Facial Expressions of Horses Using Weighted Multivariate Statistics for Assessment of Subtle Local Pain Induced by Polylactide-Based Polymers Implanted Subcutaneously
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics in the Use of Animals
2.2. Preparation of Polymers
2.3. Animals
2.4. Experimental Groups
2.5. Procedure for Implantation of Biopolymers
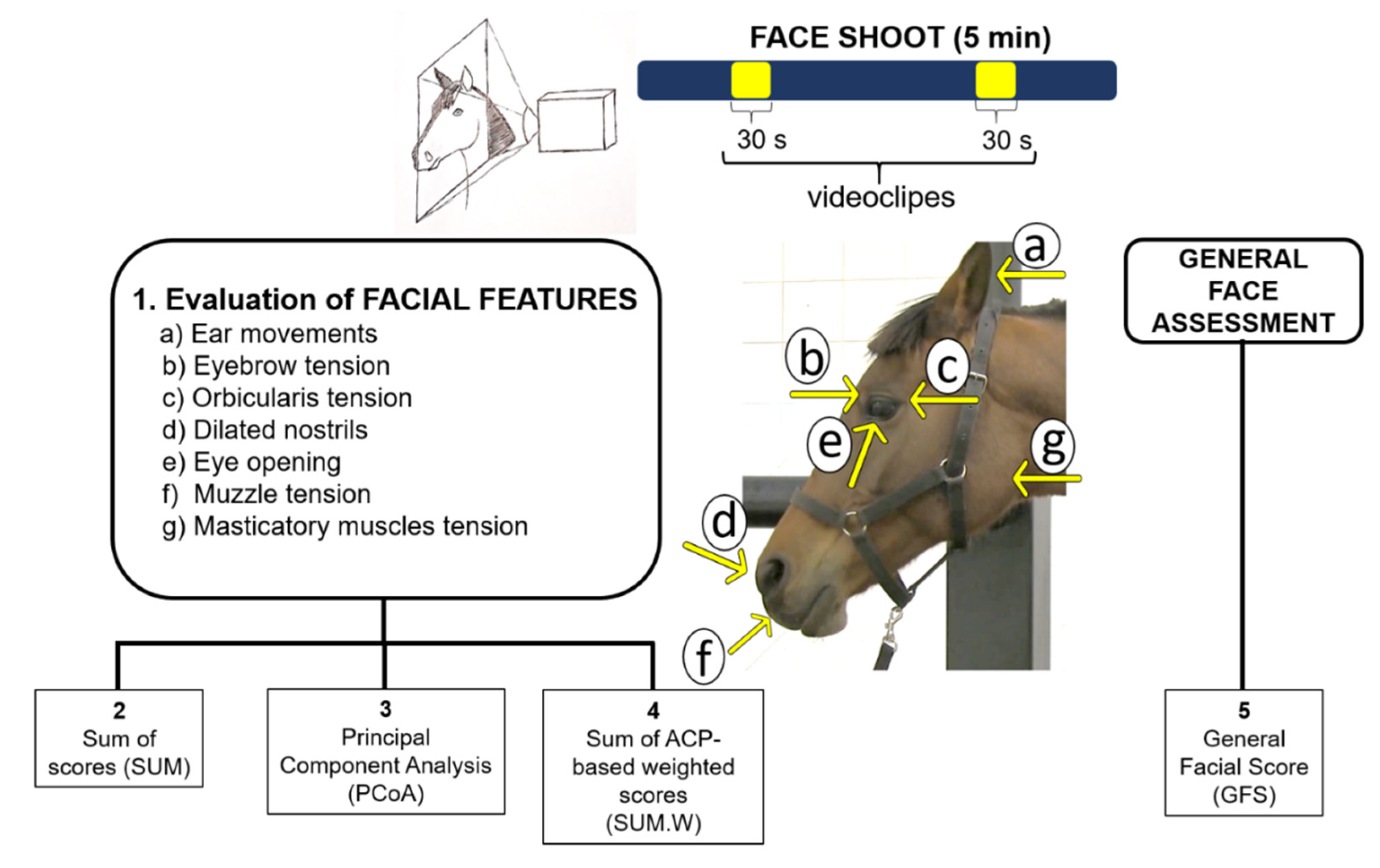
2.6. Facial Expressions Evaluation
| Facial Features | Scores | Descriptions |
|---|---|---|
| Ear movements | 0 | Ears directed forward or to the side in most of the video clip. |
| 1 | Ears directed backwards at least once or in less than half of the video clip. | |
| 2 | Ears directed backward in most of the video clip or pressed against the neck at least once. | |
| Eyebrow tension | 0 | Eyebrows not contracted during the video clip. |
| 1 | Moderately * contracted eyebrows in most of the video clip. | |
| 2 | Intensely * contracted eyebrows in most of the video clip. | |
| Orbicularis tension | 0 | Orbicularis muscle not contracted when blinking during the video clip. |
| 1 | Orbicularis muscle moderately contracted when blinking at least once during the video clip. | |
| 2 | Orbicularis muscle intensely # contracted when blinking at least once during the video clip. | |
| Dilated nostrils | 0 | Nostrils not dilated in most of the video clip. |
| 1 | Nostrils moderately dilated in most of the video clip. | |
| 2 | Nostrils intensely dilated in most of the video clip. | |
| Eye opening | 0 | Eyes completely open in most of the video clip. |
| 1 | Eyes partially closed in most of the video clip. | |
| 2 | Eyes completely closed in most of the video clip. | |
| Muzzle tension | 0 | Muzzle without tension during the video clip. |
| 1 | Muzzle moderately tense in most of the video clip. | |
| 2 | Muzzle intensely tense in most of the video clip. | |
| Masticatory muscles tension | 0 | Masticatory muscles without tension during the video clip. |
| 1 | Masticatory muscles moderately tense in most of the video clip. | |
| 2 | Masticatory muscles intensely tense in most of the video clip. |
2.7. Thermographic Evaluation
2.8. Evaluation of Mechanical Nociceptive Threshold
2.9. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Webster, J. Animal Welfare: Freedoms, Dominions and “A Life Worth Living”. Animals 2016, 6, 35. [Google Scholar] [CrossRef] [PubMed]
- Price, D.D. Psychological and Neural Mechanisms of the Affective Dimension of Pain. Science 2000, 288, 1769–1772. [Google Scholar] [CrossRef]
- McKune, C.M.; Murrel, J.C.; Nolan, A.M.; White, K.L.; Wright, B.D. Nociception and pain. In Lumb & Jones Veterinary Anesthesia and Analgesia, 5th ed.; Grimm, K.A., Lamont, L.A., Tranquilli, W.J., Greene, S.A., Robertson, S.A., Eds.; John Wiley & Sons, Inc.: Ames, IA, USA, 2015. [Google Scholar]
- Broomé, S.; Ask, K.; Rashid-Engström, M.; Andersen, P.H.; Kjellström, H. Sharing pain: Using pain domain transfer for video recognition of low grade orthopedic pain in horses. PLoS ONE 2022, 17, e0263854. [Google Scholar] [CrossRef] [PubMed]
- Mullard, J.; Berger, J.M.; Ellis, A.D.; Dyson, S. Development of an ethogram to describe facial expressions in ridden horses (FEReq). J. Veter Behav. 2017, 18, 7–12. [Google Scholar] [CrossRef]
- Gleerup, K.C.B.; Lindegaard, C. Recognition and quantification of pain in horses: A tutorial review. Equine Vet. Educ. 2015, 28, 47–57. [Google Scholar] [CrossRef]
- Trindade, P.H.E.; Taffarel, M.O.; Luna, S.P.L. Spontaneous Behaviors of Post-Orchiectomy Pain in Horses Regardless of the Effects of Time of Day, Anesthesia, and Analgesia. Animals 2021, 11, 1629. [Google Scholar] [CrossRef]
- Silva, N.E.O.F.; Trindade, P.H.E.; Oliveira, A.R.; Taffarel, M.O.; Moreira, M.A.P.; Denadai, R.; Rocha, P.B.; Luna, S.P.L. Validation of the Unesp-Botucatu composite scale to assess acute postoperative abdominal pain in sheep (USAPS). PLoS ONE 2020, 15, e0239622. [Google Scholar] [CrossRef]
- Broom, D.M. Animal welfare: Concepts and measurement2. J. Anim. Sci. 1991, 69, 4167–4175. [Google Scholar] [CrossRef]
- de Grauw, J.; van Loon, J. Systematic pain assessment in horses. Vet. J. 2016, 209, 14–22. [Google Scholar] [CrossRef]
- Taffarel, M.; Luna, S.P.L.; De Oliveira, F.A.; Cardoso, G.S.; Alonso, J.D.M.; Pantoja, J.C.; Brondani, J.T.; Love, E.; Taylor, P.; White, K.; et al. Refinement and partial validation of the UNESP-Botucatu multidimensional composite pain scale for assessing postoperative pain in horses. BMC Vet. Res. 2015, 11, 83. [Google Scholar] [CrossRef] [Green Version]
- Luna, S.P.L.; De Araújo, A.L.; Neto, P.I.D.N.; Brondani, J.T.; De Oliveira, F.A.; Azerêdo, L.M.D.S.; Telles, F.G.; Trindade, P.H.E. Validation of the UNESP-Botucatu pig composite acute pain scale (UPAPS). PLoS ONE 2020, 15, e0233552. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, F.A.; Luna, S.P.L.; Amaral, J.B.D.; Rodrigues, K.A.; Sant’Anna, A.C.; Daolio, M.; Brondani, J.T. Validation of the UNESP-Botucatu unidimensional composite pain scale for assessing postoperative pain in cattle. BMC Vet. Res. 2014, 10, 200. [Google Scholar] [CrossRef] [PubMed]
- Mott, R.O.; Hawthorne, S.J.; McBride, S.D. Blink rate as a measure of stress and attention in the domestic horse (Equus caballus). Sci. Rep. 2020, 10, 21409. [Google Scholar] [CrossRef] [PubMed]
- Cobianchi, L.; Gigliuto, C.; de Gregori, M.; Malafoglia, V.; Raffaeli, W.; Compagnone, C.; Visai, L.; Petrini, P.; Avanzini, M.A.; Muscoli, C.; et al. Pain assessment in animal models: Do we need further studies? J. Pain Res. 2014, 7, 227–236. [Google Scholar] [CrossRef]
- Guedes, A. Pain Management in Horses. Vet. Clin. Equine Pract. 2017, 33, 181–211. [Google Scholar] [CrossRef]
- Ekman, P.; Friesen, W.V. Facial Action Coding System: A Technique for the Measurement of Facial Movement, 1st ed.; Consulting Psychologists Press: London, UK, 1978. [Google Scholar]
- Jordan, A.; Hughes, J.; Pakresi, M.; Hepburn, S.; O’Brien, J.T. The utility of PAINAD in assessing pain in a UK population with severe dementia. Int. J. Geriatr. Psychiatry 2011, 26, 118–126. [Google Scholar] [CrossRef]
- Chang, J.; Versloot, J.; Fashler, S.R.; McCrystal, K.N.; Craig, K.D. Pain assessment in children: Validity of facial expression items in observational pain scales. Clin. J. Pain 2015, 31, 189–197. [Google Scholar] [CrossRef]
- Langford, D.J.; Bailey, A.L.; Chanda, M.L.; E Clarke, S.; E Drummond, T.; Echols, S.; Glick, S.; Ingrao, J.; Klassen-Ross, T.; LaCroix-Fralish, M.L.; et al. Coding of facial expressions of pain in the laboratory mouse. Nat. Methods 2010, 7, 447–449. [Google Scholar] [CrossRef]
- Leach, M.C.; Klaus, K.; Miller, A.L.; Di Perrotolo, M.S.; Sotocinal, S.G.; Flecknell, P.A. The Assessment of Post-Vasectomy Pain in Mice Using Behaviour and the Mouse Grimace Scale. PLoS ONE 2012, 7, e35656. [Google Scholar] [CrossRef]
- Matsumiya, L.C.; E Sorge, R.; Sotocinal, S.G.; Tabaka, J.M.; Wieskopf, J.S.; Zaloum, A.; King, O.D.; Mogil, J.S. Using the Mouse Grimace Scale to reevaluate the efficacy of postoperative analgesics in laboratory mice. J. Am. Assoc. Lab. Anim. Sci. 2012, 51, 42–49. [Google Scholar]
- Costa, E.D.; Pascuzzo, R.; Leach, M.C.; Dai, F.; Lebelt, D.; Vantini, S.; Minero, M. Can grimace scales estimate the pain status in horses and mice? A statistical approach to identify a classifier. PLoS ONE 2018, 13, e0200339. [Google Scholar] [CrossRef] [Green Version]
- Sotocinal, S.G.; E Sorge, R.; Zaloum, A.; Tuttle, A.H.; Martin, L.; Wieskopf, J.S.; Mapplebeck, J.C.; Wei, P.; Zhan, S.; Zhang, S.; et al. The Rat Grimace Scale: A Partially Automated Method for Quantifying Pain in the Laboratory Rat via Facial Expressions. Mol. Pain 2011, 7, 55. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.; Long, H.; Zhang, L.; Chen, H.; Zhou, Y.; Ye, N.; Lai, W. Evaluation of pain in rats through facial expression following experimental tooth movement. Eur. J. Oral Sci. 2014, 122, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Sperry, M.; Yu, Y.-H.; Welch, R.L.; Granquist, E.J.; Winkelstein, B.A. Grading facial expression is a sensitive means to detect grimace differences in orofacial pain in a rat model. Sci. Rep. 2018, 8, 13894. [Google Scholar] [CrossRef]
- Keating, S.C.J.; Thomas, A.A.; Flecknell, P.; Leach, M.C. Evaluation of EMLA Cream for Preventing Pain during Tattooing of Rabbits: Changes in Physiological, Behavioural and Facial Expression Responses. PLoS ONE 2012, 7, e44437. [Google Scholar] [CrossRef] [PubMed]
- Hedenqvist, P.; Trbakovic, A.; Thor, A.; Ley, C.; Ekman, S.; Jensen-Waern, M. Carprofen neither reduces postoperative facial expression scores in rabbits treated with buprenorphine nor alters long term bone formation after maxillary sinus grafting. Res. Vet. Sci. 2016, 107, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Pinho, R.H.; Luna, S.P.L.; Trindade, P.H.E.; Justo, A.A.; Cima, D.S.; Fonseca, M.W.; Minto, B.W.; Rocha, F.D.L.; Miller, A.; Flecknell, P.; et al. Validation of the rabbit pain behaviour scale (RPBS) to assess acute postoperative pain in rabbits (Oryctolagus cuniculus). PLoS ONE 2022, 17, e0268973. [Google Scholar] [CrossRef]
- Miller, A.L.; Clarkson, J.M.; Quigley, C.; Neville, V.; Krall, C.; Geijer-Simpson, A.; Flecknell, P.A.; Leach, M.C. Evaluating Pain and Analgesia Effectiveness Following Routine Castration in Rabbits Using Behavior and Facial Expressions. Front. Vet. Sci. 2022, 9, 782486. [Google Scholar] [CrossRef]
- Reijgwart, M.L.; Schoemaker, N.J.; Pascuzzo, R.; Leach, M.C.; Stodel, M.; De Nies, L.; Hendriksen, C.F.M.; Van Der Meer, M.; Vinke, C.M.; Van Zeeland, Y.R.A. The composition and initial evaluation of a grimace scale in ferrets after surgical implantation of a telemetry probe. PLoS ONE 2017, 12, e0187986. [Google Scholar] [CrossRef]
- MacRae, A.M.; Makowska, I.J.; Fraser, D. Initial evaluation of facial expressions and behaviours of harbour seal pups (Phoca vitulina) in response to tagging and microchipping. Appl. Anim. Behav. Sci. 2018, 205, 167–174. [Google Scholar] [CrossRef]
- Holden, E.; Calvo, G.; Collins, M.; Bell, A.; Reid, J.; Scott, E.M.; Nolan, A.M. Evaluation of facial expression in acute pain in cats. J. Small Anim. Pract. 2014, 55, 615–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evangelista, M.C.; Watanabe, R.; Leung, V.S.Y.; Monteiro, B.P.; O’Toole, E.; Pang, D.S.J.; Steagall, P.V. Facial expressions of pain in cats: The development and validation of a Feline Grimace Scale. Sci. Rep. 2019, 9, 19128. [Google Scholar] [CrossRef] [PubMed]
- Di Giminiani, P.; Brierley, V.L.M.H.; Scollo, A.; Gottardo, F.; Malcolm, E.M.; Edwards, S.A.; Leach, M.C. The Assessment of Facial Expressions in Piglets Undergoing Tail Docking and Castration: Toward the Development of the Piglet Grimace Scale. Front. Vet. Sci. 2016, 3, 100. [Google Scholar] [CrossRef] [PubMed]
- Viscardi, A.V.; Hunniford, M.; Lawlis, P.; Leach, M.; Turner, P.V. Development of a Piglet Grimace Scale to Evaluate Piglet Pain Using Facial Expressions Following Castration and Tail Docking: A Pilot Study. Front. Vet. Sci. 2017, 4, 51. [Google Scholar] [CrossRef] [PubMed]
- Navarro, E.; Mainau, E.; Manteca, X. Development of a Facial Expression Scale Using Farrowing as a Model of Pain in Sows. Animals 2020, 10, 2113. [Google Scholar] [CrossRef]
- Vullo, C.; Barbieri, S.; Catone, G.; Graïc, J.-M.; Magaletti, M.; Di Rosa, A.; Motta, A.; Tremolada, C.; Canali, E.; Costa, E.D. Is the Piglet Grimace Scale (PGS) a Useful Welfare Indicator to Assess Pain after Cryptorchidectomy in Growing Pigs? Animals 2020, 10, 412. [Google Scholar] [CrossRef]
- Gleerup, K.B.; Andersen, P.H.; Munksgaard, L.; Forkman, B. Pain evaluation in dairy cattle. Appl. Anim. Behav. Sci. 2015, 171, 25–32. [Google Scholar] [CrossRef]
- Müller, B.R.; Soriano, V.S.; Bellio, J.C.B.; Molento, C.F.M. Facial expression of pain in Nellore and crossbred beef cattle. J. Vet. Behav. 2019, 34, 60–65. [Google Scholar] [CrossRef]
- Yamada, P.H.; Codognoto, V.M.; de Ruediger, F.R.; Trindade, P.H.E.; da Silva, K.M.; Rizzoto, G.; Maestá, S.A.; Ferreira, J.C.P.; de Soutello, R.V.G.; Oba, E. Pain assessment based on facial expression of bulls during castration. Appl. Anim. Behav. Sci. 2021, 236, 105258. [Google Scholar] [CrossRef]
- McLennan, K.M.; Rebelo, C.J.; Corke, M.J.; Holmes, M.A.; Leach, M.C.; Constantino-Casas, F. Development of a facial expression scale using footrot and mastitis as models of pain in sheep. Appl. Anim. Behav. Sci. 2016, 176, 19–26. [Google Scholar] [CrossRef]
- Guesgen, M.; Beausoleil, N.; Leach, M.; Minot, E.; Stewart, M.; Stafford, K. Coding and quantification of a facial expression for pain in lambs. Behav. Process. 2016, 132, 49–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Häger, C.; Biernot, S.; Buettner, M.; Glage, S.; Keubler, L.M.; Held, N.; Bleich, E.M.; Otto, K.; Müller, C.W.; Decker, S.; et al. The Sheep Grimace Scale as an indicator of post-operative distress and pain in laboratory sheep. PLoS ONE 2017, 12, e0175839. [Google Scholar] [CrossRef] [PubMed]
- Dalla Costa, E.; Minero, M.; Lebelt, D.; Stucke, D.; Canali, E.; Leach, M.C. Development of the Horse Grimace Scale (HGS) as a Pain Assessment Tool in Horses Undergoing Routine Castration. PLoS ONE 2014, 9, e92281. [Google Scholar] [CrossRef]
- van Loon, J.P.; Van Dierendonck, M.C. Monitoring acute equine visceral pain with the Equine Utrecht University Scale for Composite Pain Assessment (EQUUS-COMPASS) and the Equine Utrecht University Scale for Facial Assessment of Pain (EQUUS-FAP): A scale-construction study. Vet. J. 2015, 206, 356–364. [Google Scholar] [CrossRef]
- Gleerup, K.B.; Forkman, B.; Lindegaard, C.; Andersen, P.H. An equine pain face. Vet. Anaesth. Analg. 2015, 42, 103–114. [Google Scholar] [CrossRef]
- Costa, E.D.; Stucke, D.; Dai, F.; Minero, M.; Leach, M.C.; Lebelt, D. Using the Horse Grimace Scale (HGS) to Assess Pain Associated with Acute Laminitis in Horses (Equus caballus). Animals 2016, 6, 47. [Google Scholar] [CrossRef]
- Dyson, S.; Berger, J.M.; Ellis, A.D.; Mullard, J. Can the presence of musculoskeletal pain be determined from the facial expressions of ridden horses (FEReq)? J. Vet. Behav. 2017, 19, 78–89. [Google Scholar] [CrossRef]
- Lansade, L.; Nowak, R.; Lainé, A.-L.; Leterrier, C.; Bonneau, C.; Parias, C.; Bertin, A. Facial expression and oxytocin as possible markers of positive emotions in horses. Sci. Rep. 2018, 8, 14680. [Google Scholar] [CrossRef]
- Trindade, P.H.E.; Hartmann, E.; Keeling, L.J.; Andersen, P.H.; Ferraz, G.D.C.; Da Costa, M.J.R.P. Effect of work on body language of ranch horses in Brazil. PLoS ONE 2020, 15, e0228130. [Google Scholar] [CrossRef]
- Coneglian, M.M.; Borges, T.D.; Weber, S.H.; Bertagnon, H.G.; Michelotto, P.V. Use of the horse grimace scale to identify and quantify pain due to dental disorders in horses. Appl. Anim. Behav. Sci. 2020, 225, 104970. [Google Scholar] [CrossRef]
- Haunhorst, F.R.; Hopster, K.; Schmicke, M.; Bienert-Zeit, A.; Kästner, S. Clinical effect of buprenorphine or butorphanol, in combination with detomidine and diazepam, on sedation and postoperative pain after cheek tooth extraction in horses. Can. Vet. J. 2022, 63, 39–46. [Google Scholar] [PubMed]
- Kosiara, S.; Harrison, A.P. The Effect of Aromatherapy on Equine Facial Expression, Heart Rate, Respiratory Tidal Volume and Spontaneous Muscle Contractures in M. Temporalis and M. Cleidomastoideus. Open J. Vet. Med. 2021, 11, 87–103. [Google Scholar] [CrossRef]
- Lundblad, J.; Rashid, M.; Rhodin, M.; Andersen, P.H. Effect of transportation and social isolation on facial expressions of healthy horses. PLoS ONE 2021, 16, e0241532. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, A.R.; Gozalo-Marcilla, M.; Ringer, S.K.; Schauvliege, S.; Fonseca, M.W.; Trindade, P.H.E.; Filho, J.N.P.P.; Luna, S.P.L. Development and validation of the facial scale (FaceSed) to evaluate sedation in horses. PLoS ONE 2021, 16, e0251909. [Google Scholar] [CrossRef]
- Ask, K.; Rhodin, M.; Tamminen, L.-M.; Hernlund, E.; Andersen, P.H. Identification of Body Behaviors and Facial Expressions Associated with Induced Orthopedic Pain in Four Equine Pain Scales. Animals 2020, 10, 2155. [Google Scholar] [CrossRef] [PubMed]
- Gregory, N.S.; Harris, A.L.; Robinson, C.R.; Dougherty, P.M.; Fuchs, P.N.; Sluka, K.A. An Overview of Animal Models of Pain: Disease Models and Outcome Measures. J. Pain 2013, 14, 1255–1269. [Google Scholar] [CrossRef] [PubMed]
- Gozalo-Marcilla, M.; Luna, S.; Gasthuys, F.; Schauvliege, S. Thermal, mechanical and electrical stimuli in antinociceptive studies in standing horses: An update. Vet. Anaesth. Analg. 2020, 47, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Casas-Alvarado, A.; Mota-Rojas, D.; Hernández-Ávalos, I.; Mora-Medina, P.; Olmos-Hernández, A.; Verduzco-Mendoza, A.; Reyes-Sotelo, B.; Martínez-Burnes, J. Advances in infrared thermography: Surgical aspects, vascular changes, and pain monitoring in veterinary medicine. J. Therm. Biol. 2020, 92, 102664. [Google Scholar] [CrossRef]
- McDonnell, S. The Equid Ethogram: A Practical Field Guide to Horse Behavior, 1st ed.; Eclipse Press: Amman, Jordan, 2003. [Google Scholar]
- Descovich, K.A.; Wathan, J.; Leach, M.C.; Buchanan-Smith, H.M.; Flecknell, P.; Farningham, D.; Vick, S.J. Facial expression: An under-utilised tool for the assessment of welfare in mammals. ALTEX 2017, 34, 409–429. [Google Scholar] [CrossRef]
- Trindade, P.H.E.; Costa, F.D.O.; Da Costa, M.J.R.P. Expressões faciais em cavalos: Uma abordagem sobre os métodos de avaliação e as limitações de seu uso. Rev. Acad. Ciên. Anim. 2018, 16, e161109. [Google Scholar] [CrossRef]
- Rashid, M.; Silventoinen, A.; Gleerup, K.B.; Andersen, P.H. Equine Facial Action Coding System for determination of pain-related facial responses in videos of horses. PLoS ONE 2020, 15, e0231608. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, J.R.G.; Conde, G.; Antonioli, M.L.; Dias, P.P.; Vasconcelos, R.O.; Taboga, S.R.; Canola, P.A.; Chinelatto, M.A.; Pereira, G.T.; Ferraz, G.C. Biocompatibility and biodegradation of poly(lactic acid) (PLA) and an immiscible PLA/poly(ε-caprolactone) (PCL) blend compatibilized by poly(ε-caprolactone-b-tetrahydrofuran) implanted in horses. Polym. J. 2020, 52, 629–643. [Google Scholar] [CrossRef]
- Dias, P.P.; Chinelatto, M.A. Effect of Poly(ε-caprolactone-b-tetrahydrofuran) Triblock Copolymer Concentration on Morphological, Thermal and Mechanical Properties of Immiscible PLA/PCL Blends. J. Renew. Mater. 2019, 7, 129–138. [Google Scholar] [CrossRef]
- Martin, P.; Bateson, P.P.G. Measuring Behaviour: An Introductory Guide, 3rd ed.; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar] [CrossRef]
- Wang, F.K.; Chen, J.C. Capability index using principal components analysis. Qual. Eng. 1998, 11, 21–27. [Google Scholar] [CrossRef]
- Kubrusly, L.S. Um procedimento para calcular índices a partir de uma base de dados multivariados. Pesqui. Oper. 2001, 21, 107–117. [Google Scholar] [CrossRef]
- Wathan, J.; Burrows, A.M.; Waller, B.M.; McComb, K. EquiFACS: The Equine Facial Action Coding System. PLoS ONE 2015, 10, e0131738. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. An Application of Hierarchical Kappa-type Statistics in the Assessment of Majority Agreement among Multiple Observers. Biometrics 1977, 33, 363–374. [Google Scholar] [CrossRef]
- Nóbrega, F.S.; Ferreira, M.P.; Facó, L.L.; Selim, M.B.; De Zoppa, A.L.V. Uso da termografia para avaliação da resposta tecidual após implante de polímero a base de poliuretana de mamona em osso III metacarpiano de equinos. Acta. Sci. Vet. 2014, 42, 1–5. [Google Scholar]
- Carvalho, J.R.G.; Conde, G.; Antonioli, M.L.; Santana, C.H.; Littiere, T.O.; Dias, P.P.; Chinelatto, M.A.; Canola, P.A.; Zara, F.J.; Ferraz, G.C. Long-Term Evaluation of Poly(lactic acid) (PLA) Implants in a Horse: An Experimental Pilot Study. Molecules 2021, 26, 7224. [Google Scholar] [CrossRef]
- Li, R.-Y.; Liu, Z.-G.; Liu, H.-Q.; Chen, L.; Liu, J.-F.; Pan, Y.-H. Evaluation of biocompatibility and toxicity of biodegradable poly (DL-lactic acid) films. Am. J. Transl. Res. 2015, 7, 1357–1370. [Google Scholar]
- Heber, S.; Ciotu, C.I.; Hartner, G.; Gold-Binder, M.; Ninidze, N.; Gleiss, A.; Kress, H.-G.; Fischer, M.J. TRPV1 antagonist BCTC inhibits pH 6.0-induced pain in human skin. Pain 2020, 161, 1532–1541. [Google Scholar] [CrossRef] [PubMed]
- Bussières, G.; Jacques, C.; Lainay, O.; Beauchamp, G.; Leblond, A.; Cadoré, J.-L.; Desmaizières, L.-M.; Cuvelliez, S.; Troncy, E. Development of a composite orthopaedic pain scale in horses. Res. Vet. Sci. 2008, 85, 294–306. [Google Scholar] [CrossRef]
- Hayashi, K.; Ikemoto, T.; Ueno, T.; Arai, Y.-C.P.; Shimo, K.; Nishihara, M.; Suzuki, S.; Ushida, T. Discordant Relationship Between Evaluation of Facial Expression and Subjective Pain Rating Due to the Low Pain Magnitude. Basic Clin. Neurosci. J. 2018, 9, 43–50. [Google Scholar] [CrossRef]
- Lindegaard, C.; Vaabengaard, D.; Christophersen, M.T.; Ekstøm, C.T.; Fjeldborg, J. Evaluation of pain and inflammation associated with hot iron branding and microchip transponder injection in horses. Am. J. Vet. Res. 2009, 70, 840–847. [Google Scholar] [CrossRef]
- Erber, R.; Wulf, M.; Becker-Birck, M.; Kaps, S.; Aurich, J.; Möstl, E.; Aurich, C. Physiological and behavioural responses of young horses to hot iron branding and microchip implantation. Vet. J. 2012, 191, 171–175. [Google Scholar] [CrossRef]
- Hair, J.J.F.; Black, W.C.; Babin, B.J.; Anderson, R.E. Multivariate Data Analysis, 7th ed.; Pearson Education: Upper Saddle River, NJ, USA, 2014. [Google Scholar]
| Behavioral Categories | Descriptions |
|---|---|
| Tail movement | Moves the tail to the left or right above or below the groin or up and down at least 45 degrees quickly. |
| Ear movement | Both ears are turned laterally and dorsally/caudally. The inner ear opening faces outward. |
| Head movement | Throws the head up or down, with or without moving the neck in the same direction simultaneously. Turns the head to the right or to the left, leaving the whites of the eye visible. Turns the head toward the stimulus. |
| Kick or step sideways | Moves with two legs, forward, backward, or sideways, resulting in a new position, or raises the pelvic member and vigorously moves it backwards or sideways. |
| 95% Confidence Interval | |||
|---|---|---|---|
| Facial Features | Weighted Kappa | Minimum | Maximum |
| Ear movements | 0.78 | 0.39 | 1.00 |
| Eyebrow tension | 0.71 | 0.47 | 0.96 |
| Orbicularis tension | 1.00 | 1.00 | 1.00 |
| Dilated nostrils | 1.00 | 1.00 | 1.00 |
| Eye opening | 1.00 | 1.00 | 1.00 |
| Muzzle tension | 0.86 | 0.66 | 1.00 |
| Masticatory muscles tension | 1.00 | 1.00 | 1.00 |
| Video Clip 1 | Video Clip 2 | |||
|---|---|---|---|---|
| Median | Range | Median | Range | |
| Ear movements | 0 | 0–2 | 0 | 0–2 |
| Eyebrow tension | 1 | 0–2 | 0 | 0–2 |
| Orbicularis tension | 0 | 0–1 | 0 | 0–1 |
| Dilated nostrils | 1 | 0–2 | 1 | 0–2 |
| Eye opening | 0 | 0–2 | 0 | 0–2 |
| Muzzle tension | 0 | 0–2 | 0 | 0–2 |
| Masticatory muscles tension | 0 | 0–2 | 0 | 0–2 |
| GFS | 0 | 0–2 | 0 | 0–2 |
| A | |||||||
|---|---|---|---|---|---|---|---|
| Before | 24 h After | 48 h After | |||||
| Groups | Variables | Median | Amplitude | Median | Amplitude | Median | Amplitude |
| S | Ear movements | 0.00 | 0–1 | 0.00 | 0–2 | 1.00 | 0–2 |
| Eyebrow tension | 0.00 | 0–1 | 0.00 | 0–1 | 0.00 | 0–2 | |
| Orbicularis tension | 0.00 | 0–0 | 0.00 | 0–1 | 0.00 | 0–0 | |
| Dilated nostrils | 0.00 | 0–1 | 0.00 | 0–1 | 0.50 | 0–2 | |
| Eye opening | 1.00 | 0–1 | 0.50 | 0–2 | 1.00 | 0–1 | |
| Muzzle tension | 0.00 | 0–1 | 0.00 | 0–1 | 0.00 | 0–2 | |
| Masticatory muscles tension | 0.00 | 0–1 | 0.00 | 0–2 | 0.00 | 0–2 | |
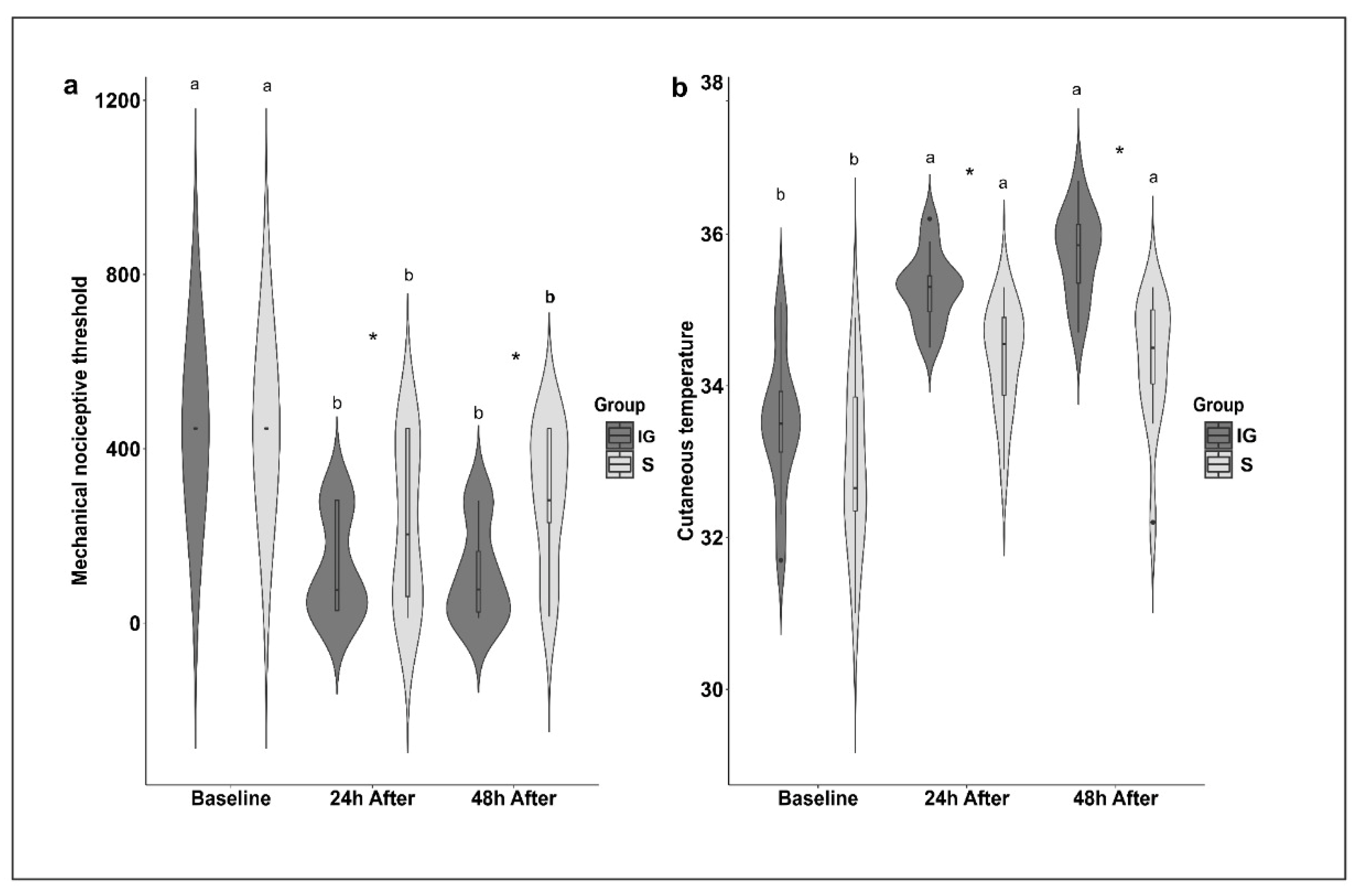
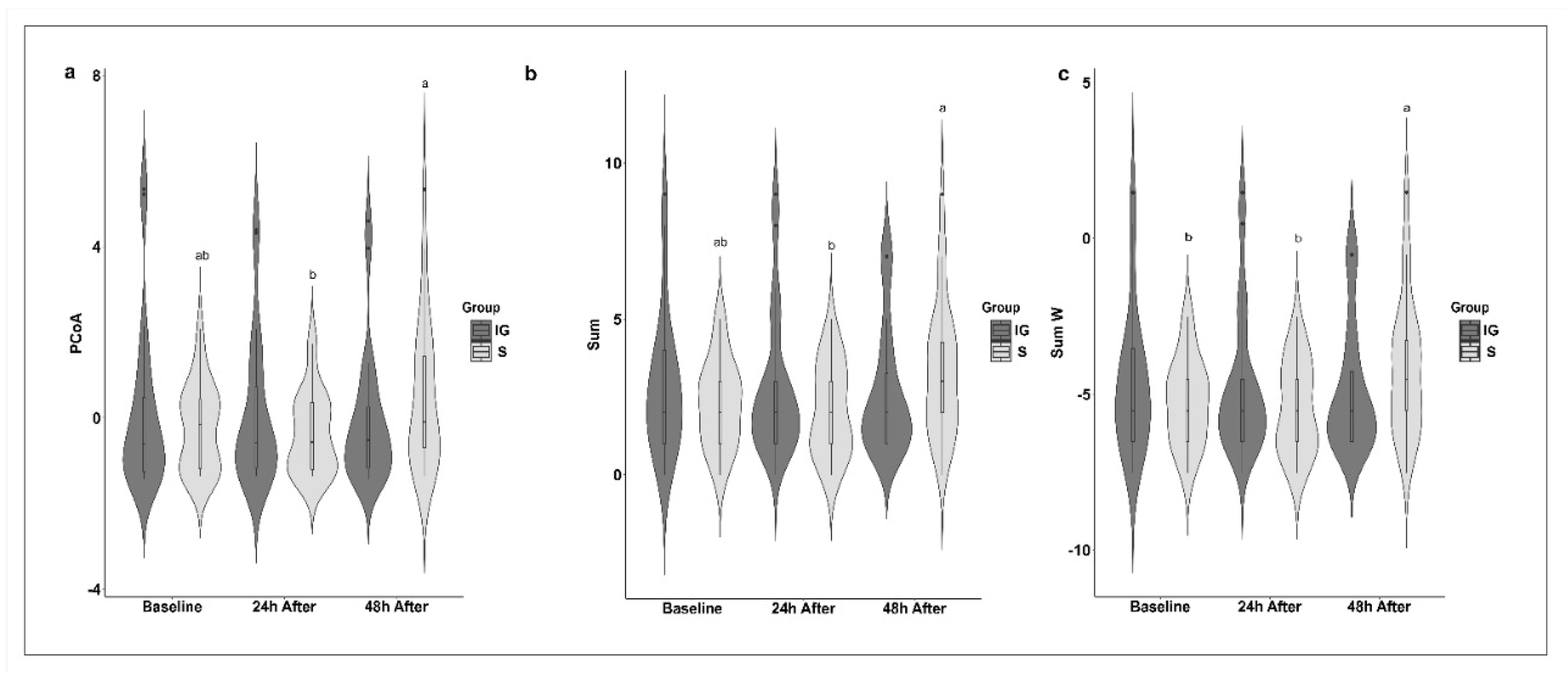
| SUM | 2.00 ab | 0–5 | 2.00 b | 0–5 | 3.00 a | 0–9 | |
| PCoA | −0.15 ab | −1.35–2.08 | −0.56 b | −1.35–1.73 | −0.08 a | −1.35–5.34 | |
| SUM.W | −5.54 b | −7.54–−2.54 | −5.54 b | −7.54–−2.54 | −4.54 a | −7.54–1.46 | |
| GFS | 0.00 | 0–2 | 0 | 0–1 | 0.5 | 0–2 | |
| MNT | 446.68 a | 446.68–446.68 | 203.87 bA | 11.75–446.68 | 281.84 bA | 15.14–446.68 | |
| IG | Ear movements | 0.00 | 0–2 | 0.00 | 0–2 | 0.00 | 0–2 |
| Eyebrow tension | 1.00 | 0–2 | 1.00 | 0–2 | 0.00 | 0–2 | |
| Orbicularis tension | 0.00 | 0–0 | 0.00 | 0–0 | 0.00 | 0–1 | |
| Dilated nostrils | 0.00 | 0–2 | 0.00 | 0–2 | 0.00 | 0–2 | |
| Eye opening | 0.00 | 0–2 | 0.50 | 0–2 | 1.00 | 0–2 | |
| Muzzle tension | 0.00 | 0–2 | 0.00 | 0–2 | 0.00 | 0–2 | |
| Masticatory muscles tension | 0.00 | 0–2 | 0.00 | 0–2 | 0.00 | 0–2 | |
| SUM | 2.00 | 0–9 | 2.00 | 0–9 | 2.00 | 1–7 | |
| PCoA | −0.59 | −1.42–5.34 | −0.57 | −1.35–4.39 | −0.52 | −1.42–4.60 | |
| SUM.W | −5.54 | −7.54–1.46 | −5.54 | −7.54–1.46 | −5.54 | −6.54–−0.54 | |
| GFS | 0.00 | 0–2 | 0.00 | 0–2 | 0.00 | 0–2 | |
| MNT | 446.68 a | 446.68–446.68 | 75.86 bB | 28.84–281.84 | 77.37 bB | 11.75–281.84 | |
| B | |||||||
| Before | 24 h After | 48 h After | |||||
| Mean | SD | Mean | SD | Mean | SD | ||
| S | CT | 32.92 b | 1.10 | 34.38 aB | 0.69 | 34.32 aB | 0.87 |
| IG | CT | 33.46 b | 0.92 | 35.29 aA | 0.48 | 35.75 aA | 0.58 |
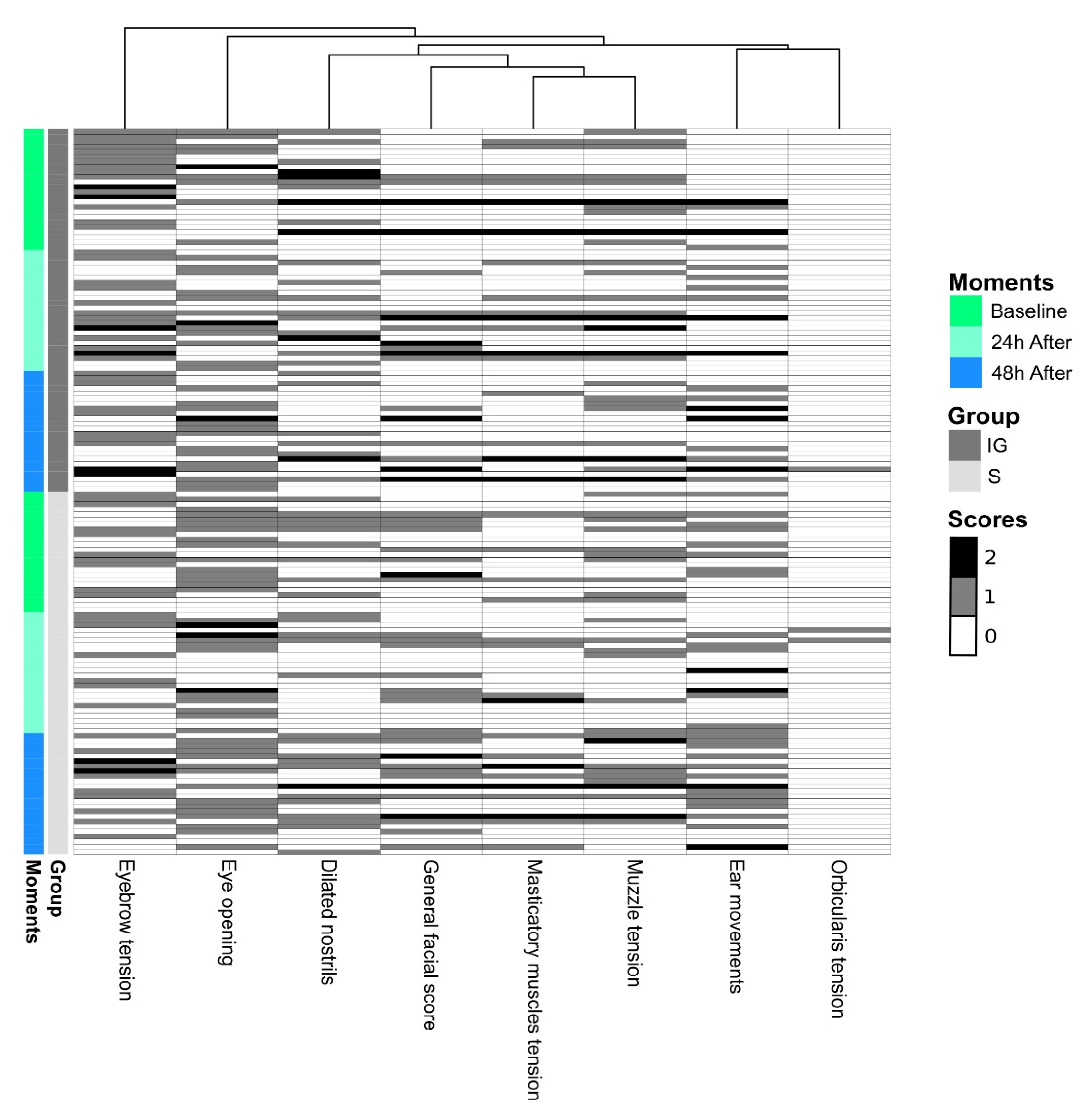
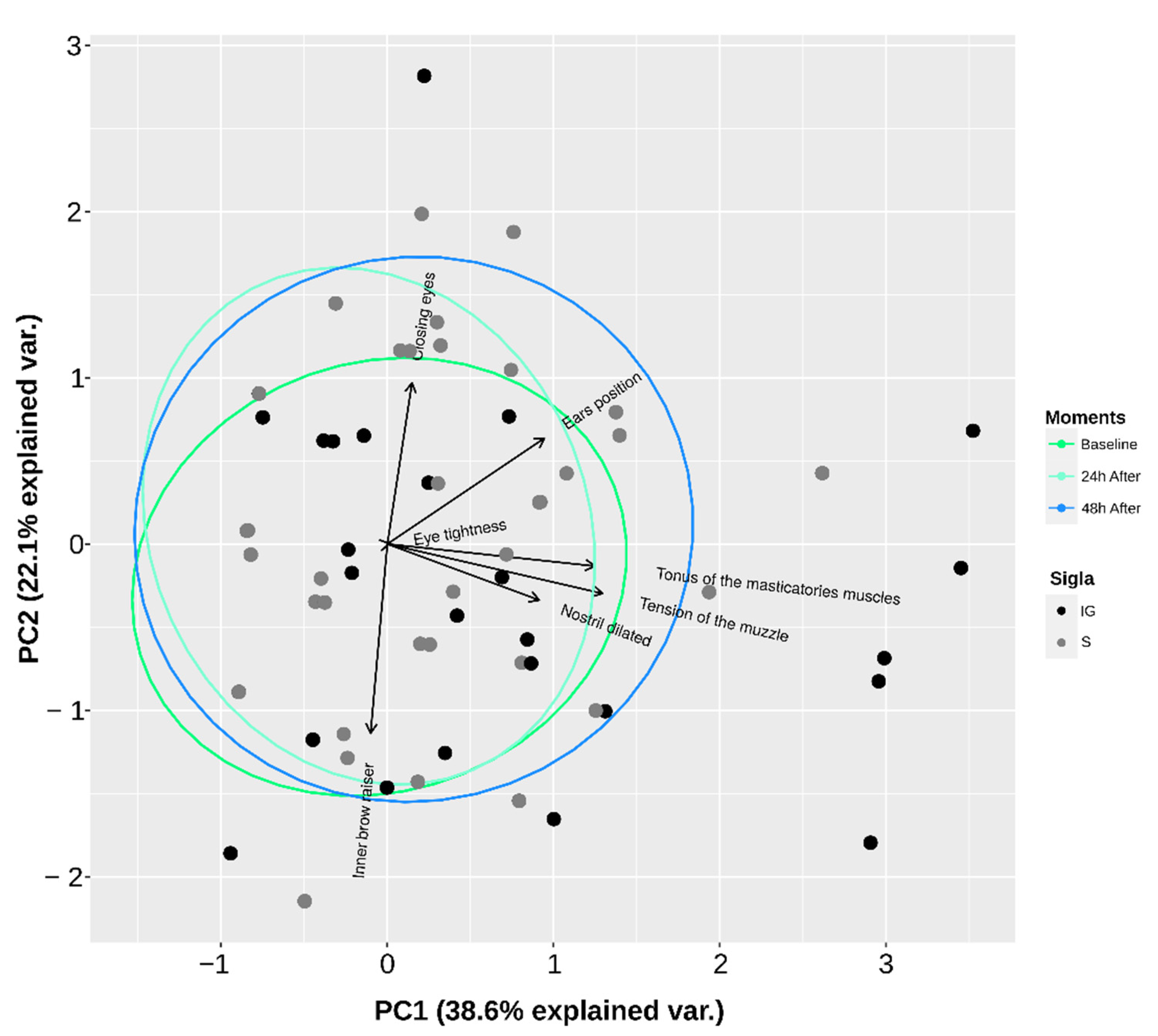
| Dimension 1 | Dimension 2 | |
|---|---|---|
| Ear movements | 0.59 | 0.39 |
| Eyebrow tension | −0.06 | −0.76 |
| Orbicularis tension | 0.06 | 0.03 |
| Dilated nostrils | 0.10 | 0.75 |
| Eye opening | 0.87 | −0.07 |
| Muzzle tension | 0.67 | −0.22 |
| Masticatory muscles tension | 0.85 | −0.17 |
| Eigenvalue | 2.29 | 1.36 |
| Variance (%) | 32.83 | 19.52 |
| MNT | CT | Ear Movements | Eyebrow Tension | Orbicularis Tension | Dilated Nostrils | Eye Opening | Muzzle Tension | Masticatory Muscles Tension | GFS | SUM | PC | SUM.W | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MNT | |||||||||||||
| CT | −0.55 * | ||||||||||||
| Ear movements | −0.13 | 0.02 | |||||||||||
| Eyebrow tension | 0.05 | −0.08 | −0.22 * | ||||||||||
| Orbicularis tension | −0.13 | 0.06 | 0.03 | 0.00 | |||||||||
| Dilated nostrils | −0.02 | −0.10 | 0.13 | 0.07 | −0.01 | ||||||||
| Eye opening | −0.20 * | 0.03 | 0.18 * | −0.30* | 0.03 | 0.02 | |||||||
| Muzzle tension | 0.03 | −0.06 | 0.27 * | 0.07 | 0.08 | 0.38 * | 0.02 | ||||||
| Masticatory muscles tension | −0.16 | −0.06 | 0.28 * | −0.06 | 0.03 | 0.40 * | 0.02 | 0.62 * | |||||
| GFS | −0.24 * | −0.02 | 0.45 * | −0.08 | 0.12 | 0.34 * | 0.27 * | 0.54 * | 0.62 * | ||||
| SUM | −0.13 | −0.07 | 0.51 * | 0.22* | 0.09 | 0.61 * | 0.36 * | 0.69 * | 0.62 * | 0.63 * | |||
| PCoA | −0.11 | −0.05 | 0.52 * | −0.14 | 0.09 | 0.62 * | 0.26 * | 0.79 * | 0.71 * | 0.65 * | 0.87 * | ||
| SUM.W | −0.13 | −0.07 | 0.51 * | 0.22* | 0.09 | 0.61 * | 0.36 * | 0.69 * | 0.62 * | 0.63 * | 1.00 * | 0.87 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvalho, J.R.G.; Trindade, P.H.E.; Conde, G.; Antonioli, M.L.; Funnicelli, M.I.G.; Dias, P.P.; Canola, P.A.; Chinelatto, M.A.; Ferraz, G.C. Facial Expressions of Horses Using Weighted Multivariate Statistics for Assessment of Subtle Local Pain Induced by Polylactide-Based Polymers Implanted Subcutaneously. Animals 2022, 12, 2400. https://doi.org/10.3390/ani12182400
Carvalho JRG, Trindade PHE, Conde G, Antonioli ML, Funnicelli MIG, Dias PP, Canola PA, Chinelatto MA, Ferraz GC. Facial Expressions of Horses Using Weighted Multivariate Statistics for Assessment of Subtle Local Pain Induced by Polylactide-Based Polymers Implanted Subcutaneously. Animals. 2022; 12(18):2400. https://doi.org/10.3390/ani12182400
Chicago/Turabian StyleCarvalho, Júlia R. G., Pedro H. E. Trindade, Gabriel Conde, Marina L. Antonioli, Michelli I. G. Funnicelli, Paula P. Dias, Paulo A. Canola, Marcelo A. Chinelatto, and Guilherme C. Ferraz. 2022. "Facial Expressions of Horses Using Weighted Multivariate Statistics for Assessment of Subtle Local Pain Induced by Polylactide-Based Polymers Implanted Subcutaneously" Animals 12, no. 18: 2400. https://doi.org/10.3390/ani12182400
APA StyleCarvalho, J. R. G., Trindade, P. H. E., Conde, G., Antonioli, M. L., Funnicelli, M. I. G., Dias, P. P., Canola, P. A., Chinelatto, M. A., & Ferraz, G. C. (2022). Facial Expressions of Horses Using Weighted Multivariate Statistics for Assessment of Subtle Local Pain Induced by Polylactide-Based Polymers Implanted Subcutaneously. Animals, 12(18), 2400. https://doi.org/10.3390/ani12182400











