation. |
| XP | Sexual Present | Estrous female shows her swelling to male. |
| AGGRESSIVE BEHAVIOR | ||
| BI | Bite | Bruising, puncturing, or tearing another’s skin with teeth. |
| BS | Bipedal Swagger | Swaying rhythmically in an upright posture from one foot to the other. May remain in one spot or move forward. The arms are held out from the body and the shoulders are hunched up. Often precedes a display. |
| CG | Charge | Fast movement targeted at another individual that may end with hitting or stomping on intended target. May also include running at or past another animal in aggressive context without physical contact. |
| CS | Chase | Run after a fleeing individual during aggressive dispute. |
| CH | Cough Threat | A grunt-like sound given as a warning and usually directed down the hierarchy. |
| DS | Display | Chimpanzee may charge, vocalize, run, and throw objects or make loud sounds with cage furnishings or may run or jump towards or past a targeted individual. Common in male dominance rivalry, most often chimpanzee is pilo-erect at time of display. |
| FI | Fight | Physical fighting (contact aggression) with another conspecific. Is not mutually exclusive and can include biting, hitting, grabbing, punching, poking in quick succession, often involving many individuals. |
| GB | Grab | Grasping at or cuff another, usually directed down the hierarchy. |
| HI | Hit | To use hands or feet to strike another individual in an aggressive manner. |
| INT | Intimidate | To look at, lunge at, or otherwise threaten an individual to gain access to a resource such as food or a preferred location. |
| JO | Jump On | During an aggressive dispute, one individual leaps onto the body of another (often seen as part of an attack, display, or chase). |
| OG | Other Aggressive | Negative interactions that are not defined. |
| PT | Pilo-erect | Bristling hair; hair standing on end. Usually seen prior to display or aggression. |
| PU | Push | Shove another individual out of the way. |
| SR | Scream | A sharp, high-pitched vocalization sometimes accompanied by a fear grimace. Context can include submission or excitement. |
| ST | Spit | Chimpanzee uses water or saliva to spit at another individual, human, or chimp. |
| STE | Steal | To take an item, may be food, an enrichment item, or a “possession” from an individual. The individual being stolen from is typically distraught. |
| TA | Hit At | To rapidly hit at another chimp without making contact. |
| TH | Throw feces | Using hands to toss excrement at either people or chimps. |
| TO | Throw other | Throw toys, enrichment items, or other objects besides feces. |
| SUBMISSIVE BEHAVIOR | ||
| AV | Avoid | Deliberate locomotion away from another individual (not running). |
| BB | Bob | The body moves up and down as elbows are flexed and straightened. Typically, when a high-ranking individual passes or approaches or when greeting another chimpanzee. |
| BW | Bow | Like Bob, however, this involves deep flexion of legs with arms flexed forward, so that head is lower than hips. |
| CR | Cry | High-pitched whining vocalization. |
| FL | Flee | Run away from altercation, situation, or individuals. |
| FR | Fear Grimace | Lips drawn back to expose teeth and/or gums; may be accompanied by screaming or whimpering. |
| HO | Hoo | Low-pitched vocalization, which may be repeated. Usually in the context of apprehension. |
| OS | Other Submissive | Deferential behavior that is not defined. |
| PG | Pant Grunt | A series of soft or loud grunts given during greeting by submissive chimpanzees and during submissive interactions. |
| PR | Present | Subordinate shows hindquarters to dominant animal in non-sexual manner. |
| WH | Whimper | The mouth is closed with corners retracted. Lips are curled outward and protrude, especially the upper lip. Context is usually apprehension. |
| WP | Wrist Present | An individual extends their arm and presents an outstretched bent wrist as a submissive gesture to a dominant individual. |
References
- Ross, S.R. Captive Chimpanzees. In The Ethics of Captivity; Gruen, L., Ed.; Oxford University Press: New York, NY, USA, 2014; pp. 57–76. [Google Scholar]
- Hirata, S.; Morimura, N.; Watanuki, K.; Ross, S.R. The Establishment of Sanctuaries for Former Laboratory Chimpanzees: Challenges, Successes, and Cross-Cultural Context. In Chimpanzees in Context; Hopper, L.M., Ross, S.R., Eds.; University of Chicago Press: Chicago, IL, USA, 2020; pp. 552–582. [Google Scholar]
- Bloomsmith, M.A.; Clay, A.W.; Lambeth, S.P.; Lutz, C.K.; Breaux, S.D.; Lammey, M.L.; Franklin, A.N.; Neu, K.A.; Perlman, J.E.; Reamer, L.A.; et al. Survey of behavioral indices of welfare in research chimpanzees (Pan troglodytes) in the United States. J. Am. Assoc. Lab. Anim. Sci. 2019, 58, 160–177. [Google Scholar] [CrossRef]
- Goodall, J. The Chimpanzees of Gombe: Patterns of Behavior; Belknap Press of Harvard University Press: Cambridge, MA, USA, 1986. [Google Scholar]
- Mitani, J.C.; Amsler, S.J.; Sobolewski, M.E. Chimpanzee Minds in Nature. In The Mind of the Chimpanzee; Lonsdorf, E.V., Ross, S.R., Matsuzawa, T., Eds.; University of Chicago Press: Chicago, IL, USA, 2010; pp. 181–191. [Google Scholar]
- Matsuzawa, T. The Chimpanzee Mind: Bridging Fieldwork and Laboratory Work. In The Mind of the Chimpanzee; Lonsdorf, E.V., Ross, S.R., Matsuzawa, T., Eds.; University of Chicago Press: Chicago, IL, USA, 2010; pp. 1–19. [Google Scholar]
- AZA Ape TAG. Chimpanzee (Pan troglodytes) Care Manual; Association of Zoos and Aquariums: Silver Spring, MD, USA, 2010. [Google Scholar]
- National Institutes of Health Council of Councils. Use of chimpanzees in NIH-supported research. 2013. Available online: http://dpcpsi.nih.gov/council/chimpanzee_research (accessed on 13 June 2022).
- ChimpCARE. Available online: https://chimpcare.org/map (accessed on 21 June 2022).
- Brent, L.; Lee, D.R.; Eichberg, J.W. The effects of single caging on chimpanzee behavior. Lab. Anim. Sci. 1989, 39, 345–346. [Google Scholar] [PubMed]
- Jacobson, S.L.; Freeman, H.D.; Santymire, R.M.; Ross, S.R. Atypical experiences of captive chimpanzees (Pan troglodytes) are associated with higher hair cortisol concentrations as adults. R. Soc. Open Sci. 2017, 4, 170932. [Google Scholar] [CrossRef] [PubMed]
- United States Department of Agriculture; Animal and Plant Health Inspection Service. USDA Animal Care: Animal Welfare Act and Animal Welfare Regulations. 2017. Available online: https://naldc.nal.usda.gov/catalog/5969370 (accessed on 5 September 2022).
- Webb, S.J.N.; Hau, J.; Schapiro, S.J. Does group size matter? Captive chimpanzee (Pan troglodytes) behavior as a function of group size and composition. Am. J. Primatol. 2019, 81, e22947. [Google Scholar] [CrossRef]
- Thunstrom, M.; Persson, T.; Bjorklund, M. Integration of a hand-reared chimpanzee (Pan troglodytes) infant into a social group of conspecifics. Primates 2013, 54, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Clay, A.W.; Ross, S.R.; Lambeth, S.; Vazquez, M.; Breaux, S.; Pietsch, R.; Fultz, A.; Lammey, M.; Jacobson, S.L.; Perlman, J.E.; et al. Chimpanzees (Pan troglodytes) in U.S. zoos, sanctuaries, and research facilities: A survey-based comparison of species-typical behaviors. Animals 2022. submitted. [Google Scholar]
- Ross, S.R. Chimpanzee Welfare in the Context of Science, Policy, and Practice. In Chimpanzees in Context; Hopper, L.M., Ross, S.R., Eds.; University of Chicago Press: Chicago, IL, USA, 2020; pp. 552–582. [Google Scholar]
- Kutsukake, N.; Teramoto, M.; Honma, S.; Mori, Y.; Ikeda, K.; Yamamoto, R.; Ishida, T.; Hasegawa, T. Behavioural and hormonal changes during group formation by male chimpanzees. Behaviour 2019, 156, 109–129. [Google Scholar] [CrossRef]
- Yamanashi, Y.; Teramoto, M.; Morimura, N.; Hirata, S.; Inoue-Murayama, M.; Idani, G. Effects of relocation and individual and environmental factors on the long-term stress levels in captive chimpanzees (Pan troglodytes): Monitoring hair cortisol and behaviors. PLoS ONE 2016, 11, e0160029. [Google Scholar] [CrossRef]
- Yamanashi, Y.; Teramoto, M.; Morimura, N.; Nogami, E.; Hirata, S. Social relationship and hair cortisol level in captive male chimpanzees (Pan troglodytes). Primates 2018, 59, 145–152. [Google Scholar] [CrossRef]
- Alford, P.L.; Bloomsmith, M.A.; Keeling, M.E.; Beck, T.F. Wounding aggression during the formation and maintenance of captive, multimale chimpanzee groups. Zoo Biol. 1995, 14, 347–359. [Google Scholar] [CrossRef]
- Williams, R.C.; Nash, L.T.; Scarry, C.J.; Videan, E.N.; Fritz, J. Factors affecting wounding aggression in a colony of captive chimpanzees (Pan troglodytes). Zoo Biol. 2010, 29, 351–364. [Google Scholar] [CrossRef] [PubMed]
- Brent, L. The Care and Management of Captive Chimpanzees; American Society of Primatologists: San Antonio, TX, USA, 2001. [Google Scholar]
- Seres, M.; Aureli, F.; de Waal, F.B.M. Successful formation of a large chimpanzee group out of two preexisting subgroups. Zoo Biol. 2001, 20, 501–515. [Google Scholar] [CrossRef]
- Feliu, O.; Masip, M.; Maté, C.; Sánchez-López, S.; Crailsheim, D.; Kalcher-Sommersguter, E. Behavioural Development of Three Former Pet Chimpanzees a Decade after Arrival at the MONA Sanctuary. Animals 2022, 12, 138. [Google Scholar] [CrossRef] [PubMed]
- Koski, S.E.; de Vries, H.; van de Kraats, A.; Sterck, E.H.M. Stability and change of social relationship quality in captive chimpanzees (Pan troglodytes). Int. J. Primatol. 2012, 33, 905–921. [Google Scholar] [CrossRef]
- Schel, A.M.; Rawlings, B.; Claidiere, N.; Wilke, C.; Wathan, J.; Richardson, J.; Pearson, S.; Herrelko, E.S.; Whiten, A.; Slocombe, K. Network analysis of social changes in a captive chimpanzee community following the successful integration of two adult groups. Am. J. Primatol. 2013, 75, 254–266. [Google Scholar] [CrossRef]
- Kalcher-Sommersguter, E.; Preuschoft, S.; Franz-Schaider, C.; Hemelrijk, C.K.; Crailsheim, K.; Massen, J.J.M. Early maternal loss affects social integration of chimpanzees throughout their lifetime. Sci. Rep. 2015, 5, 16439. [Google Scholar] [CrossRef]
- Llorente, M.; Riba, D.; Ballesta, S.; Feliu, O.; Rostan, C. Rehabilitation and socialization of chimpanzees (Pan troglodytes) used for entertainment and as ets: An 8-year study at Fundació Mona. Int. J. Primatol. 2015, 36, 605–624. [Google Scholar] [CrossRef]
- Anestis, S.F. Urinary cortisol responses to unusual events in captive chimpanzees (Pan troglodytes). Stress 2009, 12, 49–57. [Google Scholar] [CrossRef]
- Bashaw, M.J.; Gullott, R.L.; Gill, E.C. What defines successful integration into a social group for hand-reared chimpanzee infants? Primates 2010, 51, 139–147. [Google Scholar] [CrossRef]
- Fritz, J. Resocialization of Captive Chimpanzees: An Amelioration Procedure. Available online: https://awionline.org/lab-animal-search/fritz-j-1989-resocialization-captive-chimpanzees-amelioration-procedure-american (accessed on 5 September 2022).
- Mukoda, N.; Tweheyo, M. Understanding the integration process of captive chimpanzees (Pan troglodytes) in the Uganda Wildlife Education Centre. Acta Zool. Sin. 2007, 53, 399–407. [Google Scholar]
- Goldsborough, Z.; Webb, C.E.; de Waal, F.B.; van Leeuwen, E.J. Zoo-housed female chimpanzee adopts local female-specific tradition upon immigrating into a new group. Behaviour 2021, 158, 547–564. [Google Scholar] [CrossRef]
- Fultz, A.; Brent, L.; Panu, L.D. Factors associated with the formation of a large chimpanzee social group. Am. J. Primatol. 2006, 68, 98. [Google Scholar]
- Fultz, A.; Panu, L.D.; Landon, F.; Brent, L. The preliminary response of retired captive chimpanzees to increases in enclosure and group size. Int. J. Primatol. 2006, 27, 38. [Google Scholar]
- Fultz, A.; Orchard, E.A.; Brent, L. Wounding data on newly formed adult chimpanzee (Pan troglodytes) groups. Am. J. Primatol. 2007, 69, 88. [Google Scholar]
- Fritz, J.; Howell, S. Captive chimpanzee social group formations. In The Care and Management of Captive Chimpanzees, Special Topics in Primatology; Brent, L., Ed.; The American Society of Primatologists: San Antonio, TX, USA, 2001; Volume 2, pp. 173–203. [Google Scholar]
- Bloomsmith, M.A.; Baker, K.C. Social management of captive chimpanzees. In The Care and Management of Captive Chimpanzees, Special Topics in Primatology; Brent, L., Ed.; The American Society of Primatologists: San Antonio, TX, USA, 2001; Volume 2, pp. 204–241. [Google Scholar]
- McNary, J.K. Integration of chimpanzees (Pan troglodytes) in captivity. In The Care and Management of Chimpanzees (Pan Troglodytes) in Captive Environments; A Husbandry Manual Developed for the Chimpanzee Species Survival Plan; Fulk, R., Garland, C., Eds.; North Carolina Zoological Society: Asheboro, NC, USA, 1992; pp. 88–92. [Google Scholar]
- Brent, L. The influence of rearing condition on chimpanzee introductions. In Proceedings of the Apes: Challenges for the 21st Century Conference, Brookfield, IL, USA, 10–13 May 2000; pp. 103–104. [Google Scholar]
- Brent, L.; Kessel, A.L.; Barrera, H. Evaluation of introduction procedures in captive chimpanzees. Zoo Biol. 1997, 16, 335–342.1. [Google Scholar] [CrossRef]
- Project Chimps Charts Final Path to Bring All NIRC Chimps to Sanctuary. Available online: https://projectchimps.org/project-chimps-charts-final-path/#:~:text=After%20two%20years%20of%20pandemic,its%20Blue%20Ridge%2C%20GA%20sanctuary (accessed on 28 June 2022).
- The NIH Is ‘Largely Finished’ Moving Its Former Research Chimps to a Sanctuary. Available online: https://www.npr.org/2022/01/27/1075856486/nih-is-largely-finished-moving-its-former-research-chimps-to-a-sanctuary (accessed on 28 June 2022).
- OKC Zoo’s Endangered Chimpanzee, Nia, Is Pregnant. Available online: https://www.aza.org/default.aspx?p=143782&naid=28099&locale=en (accessed on 28 June 2022).
- What Do We Owe Former Lab Chimps? Available online: https://www.nationalgeographic.com/animals/article/what-do-we-owe-former-lab-chimps#:~:text=Wildlife%20Waystation%E2%80%94a%20160%2Dacre,urgent%20need%20of%20new%20homes (accessed on 28 June 2022).
- Bloomsmith, M.A.; Baker, K.C.; Ross, S.R.; Lambeth, S.P. Chimpanzee Behavior during the Process of Social Introductions. Available online: https://awionline.org/lab-animal-search/bloomsmith-m-ross-s-k-baker-k-c-2000-control-over-computer-assisted-enrichment (accessed on 5 September 2022).
- De Waal, F. Chimpanzee Politics: Power and Sex Among the Great Apes; John Hopkins University Press: Baltimore, MD, USA, 1988. [Google Scholar]
- Nishida, T.; Zamma, K.; Matsusaka, T.; Inaba, A.; McGrew, W.C. Chimpanzee Behavior in the Wild; Springer: Tokyo, Japan, 2010. [Google Scholar] [CrossRef]
- Wilson, M.L.; Wallauer, W.R.; Pusey, A.E. New cases of intergroup violence among chimpanzees in Gombe National Park, Tanzania. Int. J. Primatol. 2004, 25, 523–549. [Google Scholar] [CrossRef]
- De Waal, F.B.M. The brutal elimination of a rival among captive male chimpanzees. Ethol. Sociobiol. 1986, 7, 237–251. [Google Scholar] [CrossRef]
- Boesch, C.; Head, J.; Tagg, N.; Arandjelovic, M.; Vigilant, L.; Robbins, M.M. Fatal chimpanzee attack in Loango National Park, Gabon. Int. J. Primatol. 2007, 28, 1025–1034. [Google Scholar] [CrossRef]
- Mitani, J.C.; Watts, D.P.; Amsler, S.J. Lethal intergroup aggression leads to territorial expansion in wild chimpanzees. Curr. Biol. 2010, 20, R507–R508. [Google Scholar] [CrossRef]
- Kahlenberg, S.M.; Thompson, M.E.; Muller, M.N.; Wrangham, R.W. Immigration costs for female chimpanzees and male protection as an immigrant counterstrategy to intrasexual aggression. Anim. Behav. 2008, 76, 1497–1509. [Google Scholar] [CrossRef]
- Lehmann, J.; Boesch, C. Sociality of the dispersing sex: The nature of social bonds inWest African female chimpanzees, Pan troglodytes. Anim. Behav. 2009, 77, 377–387. [Google Scholar] [CrossRef]
- Koyama, N.F.; Caws, C.; Aureli, F. Interchange of grooming and agonistic support in chimpanzees. Int. J. Primatol. 2006, 27, 1293–1309. [Google Scholar] [CrossRef]
- Preis, A.; Samuni, L.; Deschner, T.; Crockford, C.; Wittig, R.M. Urinary cortisol, aggression, dominance and competition in wild, West African male chimpanzees. Front. Ecol. Evol. 2019, 7, 107. [Google Scholar] [CrossRef]
- Reimers, M.; Schwarzenberger, F.; Preuschoft, S. Rehabilitation of research chimpanzees: Stress and coping after long-term isolation. Horm. Behav. 2007, 51, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Bloomsmith, M.A.; Pazol, K.A.; Alford, P.L. Juvenile and Adolescent Chimpanzee Behavioral Development in Complex Groups. Available online: https://www.sciencedirect.com/science/article/abs/pii/0168159194900175 (accessed on 5 September 2022).
- Bloomsmith, M.A.; Else, J.G. Behavioral management of chimpanzees in biomedical research facilities: The state of the science. ILAR J. 2005, 46, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Díaz, S.; Murray, L.; Roberts, S.G.B.; Rodway, P. Social network analysis of a chimpanzee (Pan troglodytes) group in captivity following the integration of a new adult member. Int. J. Primatol. 2020, 41, 683–700. [Google Scholar] [CrossRef]
- Watts, D.P. Conflict resolution in chimpanzees and the valuable-relationships hypothesis. Int. J. Primatol. 2006, 27, 1337–1364. [Google Scholar] [CrossRef]
- Lonsdorf, E.V.; Eberly, L.E.; Pusey, A.E. Sex differences in learning in chimpanzees. Nature 2004, 428, 715. [Google Scholar] [CrossRef]
- Lehmann, J.; Boesch, C. Sexual differences in chimpanzee sociality. Int. J. Primatol. 2008, 29, 65–81. [Google Scholar] [CrossRef]
- Lonsdorf, E.V.; Anderson, K.E.; Stanton, M.A.; Shender, M.; Heintz, M.R.; Goodall, J.; Murray, C.M. Boys will be boys: Sex differences in wild infant chimpanzee social interactions. Anim. Behav. 2014, 88, 79–83. [Google Scholar] [CrossRef]
- Pepper, J.W.; Mitani, J.C.; Watts, D.P. General gregariousness and specific social preferences among wild chimpanzees. Int. J. Primatol. 1999, 20, 613–632. [Google Scholar] [CrossRef]
- Muller, M.N.; Enigk, D.K.; Fox, S.A.; Lucore, J.; Machanda, Z.P.; Wrangham, R.W.; Emery Thompson, M. Aggression, glucocorticoids, and the chronic costs of status competition for wild male chimpanzees. Horm. Behav. 2021, 130, 104965. [Google Scholar] [CrossRef] [PubMed]
- Muller, M.N.; Wrangham, R.W. Dominance, aggression and testosterone in wild chimpanzees: A test of the ‘challenge hypothesis’. Anim. Behav. 2004, 67, 113–123. [Google Scholar] [CrossRef]
- Reynolds, V. The Chimpanzees of the Budongo Forest; Oxford University Press: Oxford, NY, USA, 2005; p. 131. [Google Scholar]
- Noë, R.; de Waal, F.B.M.; van Hooff, J.A.R.A.M. Types of dominance in a chimpanzee colony. Folia Primatol. 1980, 34, 90–110. [Google Scholar] [CrossRef]
- Crailsheim, D.; Stüger, H.P.; Kalcher-Sommersguter, E.; Llorente, M. Early life experience and alterations of group composition shape the social grooming networks of former pet and entertainment chimpanzees (Pan troglodytes). PLoS ONE 2020, 15, e0226947. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.E. The effects of rearing conditions on grooming and play behaviour in captive chimpanzees. Anim. Welf. 2005, 14, 125–133. [Google Scholar]
- Clay, A.W.; Bard, K.A.; Bloomsmith, M.A. Effects of sex and early rearing condition on adult behavior, health, and well-being in captive chimpanzees (Pan troglodytes). Behav. Process. 2017, 156, 58–76. [Google Scholar] [CrossRef]
- Mitani, J.C.; Merriwether, D.A.; Zhang, C. Male affiliation, cooperation and kinship in wild chimpanzees. Anim. Behav. 2000, 59, 885–893. [Google Scholar] [CrossRef]
- Gilby, I.C.; Wrangham, R.W. Association patterns among wild chimpanzees (Pan troglodytes schweinfurthii) reflect sex differences in cooperation. Behav. Ecol. Sociobiol. 2008, 62, 1831. [Google Scholar] [CrossRef]
- Levé, M.; Sueur, C.; Petit, O.; Matsuzawa, T.; Hirata, S. Social grooming network in captive chimpanzees: Does the wild or captive origin of group members affect sociality? Primates 2016, 57, 73–82. [Google Scholar] [CrossRef]
- Neal Webb, S.J.; Hau, J.; Schapiro, S.J. Captive chimpanzee (Pan troglodytes) behavior as a function of space per animal and enclosure type. Am. J. Primatol. 2018, 80, e22749. [Google Scholar] [CrossRef] [PubMed]
- Herrelko, E.S.; Buchanan-Smith, H.M.; Vick, S.J. Perception of available space during chimpanzee introductions: Number of accessible areas is more important than enclosure size. Zoo Biol. 2015, 34, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Ivory, A.; Carter, S. Male/male and male/female relationships during and after the integration of two chimpanzee social groups. In AZA Annual Conference Proceedings; Miami University: Oxford, UK, 1999; pp. 287–293. [Google Scholar]
- Murray, L.E. The effects of group structure and rearing strategy on personality in Chimpanzees (Pan troglodytes) at Chester, London ZSL and Twycross Zoos. Int. Zoo Yearb. 1998, 36, 97–108. [Google Scholar] [CrossRef]
- Ross, S.R.; Bloomsmith, M.A.; Bettinger, T.L.; Wagner, K.E. The influence of captive adolescent male chimpanzees on wounding: Management and welfare implications. Zoo Biol. 2009, 28, 623–634. [Google Scholar] [CrossRef] [PubMed]
- McClung, J.S.; Goetschi, F.; Baumeyer, A.; Zuberbühler, K. Chimpanzee immigration: Complex social strategies differ between zoo-based and wild animals. J. Zoo Aquar. Res. 2020, 8, 10–17. [Google Scholar] [CrossRef]
- Baker, K.C. Advanced age influences chimpanzee behavior in small social groups. Zoo Biol. 2007, 19, 111–119. [Google Scholar] [CrossRef]
- Neal Webb, S.J.; Hau, J.; Lambeth, S.P.; Schapiro, S.J. Differences in behavior between elderly and nonelderly captive chimpanzees and the effects of the social environment. J. Am. Assoc. Lab. Anim. Sci. 2019, 58, 783–789. [Google Scholar] [CrossRef] [PubMed]




| Behavior | Significant Factor | Factor Level a | Estimate | S.E. | Z | p Value |
|---|---|---|---|---|---|---|
| Aggressive | Sex | Female | Reference | |||
| Male *** | 0.623 | 0.099 | 6.28 | 0.0000 | ||
| Age | (Continuous) ** | 0.017 | 0.006 | 2.69 | 0.0073 | |
| Birth status | Captive born | Reference | ||||
| Wild born (*) | −0.404 | 0.212 | −1.90 | 0.0570 | ||
| Unknown | −0.2087 | 0.216 | −0.97 | 0.3300 | ||
| Rearing history | Nursery | Reference | ||||
| Enculturated | 0.521 | 0.382 | 1.36 | 0.1723 | ||
| Human-reared (*) | 0.530 | 0.274 | 1.93 | 0.0530 | ||
| Mother-reared | 0.183 | 0.141 | 1.30 | 0.1947 | ||
| Unknown | −0.086 | 0.139 | −0.62 | 0.5343 | ||
| Location | Close | Reference | ||||
| Open * | 0.3207 | 0.127 | 2.53 | 0.0113 | ||
| Duration | (Continuous) * | 0.674 | 0.288 | 2.34 | 0.0192 | |
| Submissive | Sex | Female | Reference | |||
| Male *** | 0.378 | 0.080 | 4.76 | 0.0000 | ||
| Rearing history | Nursery | Reference | ||||
| Enculturated | −0.086 | 0.322 | −0.27 | 0.7889 | ||
| Human-reared | −0.254 | 0.225 | −1.13 | 0.2593 | ||
| Mother-reared (*) | −0.154 | 0.093 | −1.66 | 0.0978 | ||
| Unknown | −0.160 | 0.104 | −1.53 | 0.1259 | ||
| Group size | (Continuous) *** | −0.050 | 0.010 | −5.03 | 0.0000 | |
| Duration | (Continuous) *** | 0.735 | 0.185 | 3.98 | 0.0001 | |
| Affiliative | Sex | Female | Reference | |||
| Male *** | 0.264 | 0.071 | 3.74 | 0.0002 | ||
| Location | Close | Reference | ||||
| Open *** | 0.30598 | 0.081 | 3.79 | 0.0002 | ||
| Group size | (Continuous) *** | −0.05388 | 0.009 | −5.88 | 0.0000 | |
| Duration | (Continuous) *** | 0.457 | 0.160 | 2.85 | 0.0043 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fultz, A.; Yanagi, A.; Breaux, S.; Beaupre, L. Aggressive, Submissive, and Affiliative Behavior in Sanctuary Chimpanzees (Pan Troglodytes) During Social Integration. Animals 2022, 12, 2421. https://doi.org/10.3390/ani12182421
Fultz A, Yanagi A, Breaux S, Beaupre L. Aggressive, Submissive, and Affiliative Behavior in Sanctuary Chimpanzees (Pan Troglodytes) During Social Integration. Animals. 2022; 12(18):2421. https://doi.org/10.3390/ani12182421
Chicago/Turabian StyleFultz, Amy, Akie Yanagi, Sarah Breaux, and Leilani Beaupre. 2022. "Aggressive, Submissive, and Affiliative Behavior in Sanctuary Chimpanzees (Pan Troglodytes) During Social Integration" Animals 12, no. 18: 2421. https://doi.org/10.3390/ani12182421
APA StyleFultz, A., Yanagi, A., Breaux, S., & Beaupre, L. (2022). Aggressive, Submissive, and Affiliative Behavior in Sanctuary Chimpanzees (Pan Troglodytes) During Social Integration. Animals, 12(18), 2421. https://doi.org/10.3390/ani12182421





