Vitamin D3 Metabolic Enzymes in Plateau Zokor (Myospalax baileyi) and Plateau Pika (Ochotona curzoniae): Expression and Response to Hypoxia
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Procedures
2.2. Ultra Performance Liquid Chromatography-Tandem Mass Spectrometry (UPLC-MS/MS)
2.2.1. Quantitative Study of 25(OH)D3
2.2.2. Quantitative Study of LCA
2.3. Calcium Concentration Analysis
2.4. Total RNA Isolation and qRT-PCR
2.5. Western Blotting
2.6. Statistical Analysis
3. Results
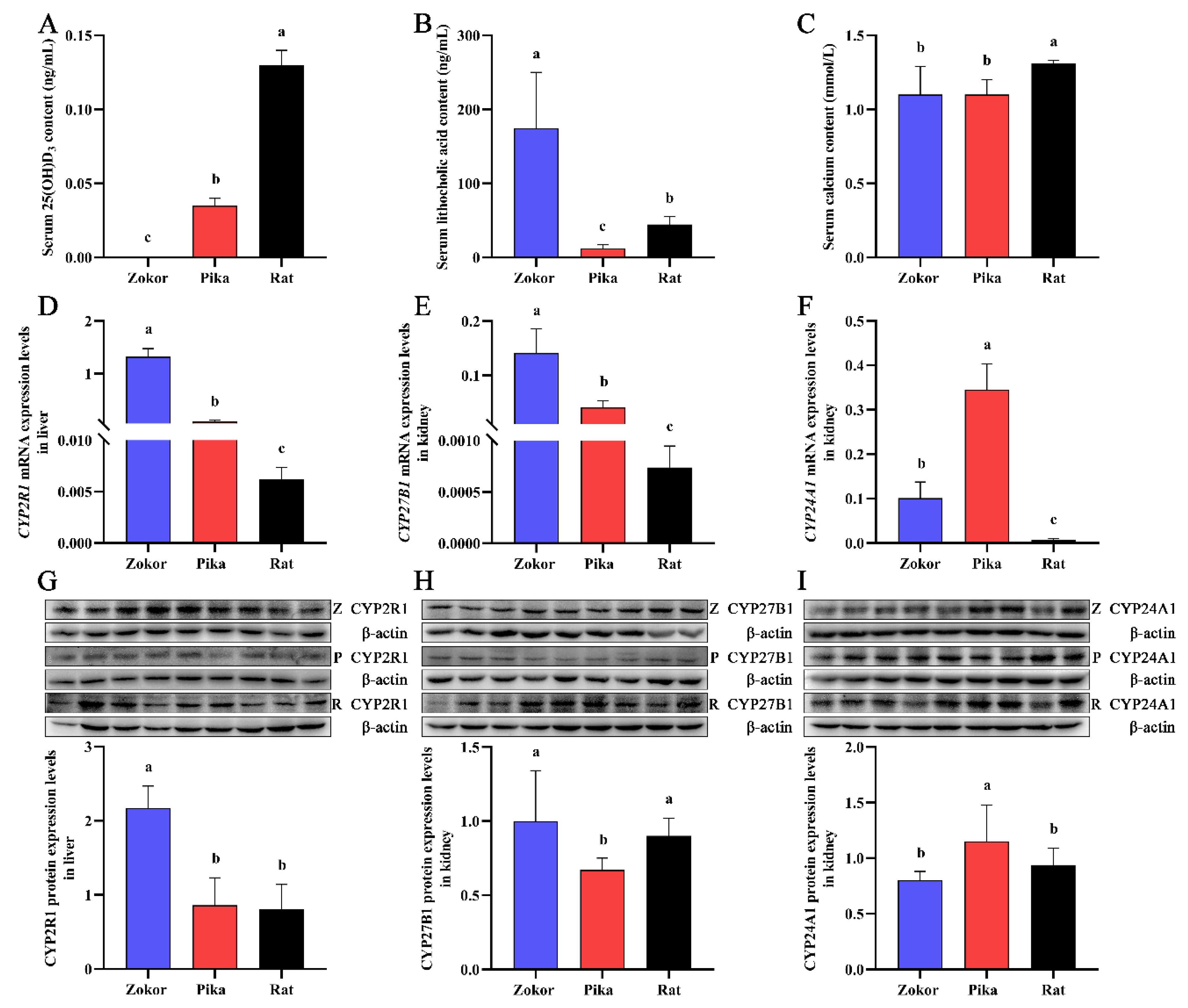
3.1. Serum Levels of 25(OH)D3, LCA, and Calcium and the Expression of Vitamin D3 Metabolism-Related Genes in Plateau Zokor, Plateau Pika, and SD Rats
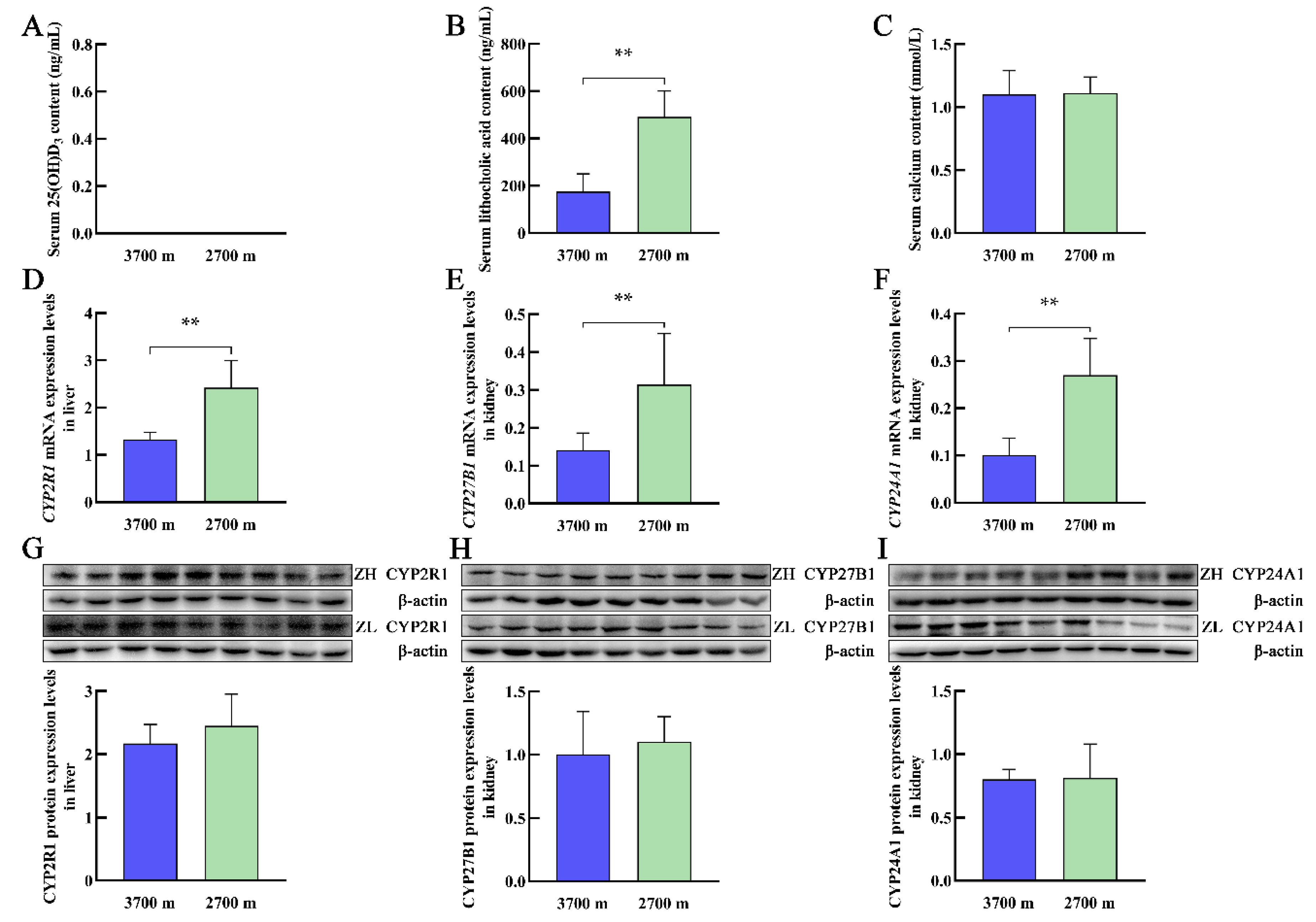
3.2. Serum Levels of 25(OH)D3, LCA, and Calcium and the Expression of Vitamin D3 Metabolism-Related Genes in Plateau Zokors at Different Altitudes
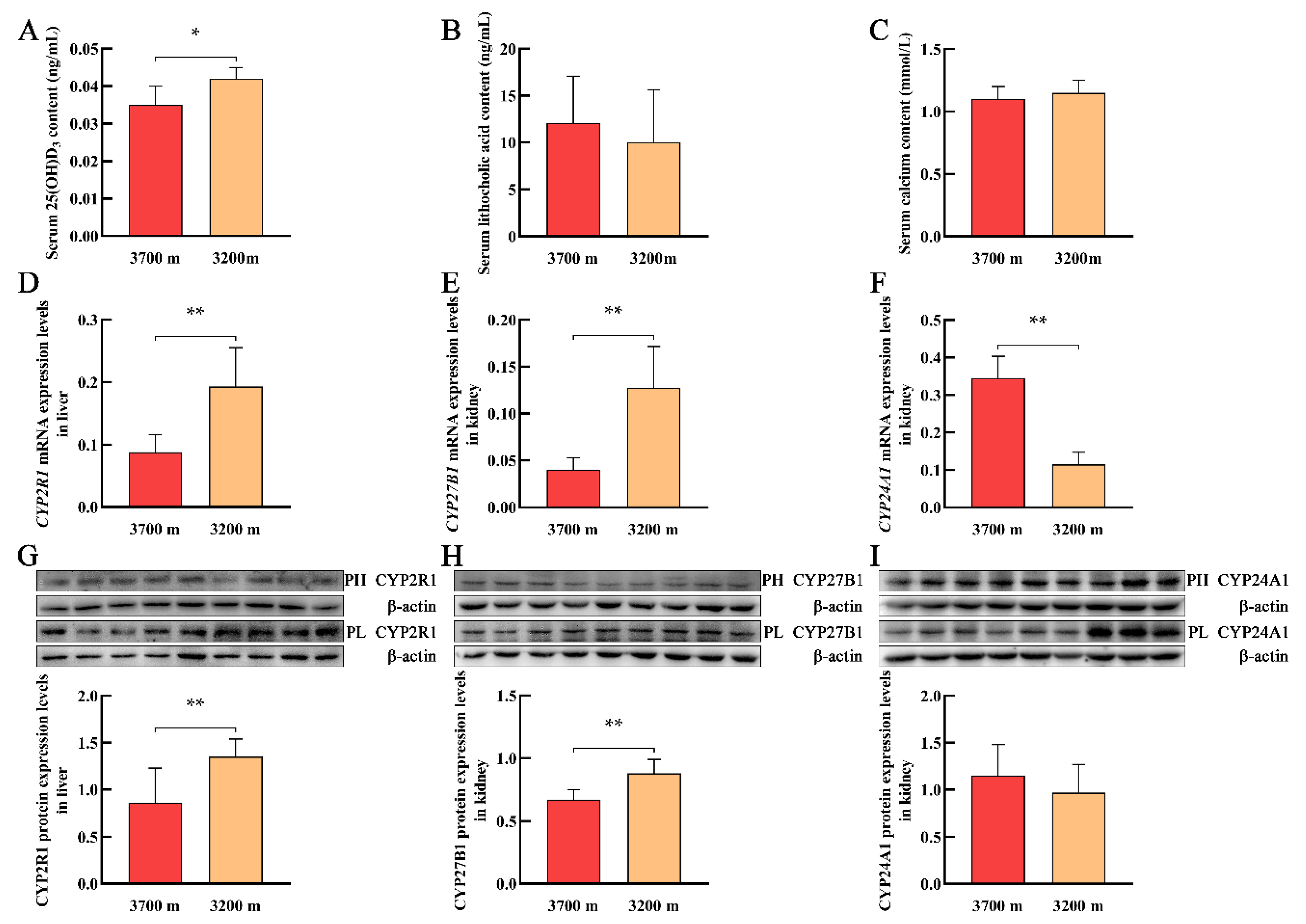
3.3. Serum Levels of 25(OH)D3, LCA, and Calcium and the Expression of Vitamin D3 Metabolism-Related Genes in Plateau Pikas at Different Altitudes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bikle, D.D. Vitamin D: An ancient hormone. Exp. Dermatol. 2011, 20, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, molecular mechanism of action, and pleiotropic effects. Physiol. Rev. 2016, 96, 365–408. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.; Christakos, S. New aspects of vitamin D metabolism and action-addressing the skin as source and target. Nat. Rev. Endocrinol. 2020, 16, 234–252. [Google Scholar] [CrossRef] [PubMed]
- Christakos, S. Vitamin D: A critical regulator of intestinal physiology. JBMR Plus 2021, 5, e10554. [Google Scholar] [CrossRef] [PubMed]
- Jones, G. 100 years of vitamin D: Historical aspects of vitamin D. Endocr. Connect. 2022, 11, e210594. [Google Scholar] [CrossRef] [PubMed]
- Baeke, F.; Takiishi, T.; Korf, H.; Gysemans, C.; Mathieu, C. Vitamin D: Modulator of the immune system. Curr. Opin. Pharmacol. 2010, 10, 482–496. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Mitri, J.; Mathieu, C.; Badenhoop, K.; Tamer, G.; Orio, F.; Mezza, T.; Vieth, R.; Colao, A.; Pittas, A. Mechanisms in endocrinology: Vitamin D as a potential contributor in endocrine health and disease. Eur. J. Endocrinol. 2014, 171, R101–R110. [Google Scholar] [CrossRef]
- Mungai, L.N.W.; Mohammed, Z.; Maina, M.; Anjumanara, O. Vitamin D review: The low hanging fruit for human health. J. Nutr. Metab. 2021, 2021, 6335681. [Google Scholar] [CrossRef]
- Lee, D.B.; Walling, M.M.; Levine, B.S.; Gafter, U.; Silis, V.; Hodsman, A.; Coburn, J.W. Intestinal and metabolic effect of 1, 25-dihydroxyvitamin D3 in normal adult rat. Am. J. Physiol. 1981, 240, G90–G96. [Google Scholar] [CrossRef]
- Favus, M.J. Factors that influence absorption and secretion of calcium in the small intestine and colon. Am. J. Physiol. 1985, 248, G147–G157. [Google Scholar] [CrossRef]
- Favus, M.J.; Angeid-Backman, E. Effects of 1, 25(OH)2D3 and calcium channel blockers on cecal calcium transport in the rat. Am. J. Physiol. 1985, 248, G676–G681. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, R.H. Vitamin D and the dual processes of intestinal calcium absorption. J. Nutr. 2004, 134, 3137–3139. [Google Scholar] [CrossRef] [PubMed]
- Christakos, S.; Seth, T.; Hirsch, J.; Porta, A.; Moulas, A.; Dhawan, P. Vitamin D biology revealed through the study of knockout and transgenic mouse models. Annu. Rev. Nutr. 2013, 33, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Furuse, M.; Fujita, K.; Hiiragi, T.; Fujimoto, K.; Tsukita, S. Claudin-1 and -2: Novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J. Cell. Biol. 1998, 141, 1539–1550. [Google Scholar] [CrossRef] [PubMed]
- Bronner, F. Mechanisms of intestinal calcium absorption. J. Cell. Biochem. 2003, 88, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Benn, B.S.; Ajibade, D.; Porta, A.; Dhawan, P.; Hediger, M.; Peng, J.B.; Jiang, Y.; Oh, G.T.; Jeung, E.B.; Lieben, L.; et al. Active intestinal calcium transport in the absence of transient receptor potential vanilloid type 6 and calbindin-D9k. Endocrinology 2008, 149, 3196–3205. [Google Scholar] [CrossRef] [PubMed]
- Fujita, H.; Sugimoto, K.; Inatomi, S.; Maeda, T.; Osanai, M.; Uchiyama, Y.; Yamamoto, Y.; Wada, T.; Kojima, T.; Yokozaki, H.; et al. Tight junction proteins claudin-2 and -12 are critical for vitamin D-dependent Ca2+ absorption between enterocytes. Mol. Biol. Cell. 2008, 19, 1912–1921. [Google Scholar] [CrossRef]
- Christakos, S. Recent advances in our understanding of 1, 25-dihydroxyvitamin D3 regulation of intestinal calcium absorption. Arch. Biochem. Biophys. 2012, 523, 73–76. [Google Scholar] [CrossRef]
- Lee, S.M.; Riley, E.M.; Meyer, M.B.; Benkusky, N.A.; Plum, L.A.; DeLuca, H.F.; Pike, J.W. 1, 25-dihydroxyvitamin D3 controls a cohort of vitamin D receptor target genes in the proximal intestine that is enriched for calcium-regulating components. J. Biol. Chem. 2015, 290, 18199–18215. [Google Scholar] [CrossRef]
- Adamczak, M.; Surma, S.; Więcek, A. Vitamin D and arterial hypertension: Facts and myths. Curr. Hypertens. Rep. 2020, 22. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D and the skin: Physiology and pathophysiology. Rev. Endocr. Metab. Disord. 2012, 13, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.Q.; Holick, M.F. Catalyzed thermal isomerization between previtamin D3 and vitamin D3 via β-cyclodextrin complexation. J. Biol. Chem. 1995, 270, 8706–8711. [Google Scholar] [CrossRef] [PubMed]
- DeLuca, H.F. Overview of general physiologic features and functions of vitamin D. Am. J. Clin. Nutr. 2004, 80, 1689S–1696S. [Google Scholar] [CrossRef]
- Bhattacharyya, M.H.; DeLuca, H.F. Subcellular location of rat liver calciferol-25-hydroxylase. Arch. Biochem. Biophys. 1974, 160, 58–62. [Google Scholar] [CrossRef]
- Zhu, J.G.; Ochalek, J.T.; Kaufmann, M.; Jones, G.; Deluca, H.F. CYP2R1 is a major, but not exclusive, contributor to 25-hydroxyvitamin D production in vivo. Proc. Natl. Acad. Sci. USA 2013, 110, 15650–15655. [Google Scholar] [CrossRef] [PubMed]
- Fu, G.K.; Lin, D.; Zhang, M.Y.H.; Bikle, D.D.; Shackleton, C.H.L.; Miller, W.L.; Portale, A.A. Cloning of human 25-hydroxyvitamin D-1α-hydroxylase and mutations causing vitamin D-dependent rickets type 1. Mol. Endocrinol. 1997, 11, 1961–1970. [Google Scholar] [CrossRef][Green Version]
- Cashman, K.D.; van den Heuvel, E.G.; Schoemaker, R.J.; Prévéraud, D.P.; Macdonald, H.M.; Arcot, J. 25-hydroxyvitamin D as a biomarker of vitamin D status and its modeling to inform strategies for prevention of vitamin D deficiency within the population. Adv. Nutr. 2017, 8, 947–957. [Google Scholar] [CrossRef]
- Makris, K.; Sempos, C.; Cavalier, E. The measurement of vitamin D metabolites: Part I-metabolism of vitamin D and the measurement of 25-hydroxyvitamin D. Hormones 2020, 19, 81–96. [Google Scholar] [CrossRef]
- Bosworth, C.R.; Levin, G.; Robinson-Cohen, C.; Hoofnagle, A.N.; Ruzinski, J.; Young, B.; Schwartz, S.M.; Himmelfarb, J.; Kestenbaum, B.; de Boer, I.H. The serum 24, 25-dihydroxyvitamin D concentration, a marker of vitamin D catabolism, is reduced in chronic kidney disease. Kidney Int. 2012, 82, 693–700. [Google Scholar] [CrossRef]
- Bouillon, R.; Antonio, L.; Olarte, O.R. Calcifediol (25OH vitamin D3) deficiency: A risk factor from early to old age. Nutrients 2022, 14, 1168. [Google Scholar] [CrossRef] [PubMed]
- Lovegrove, B.G.; Knight-Eloff, A. Soil and burrow temperatures, and the resource characteristics of the social mole-rat Cryptomys damarensis (Bathyergidae) in the Kalahari desert. J. Zool. 1988, 216, 403–416. [Google Scholar] [CrossRef]
- Buffenstein, R.; Yahav, S. Cholecalciferol has no effect on calcium and inorganic phosphorus balance in a naturally cholecalciferol-deplete subterranean mammal, the naked mole rat (Heterocephalus glaber). J. Endocrinol. 1991, 129, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Chu, B.; Tian, Y.L.; Zhou, J.W.; Tang, Z.S.; Dong, K.; Hua, L.M. Increased burrow oxygen levels trigger defensive burrow-sealing behavior by plateau zokors. Sci. Rep. 2021, 11. [Google Scholar] [CrossRef]
- Buffenstein, R.; Sergeev, I.N.; Pettifor, J.M. Vitamin D hydroxylases and their regulation in a naturally vitamin D-deficient subterranean mammal, the naked mole rat (Heterocephalus glaber). J. Endocrinol. 1993, 138, 59–64. [Google Scholar] [CrossRef]
- Pitcher, T.; Sergeev, I.N.; Buffenstein, R. Vitamin D metabolism in the Damara mole-rat is altered by exposure to sunlight yet mineral metabolism is unaffected. J. Endocrinol. 1994, 143, 367–374. [Google Scholar] [CrossRef]
- Lin, X.; Wang, H.Y.; Pu, X.Y. Protective mechanism of fdft1 in steroid hormone synthesis pathway in SD rats with acute hypoxic injury. Genes. Genom. 2020, 42, 1319–1326. [Google Scholar] [CrossRef]
- Goyal, R.; Billings, T.L.; Mansour, T.; Martin, C.; Baylink, D.J.; Longo, L.D.; Pearce, W.J.; Mata-Greenwood, E. Vitamin D status and metabolism in an ovine pregnancy model: Effect of long-term, high-altitude hypoxia. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E1062–E1071. [Google Scholar] [CrossRef]
- Norsang, G.; Ma, L.W.; Dahlback, A.; Zhuoma, C.; Tsoja, W.; Porojnicu, A.; Lagunova, Z.; Moan, J. The vitamin D status among Tibetans. Photochem. Photobiol. 2009, 85, 1028–1031. [Google Scholar] [CrossRef]
- Ridlon, J.M.; Kang, D.J.; Hylemon, P.B.; Bajaj, J.S. Bile acids and the gut microbiome. Curr. Opin. Gastroenterol. 2014, 30, 332–338. [Google Scholar] [CrossRef]
- Makishima, M.; Lu, T.T.; Xie, W.; Whitfield, G.K.; Domoto, H.; Evans, R.M.; Haussler, M.R.; Mangelsdorf, D.J. Vitamin D receptor as an intestinal bile acid sensor. Science 2002, 296, 1313–1316. [Google Scholar] [CrossRef]
- Nehring, J.A.; Zierold, C.; DeLuca, H.F. Lithocholic acid can carry out in vivo functions of vitamin D. Proc. Natl. Acad. Sci. USA 2007, 104, 10006–10009. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, S.N.; Luo, J.N.; Harris, D.A.; Aliakbarian, H.; Yao, L.; Paik, D.; Subramaniam, R.; Adhikari, A.A.; Vernon, A.H.; Kiliç, A.; et al. A microbial metabolite remodels the gut-liver axis following bariatric surgery. Cell. Host Microbe. 2021, 29, 408–424. [Google Scholar] [CrossRef] [PubMed]
- Peacock, A.J. ABC of oxygen: Oxygen at high altitude. BMJ 1998, 317, 1063–1066. [Google Scholar] [CrossRef] [PubMed]
- Butaric, L.N.; Klocke, R.P. Nasal variation in relation to high-altitude adaptations among Tibetans and Andeans. Am. J. Hum. Biol. 2018, 30, e23104. [Google Scholar] [CrossRef]
- Song, D.S.; Navalsky, B.E.; Guan, W.; Ingersoll, C.; Wang, T.; Loro, E.; Eeles, L.; Matchett, K.B.; Percy, M.J.; Walsby-Tickle, J.; et al. Tibetan PHD2, an allele with loss-of-function properties. Proc. Natl. Acad. Sci. USA 2020, 117, 12230–12238. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Zhang, Z.B.; Liu, J.K. Burrowing rodents as ecosystem engineers: The ecology and management of plateau zokors Myospalax fontanierii in alpine meadow ecosystems on the Tibetan Plateau. Mammal. Rev. 2003, 33, 284–294. [Google Scholar] [CrossRef]
- Smith, A.T.; Wang, X.G. Social relationships of adult black-lipped pikas (Ochotona curzoniae). J. Mammal. 1991, 72, 231–247. [Google Scholar] [CrossRef]
- Dobson, F.S.; Smith, A.T.; Wang, X.G. The mating system and gene dynamics of plateau pikas. Behav. Processes 2000, 51, 101–110. [Google Scholar] [CrossRef]
- Yin, B.F.; Yang, S.M.; Wei, W.H.; Zhang, Y.M. Male reproductive success in plateau pikas (Ochotona curzoniae): A microsatellite analysis. Mamm. Biol. 2009, 74, 344–350. [Google Scholar] [CrossRef]
- Sun, S.Z.; Wei, L.; Wei, D.B.; Wang, D.W.; Ma, B.Y. Differences of glycolysis in skeletal muscle and lactate metabolism in liver between plateau zokor (Myospalax baileyi) and plateau pika (Ochotona curzoniae). Acta Physiol. Sin. 2013, 65, 276–284. [Google Scholar] [CrossRef]
- Shao, Y.; Li, J.X.; Ge, R.L.; Zhong, L.; Irwin, D.M.; Murphy, R.W.; Zhang, Y.P. Genetic adaptations of the plateau zokor in high-elevation burrows. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Chen, J.; Zhang, J.; Guo, Y.T.; Zhou, X.; Li, M.W.; Zheng, Z.Z.; Zhang, T.Z.; Murphy, R.W.; Nevo, E.; et al. Phenotypic and genomic adaptations to the extremely high elevation in plateau zokor (Myospalax baileyi). Mol. Ecol. 2021, 30, 5765–5779. [Google Scholar] [CrossRef] [PubMed]
- Li, S.H.; Xu, B.; An, Z.F.; Wang, Z.J.; Li, Y.X.; Wei, L.; Wei, D.B. Evolutionary analysis of TSP-1 gene in plateau zokor (Myospalax Baileyi) and its expression pattern under hypoxia. Cell. Mol. Biol. 2019, 65, 48–57. [Google Scholar]
- Bikle, D.D. Vitamin D metabolism, mechanism of action, and clinical applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Fan, N.C.; Shi, Y.Z. A revision of the zokors of subgenus. Acta Theriol. Sin. 1982, 2, 183–199. [Google Scholar] [CrossRef]
- Endres, B.; Kato, S.; DeLuca, H.F. Metabolism of 1α, 25-dihydroxyvitamin D3 in vitamin D receptor-ablated mice in vivo. Biochemistry 2000, 39, 2123–2129. [Google Scholar] [CrossRef]
- Ishizawa, M.; Akagi, D.; Makishima, M. Lithocholic acid is a vitamin D receptor ligand that acts preferentially in the ileum. Int. J. Mol. Sci. 2018, 19, 1975. [Google Scholar] [CrossRef]
- Skinner, D.C.; Moodley, G.; Buffenstein, R. Is vitamin D3 essential for mineral metabolism in the Damara mole-rat (Cryptomys damarensis)? Gen. Comp. Endocrinol. 1991, 81, 500–505. [Google Scholar] [CrossRef]
- Hashimoto, N.; Matsui, I.; Ishizuka, S.; Inoue, K.; Matsumoto, A.; Shimada, K.; Hori, S.; Lee, D.G.; Yasuda, S.; Katsuma, Y. Lithocholic acid increases intestinal phosphate and calcium absorption in a vitamin D receptor dependent but transcellular pathway independent manner. Kidney Int. 2020, 97, 1164–1180. [Google Scholar] [CrossRef]
- Zeng, J.X.; Wang, Z.W.; Shi, Z.X. Metabolic characteristics and some physiological parameters of mole rat (Myospalax baileyi) in alpine area. Acta Biol. Plat. Sin. 1984, 3, 163–171. [Google Scholar]
- Van Cromphaut, S.J.; Dewerchin, M.; Hoenderop, J.G.; Stockmans, I.; Van Herck, E.; Kato, S.; Bindels, R.J.; Collen, D.; Carmeliet, P.; Bouillon, R.; et al. Duodenal calcium absorption in vitamin D receptor-knockout mice: Functional and molecular aspects. Proc. Natl. Acad. Sci. USA 2001, 98, 13324–13329. [Google Scholar] [CrossRef] [PubMed]
- Replogle, R.A.; Li, Q.; Wang, L.B.; Zhang, M.; Fleet, J.C. Gene-by-diet interactions influence calcium absorption and bone density in mice. J. Bone Miner. Res. 2014, 29, 657–665. [Google Scholar] [CrossRef] [PubMed]
| Species | Gene | Sense Primer (5′ to 3′) | Anti-Sense Primer (5′ to 3′) |
|---|---|---|---|
| Plateau Zokor | CYP2R1 | AGTTGTTCAGTGAGAATGTGGAA | CCGAGGTAAGTGAGGCTTTC |
| CYP27B1 | CCAAGCCACTGTTCTATC | TAGTCACCTACACGGATG | |
| CYP24A1 | GAGATTCGGACTCCTTCAGA | GGTGTTGAGCCTCTTGTG | |
| Plateau Pika | CYP2R1 | TTCCTCGGCAACATCTAC | CTACATCATAGCCATTCAGAAC |
| CYP27B1 | CAATGCTCTGTCTCAACTG | CGTGAAGTGGCATAGTGA | |
| CYP24A1 | TCTCGCAATCCTCAAGTC | ACATATTCCTCAAGTCCTCTG | |
| Sprague-Dawley rat | CYP2R1 | GCATATCAACTGTGGTTCTCAAT | ATCCATCCTCTGCCATATCTG |
| CYP27B1 | ATGGTGAAGAATGGCAGAG | TGTCCAGAGTTCCAGCATA | |
| CYP24A1 | TTCGCTCATCTCCCATTC | CATCTCCACAGGTTCATTG | |
| β-actin | TCACCAACTGGGACGATATG | GTTGGCCTTAGGGTTCAGAG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; An, Z.; Wei, L.; Zhang, J.; Li, J.; Wang, Z.; Gao, C.; Wei, D. Vitamin D3 Metabolic Enzymes in Plateau Zokor (Myospalax baileyi) and Plateau Pika (Ochotona curzoniae): Expression and Response to Hypoxia. Animals 2022, 12, 2371. https://doi.org/10.3390/ani12182371
Chen X, An Z, Wei L, Zhang J, Li J, Wang Z, Gao C, Wei D. Vitamin D3 Metabolic Enzymes in Plateau Zokor (Myospalax baileyi) and Plateau Pika (Ochotona curzoniae): Expression and Response to Hypoxia. Animals. 2022; 12(18):2371. https://doi.org/10.3390/ani12182371
Chicago/Turabian StyleChen, Xiaoqi, Zhifang An, Linna Wei, Jiayu Zhang, Jimei Li, Zhijie Wang, Conghui Gao, and Dengbang Wei. 2022. "Vitamin D3 Metabolic Enzymes in Plateau Zokor (Myospalax baileyi) and Plateau Pika (Ochotona curzoniae): Expression and Response to Hypoxia" Animals 12, no. 18: 2371. https://doi.org/10.3390/ani12182371
APA StyleChen, X., An, Z., Wei, L., Zhang, J., Li, J., Wang, Z., Gao, C., & Wei, D. (2022). Vitamin D3 Metabolic Enzymes in Plateau Zokor (Myospalax baileyi) and Plateau Pika (Ochotona curzoniae): Expression and Response to Hypoxia. Animals, 12(18), 2371. https://doi.org/10.3390/ani12182371






