Geographic Variation in Note Types of Alarm Calls in Japanese Tits (Parus minor)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Subjects
2.2. Dummy Experiments and Recordings
2.3. Acoustic Analysis
2.4. Note Descriptions
2.5. Statistical Data Analysis
3. Results
3.1. Note Classification
3.2. Note Discriminant Analysis
3.3. Comparison of Shared Note Types
3.3.1. Comparison of A Notes
3.3.2. Comparison of B Notes
3.3.3. Comparison of D Notes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Catchpole, C.K. Bird song, sexual selection and female choice. Trends Ecol. Evol. 1987, 2, 94–97. [Google Scholar] [CrossRef]
- Marler, P.R. Bird calls: A cornucopia for communication. In Nature’s Music: The Science of Birdsong; Marler, P.R., Slabbekoorn, H., Eds.; Elsevier: San Diego, CA, USA, 2004; pp. 132–177. [Google Scholar]
- Magrath, R.D.; Pitcher, B.J.; Gardner, J.L. A mutual understanding? Interspecific responses by birds to each other’s aerial alarm calls. Behav. Ecol. 2007, 18, 944–951. [Google Scholar] [CrossRef]
- Nicholls, J.A.; Goldizen, A.W. Habitat type and density influence vocal signal design in satin bowerbirds. J. Anim. Ecol. 2006, 75, 549–558. [Google Scholar] [CrossRef]
- Ballentine, B. Morphological adaptation influences the evolution of a mating signal. Evolution 2006, 60, 1936–1944. [Google Scholar] [CrossRef] [PubMed]
- Irwin, D.E.; Thimgan, M.P.; Irwin, J.H. Call divergence is correlated with geographic and genetic distance in greenish warblers (Phylloscopus trochiloides): A strong role for stochasticity in signal evolution? J. Evol. Biol. 2008, 21, 435–448. [Google Scholar] [CrossRef]
- Beecher, M.D.; Brenowitz, E.A. Functional aspects of song learning in songbirds. Trends Ecol. Evol. 2005, 20, 143–149. [Google Scholar] [CrossRef]
- Freeberg, T.M. Geographic variation in note composition and use of chick-a-dee calls of Carolina Chickadees (Poecile carolinensis). Ethology 2012, 118, 555–565. [Google Scholar] [CrossRef]
- Fuisz, T.I.; de Kort, S.R. Habitat-dependent call divergence in the common cuckoo: Is it a potential signal for assortative mating? Proc. R. Soc. B-Biol. Sci. 2007, 274, 2093–2097. [Google Scholar] [CrossRef]
- Irwin, D.E. Song variation in an avian ring species. Evolution 2000, 54, 998–1010. [Google Scholar] [CrossRef]
- Ippi, S.; Vasquez, R.A.; van Dongen, W.F.D.; Lazzoni, I. Geographical variation in the vocalizations of the suboscine Thorn-tailed Rayadito Aphrastura spinicauda. Ibis 2011, 153, 789–805. [Google Scholar] [CrossRef]
- Wei, C.; Jia, C.; Dong, L.; Wang, D.; Xia, C.; Zhang, Y.; Liang, W. Geographic variation in the calls of the Common Cuckoo (Cuculus canorus): Isolation by distance and divergence among subspecies. J. Ornithol. 2015, 156, 533–542. [Google Scholar] [CrossRef]
- Podos, J.; Warren, P.S. The evolution of geographic variation in birdsong. Adv. Study Behav. 2007, 37, 403–458. [Google Scholar]
- Slater, P.J.B. Bird song learning: Causes and consequences. Ethol. Ecol. Evol. 1989, 1, 19–46. [Google Scholar] [CrossRef]
- Luttrell, S.A.M.; Lohr, B. Geographic variation in call structure, likelihood, and call-song associations across subspecies boundaries, migratory patterns, and habitat types in the Marsh Wren (Cistothorus palustris). Auk 2018, 135, 127–151. [Google Scholar] [CrossRef]
- Bradbury, J.W.; Cortopassi, K.A.; Clemmons, J.R. Geographical variation in the contact calls of orange-fronted Parakeets. Auk 2001, 118, 958–972. [Google Scholar] [CrossRef]
- Naguib, M.; Hammerschmidt, K.; Wirth, J. Microgeographic variation, habitat effects and individual signature cues in calls of chiffchaffs Phylloscopus collybita canarensis. Ethology 2001, 107, 341–355. [Google Scholar] [CrossRef]
- Freeberg, T.M. Complexity in the chick-a-dee call of Carolina Chickadees (Poecile carolinensis): Associations of context and signaler behavior to call structure. Auk 2008, 125, 896–907. [Google Scholar] [CrossRef]
- Gill, S.A.; Bierema, A.M.K. On the meaning of alarm calls: A review of functional reference in avian alarm calling. Ethology 2013, 119, 449–461. [Google Scholar] [CrossRef]
- Griesser, M. Mobbing calls signal predator category in a kin group-living bird species. Proc. R. Soc. B-Biol. Sci. 2009, 276, 2887–2892. [Google Scholar] [CrossRef]
- Templeton, C.N.; Greene, E.; Davis, K. Allometry of alarm calls: Black-capped chickadees encode information about predator size. Science 2005, 308, 1934–1937. [Google Scholar] [CrossRef]
- Templeton, C.N.; Greene, E. Nuthatches eavesdrop on variations in heterospecific chickadee mobbing alarm calls. Proc. Natl. Acad. Sci. USA 2007, 104, 5479–5482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, E.D.; Farabaugh, S.M. Macrogeographic variation in alarm calls of the Australian Magpie Gymnorhina-Tibicen. Bird Behav. 1991, 9, 64–68. [Google Scholar] [CrossRef]
- Habel, J.C.; Husemann, M.; Ulrich, W. Evolution of contact and alarm calls in the Kenyan endemic Hinde’s babbler (Aves: Passeriformes). BMC Evol. Biol. 2018, 18, 112. [Google Scholar] [CrossRef] [PubMed]
- Courter, J.R.; Ritchison, G. Alarm calls of tufted titmice convey information about predator size and threat. Behav. Ecol. 2010, 21, 936–942. [Google Scholar] [CrossRef]
- Suzuki, T.N. Assessment of predation risk through referential communication in incubating birds. Sci. Rep. 2015, 5, 10239. [Google Scholar] [CrossRef]
- Dutour, M.; Lena, J.P.; Lengagne, T. Mobbing behaviour varies according to predator dangerousness and occurrence. Anim. Behav. 2016, 119, 119–124. [Google Scholar] [CrossRef]
- Carlson, N.V.; Healy, S.D.; Templeton, C.N. A comparative study of how British tits encode predator threat in their mobbing calls. Anim. Behav. 2017, 125, 77–92. [Google Scholar] [CrossRef]
- Bloomfield, L.L.; Phillmore, L.S.; Weisman, R.G.; Sturdy, C.B. Note types and coding in parid vocalizations. III: The chick-a-dee call of the Carolina chickadee (Poecile carolinensis). Can. J. Zool. 2005, 83, 820–833. [Google Scholar] [CrossRef]
- Freeberg, T.M.; Lucas, J.R.; Krams, I. The complex call of the Carolina Chickadee. What can the chick-a-dee call teach us about communication and language? Am. Sci. 2012, 100, 398–407. [Google Scholar] [CrossRef]
- Haftorn, S.; Hailman, J.P. Do the Siberian tits Parus cinctus in Scandinavia and Siberia speak the same language? Bioacoustics 1997, 8, 223–247. [Google Scholar] [CrossRef]
- Hailman, J.P.; Ficken, M.S.; Ficken, R.W. The ‘chick-a-dee’ calls of Parus atricapillus: A recombinant system of animal communication compared with written English. Semiotica 1985, 56, 191–224. [Google Scholar] [CrossRef]
- Yu, J.P.; Xing, X.Y.; Jiang, Y.L.; Liang, W.; Wang, H.T.; Moller, A.P. Alarm call-based discrimination between common cuckoo and Eurasian sparrowhawk in a Chinese population of great tits. Ethology 2017, 123, 542–550. [Google Scholar] [CrossRef]
- Suzuki, T.N. Communication about predator type by a bird using discrete, graded and combinatorial variation in alarm calls. Anim. Behav. 2014, 87, 59–65. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, L.; Yi, G.; Zhang, K.; Yao, J.; Fang, J.; Shen, C.; Wang, H. Plastering mud around the entrance hole affects the estimation of threat levels from nest predators in Eurasian Nuthatches. Avian Res. 2021, 12, 58. [Google Scholar] [CrossRef]
- Bloomfield, L.L.; Charrier, I.; Sturdy, C.B. Note types and coding in parid vocalizations. II: The chick-a-dee call of the mountain chickadee (Poecile gambeli). Can. J. Zool. 2004, 82, 780–793. [Google Scholar] [CrossRef]
- Baptista, L.F. Geographic variation in song and dialects of puget sound white-crowned sparrow. Condor 1977, 79, 356–370. [Google Scholar] [CrossRef]
- Suzuki, T.N.; Ueda, K. Mobbing calls of Japanese tits signal predator type: Field observations of natural predator encounters. Wilson J. Ornithol. 2013, 125, 412–415. [Google Scholar] [CrossRef]
- Wilson, D.R.; Mennill, D.J. Duty cycle, not signal structure, explains conspecific and heterospecific responses to the calls of black-capped chickadees (Poecile atricapillus). Behav. Ecol. 2011, 22, 784–790. [Google Scholar] [CrossRef]
- Leavesley, A.J.; Magrath, R.D. Communicating about danger: Urgency alarm calling in a bird. Anim. Behav. 2005, 70, 365–373. [Google Scholar] [CrossRef]
- Suzuki, T.N. Referential calls coordinate multi-species mobbing in a forest bird community. J. Ethol. 2016, 34, 79–84. [Google Scholar] [CrossRef]
- Tu, H.W.; Severinghaus, L.L. Geographic variation of the highly complex Hwamei (Garrulax canorus) songs. Zool. Stud. 2004, 43, 629–640. [Google Scholar]
- Bolus, R.T. Geographic variation in songs of the Common Yellowthroat. Auk 2014, 131, 175–185. [Google Scholar] [CrossRef]
- Baker, M.C.; Baker, M.S.A.; Tilghman, L.M. Differing effects of isolation on evolution of bird songs: Examples from an island-mainland comparison of three species. Biol. J. Linn. Soc. 2006, 89, 331–342. [Google Scholar] [CrossRef]
- Lack, D.; Southern, H.N. Birds on Tenerife. Ibis 1949, 91, 607–626. [Google Scholar] [CrossRef]
- Zhao, N.; Dai, C.; Wang, W.; Zhang, R.; Qu, Y.; Song, G.; Chen, K.; Yang, X.; Zou, F.; Lei, F. Pleistocene climate changes shaped the divergence and demography of Asian populations of the great tit Parus major: Evidence from phylogeographic analysis and ecological niche models. J. Avian Biol. 2012, 43, 297–310. [Google Scholar] [CrossRef]
- Wheatcroft, D.; Price, T.D. Rates of signal evolution are associated with the nature of interspecific communication. Behav. Ecol. 2015, 26, 83–90. [Google Scholar] [CrossRef]
- Greenlaw, J.S.; Shackelford, C.E.; Brown, R.E. Call mimicry by eastern towhees and its significance in relation to auditory learning. Wilson Bull. 1998, 110, 431–434. [Google Scholar]
- Goodale, E.; Kotagama, S.W. Context-dependent vocal mimicry in a passerine bird. Proc. R. Soc. B-Biol. Sci. 2006, 273, 875–880. [Google Scholar] [CrossRef]
- Freeberg, T.M.; Lucas, J.R.; Clucas, B. Variation in chick-a-dee calls of a Carolina Chickadee population, Poecile carolinensis: Identity and redundancy within note types. J. Acoust. Soc. Am. 2003, 113, 2127–2136. [Google Scholar] [CrossRef] [PubMed]
- Proppe, D.S.; Bloomfield, L.L.; Sturdy, C.B. Acoustic transmission of the chick-a-dee call of the Black-capped Chickadee (Poecile atricapillus): Forest structure and note function. Can. J. Zool. 2010, 88, 788–794. [Google Scholar] [CrossRef]
- Slabbekoorn, H.; den Boer-Visser, A. Cities change the songs of birds. Curr. Biol. 2006, 16, 2326–2331. [Google Scholar] [CrossRef] [PubMed]
- Krams, I. Communication in crested tits and the risk of predation. Anim. Behav. 2001, 61, 1065–1068. [Google Scholar] [CrossRef]
- Krama, T.; Krams, I.; Igaune, K. Effects of cover on loud trill-call and soft seet-call use in the crested tit Parus Cristatus. Ethology 2008, 114, 656–661. [Google Scholar] [CrossRef]
- Seddon, N. Ecological adaptation and species recognition drives vocal evolution in neotropical suboscine birds. Evolution 2005, 59, 200–215. [Google Scholar] [CrossRef]
- Pohl, N.U.; Slabbekoorn, H.; Neubauer, H.; Heil, P.; Klump, G.M.; Langemann, U. Why longer song elements are easier to detect: Threshold level-duration functions in the Great Tit and comparison with human data. J. Comp. Physiol. A-Neuroethol. Sens. Neural Behav. Physiol. 2013, 199, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.C.; Becker, A.M. Mobbing calls of Black-capped Chickadees: Effects of urgency on call production. Wilson Bull. 2002, 114, 510–516. [Google Scholar] [CrossRef]

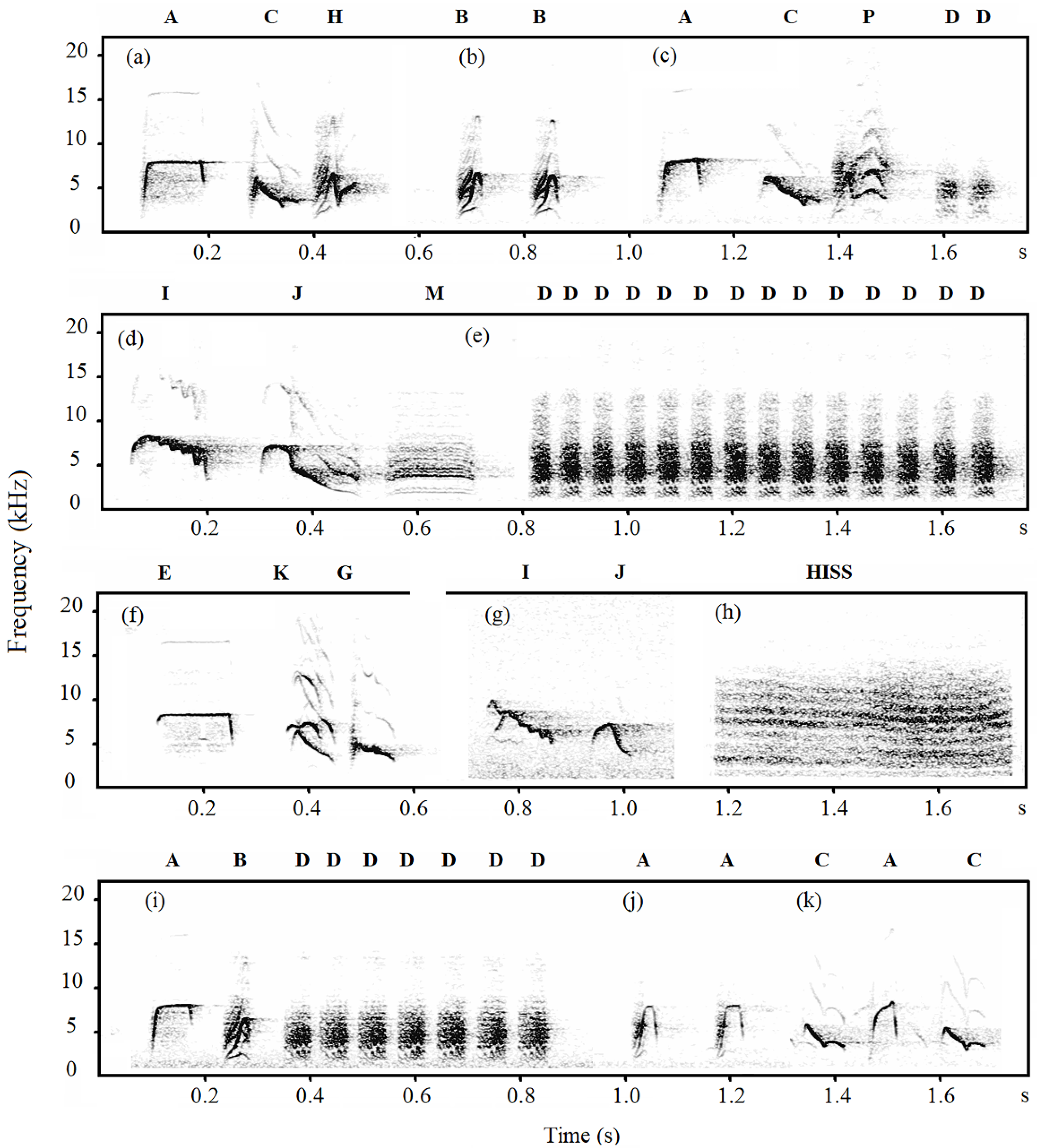
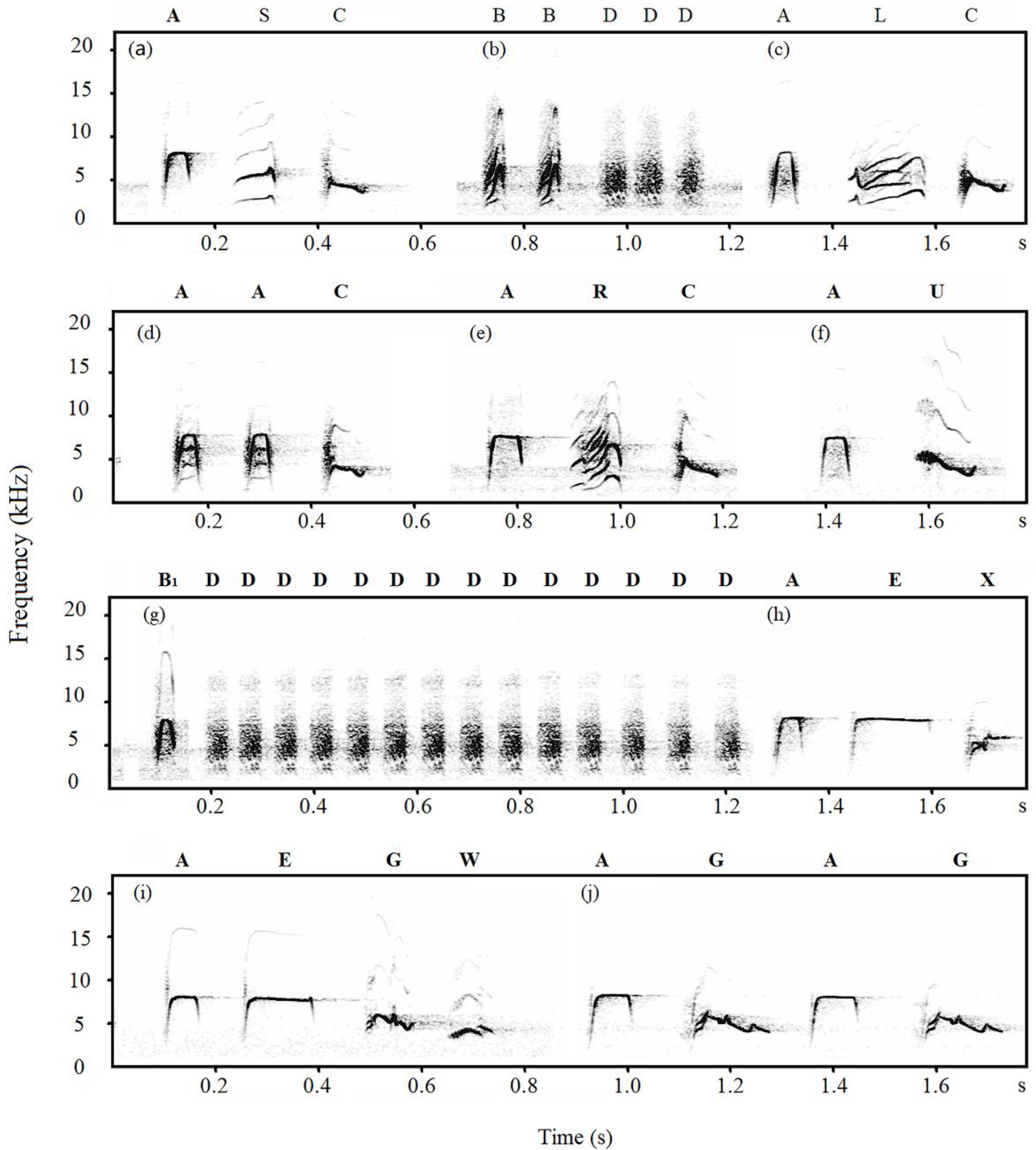
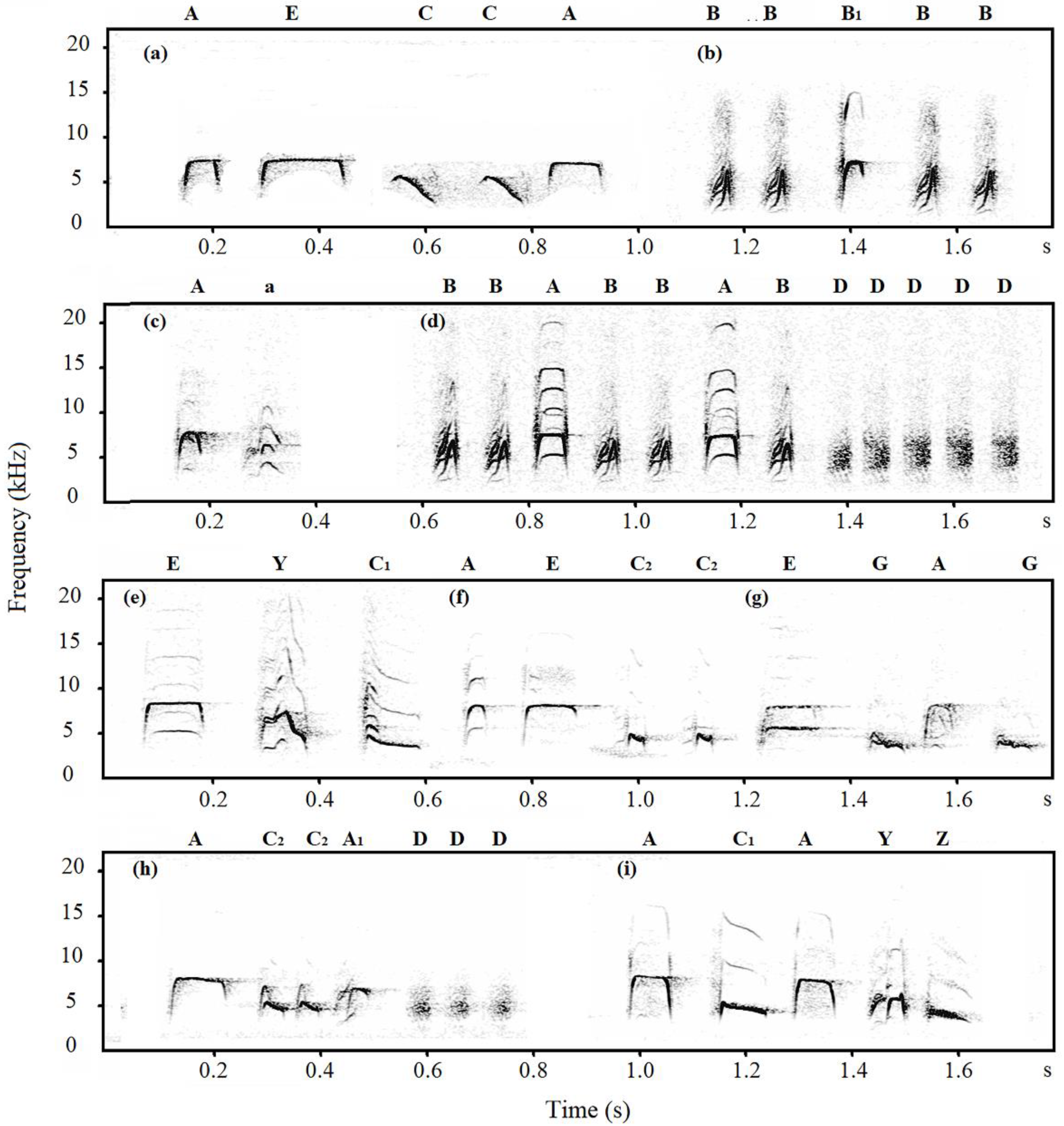
| Population | Specimen | Note Types |
|---|---|---|
| Jilin-tits | Chipmunk | A, B, C, D, E, G, H, HISS, I, J, P, Q |
| Sparrowhawk | A, B, C, D, E, G, H, I, J, K, M, P | |
| Dove | A, B, C, D, E, G, H, I, J, K, M, P | |
| Henan-tits | Chipmunk | A, B, B1, C, D, E, G, L, R, U, W, X |
| Sparrowhawk | A, B, B1, C, D, E, G, L, R, S, U, W, X | |
| Dove | A, B, B1, C, D, E, G, L, S, W, X | |
| Hainan-tits | Chipmunk | a, A, B, B1, C, C1, C2, D, E, Y, Z |
| Sparrowhawk | a, A, A1, B, B1, C, C1, C2, D, E, G, Y, Z | |
| Dove | a, A, A1, B, B1, C1, C2, D, G, Y |
| Acoustic Parameter | Population | Mean ± SE | Post-Hoc p Value | |
|---|---|---|---|---|
| Henan | Hainan | |||
| Maximum frequency (Hz) | Jilin | 8220.64 ± 10.44 | 0.704 | 0.003 ** |
| Henan | 8217.81 ± 8.41 | 0.002 ** | ||
| Hainan | 7951.81 ± 13.92 | |||
| Minimum frequency (Hz) | Jilin | 4315.34 ± 41.68 | 0.019 * | 0.323 |
| Henan | 3890.58 ± 29.64 | 0.004 ** | ||
| Hainan | 4505.45 ± 34.11 | |||
| Start frequency (Hz) | Jilin | 4408.91 ± 47.11 | 0.015 * | 0.302 |
| Henan | 3902.82 ± 30.45 | 0.003 ** | ||
| Hainan | 4614.76 ± 37.20 | |||
| End frequency (Hz) | Jilin | 5831.46 ± 39.23 | 0.013 * | 0.071 * |
| Henan | 5068.70 ± 35.63 | 0.482 | ||
| Hainan | 5185.46 ± 35.99 | |||
| Total duration (ms) | Jilin | 84.91 ± 0.79 | <0.001 ** | <0.001 ** |
| Henan | 61.26 ± 0.63 | 0.241 | ||
| Hainan | 69.31 ± 0.77 | |||
| Ascending duration (ms) | Jilin | 16.09 ± 0.17 | <0.001 ** | <0.001 ** |
| Henan | 18.00 ± 0.12 | 0.292 | ||
| Hainan | 18.57 ± 0.13 | |||
| Descending duration (ms) | Jilin | 11.44 ± 0.13 | <0.001 ** | <0.001 ** |
| Henan | 13.21 ± 0.16 | 0.169 | ||
| Hainan | 13.97 ± 0.14 | |||
| Maximum frequency duration (ms) | Jilin | 43.15 ± 1.10 | 0.062 | <0.001 ** |
| Henan | 29.84 ± 0.58 | 0.062 | ||
| Hainan | 25.79 ± 0.53 | |||
| Minimum frequency duration (ms) | Jilin | 6.74 ± 0.89 | 0.006 ** | 0.535 |
| Henan | 1.22 ± 0.35 | 0.053 | ||
| Hainan | 12.15 ± 0.96 | |||
| Acoustic Parameter | Population | Mean ± SE | Post-Hoc p Value | |
|---|---|---|---|---|
| Henan | Hainan | |||
| Maximum frequency (Hz) | Jilin | 6552.83 ± 17.69 | 0.006 ** | 0.006 ** |
| Henan | 6880.25 ± 17.61 | 0.837 | ||
| Hainan | 6971.67 ± 19.72 | |||
| Minimum frequency (Hz) | Jilin | 2228.19 ± 18.31 | 0.022 * | <0.001 ** |
| Henan | 2383.02 ± 12.70 | 0.015 * | ||
| Hainan | 2561.99 ± 12.15 | |||
| Start frequency (Hz) | Jilin | 2239.36 ± 19.79 | 0.041 * | <0.001 ** |
| Henan | 2382.42 ± 12.75 | 0.008 ** | ||
| Hainan | 2564.17 ± 12.45 | |||
| End frequency (Hz) | Jilin | 5210.94 ± 27.90 | <0.001 ** | <0.001 ** |
| Henan | 4459.36 ± 34.01 | <0.001 ** | ||
| Hainan | 3635.24 ± 22.41 | |||
| Total duration (ms) | Jilin | 48.42 ± 0.22 | 0.003 ** | <0.001 ** |
| Henan | 46.02 ± 0.25 | 0.628 | ||
| Hainan | 45.44 ± 0.17 | |||
| Ascending duration (ms) | Jilin | 25.66 ± 0.27 | 0.021 * | <0.001 ** |
| Henan | 27.77 ± 0.28 | 0.002 ** | ||
| Hainan | 31.87 ± 0.20 | |||
| Maximum frequency duration (ms) | Jilin | 33.56 ± 0.27 | 0.001 ** | <0.001 ** |
| Henan | 30.11 ± 0.24 | <0.001 ** | ||
| Hainan | 25.59 ± 0.17 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Yu, J.; Shen, C.; Yin, D.; Jin, L.; Liang, W.; Wang, H. Geographic Variation in Note Types of Alarm Calls in Japanese Tits (Parus minor). Animals 2022, 12, 2342. https://doi.org/10.3390/ani12182342
Zhang L, Yu J, Shen C, Yin D, Jin L, Liang W, Wang H. Geographic Variation in Note Types of Alarm Calls in Japanese Tits (Parus minor). Animals. 2022; 12(18):2342. https://doi.org/10.3390/ani12182342
Chicago/Turabian StyleZhang, Li, Jiangping Yu, Chao Shen, Dake Yin, Longru Jin, Wei Liang, and Haitao Wang. 2022. "Geographic Variation in Note Types of Alarm Calls in Japanese Tits (Parus minor)" Animals 12, no. 18: 2342. https://doi.org/10.3390/ani12182342
APA StyleZhang, L., Yu, J., Shen, C., Yin, D., Jin, L., Liang, W., & Wang, H. (2022). Geographic Variation in Note Types of Alarm Calls in Japanese Tits (Parus minor). Animals, 12(18), 2342. https://doi.org/10.3390/ani12182342





