The Influence of Environmental Variables on Home Range Size and Use in the Golden Snub-Nosed Monkey (Rhinopithecus roxellana) in Tangjiahe National Nature Reserve, China
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
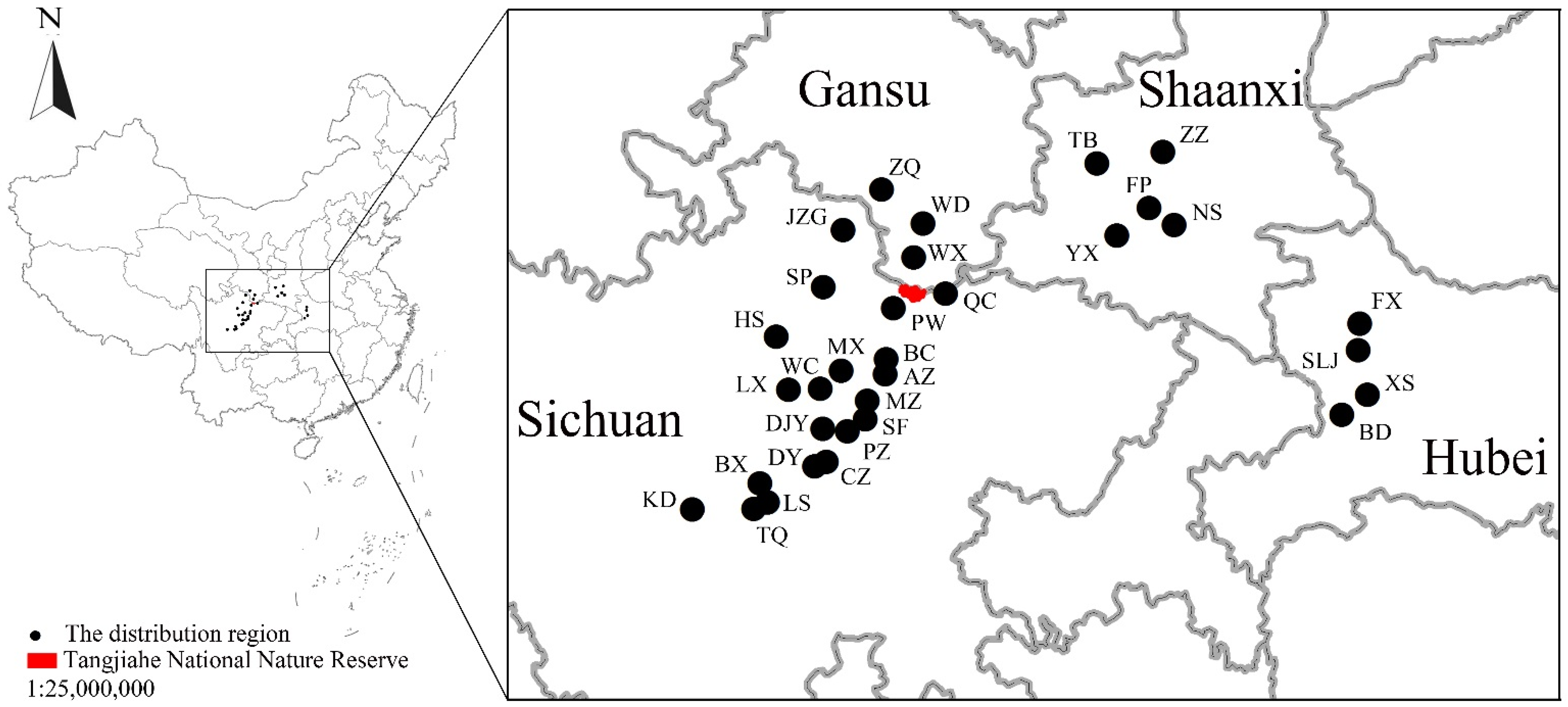
2.1. Study Area
2.2. Sampling Design
2.3. Data Analysis
3. Results
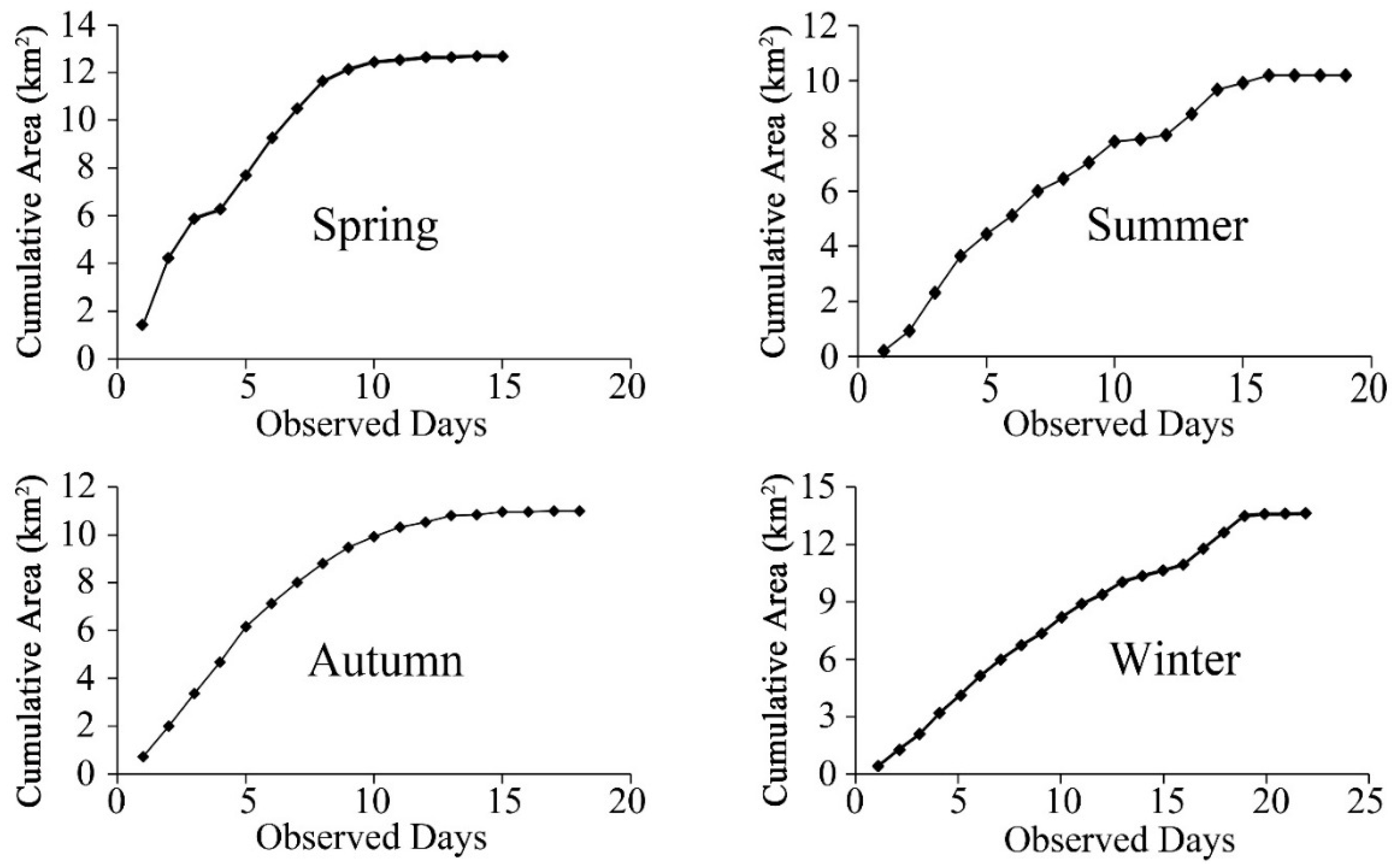
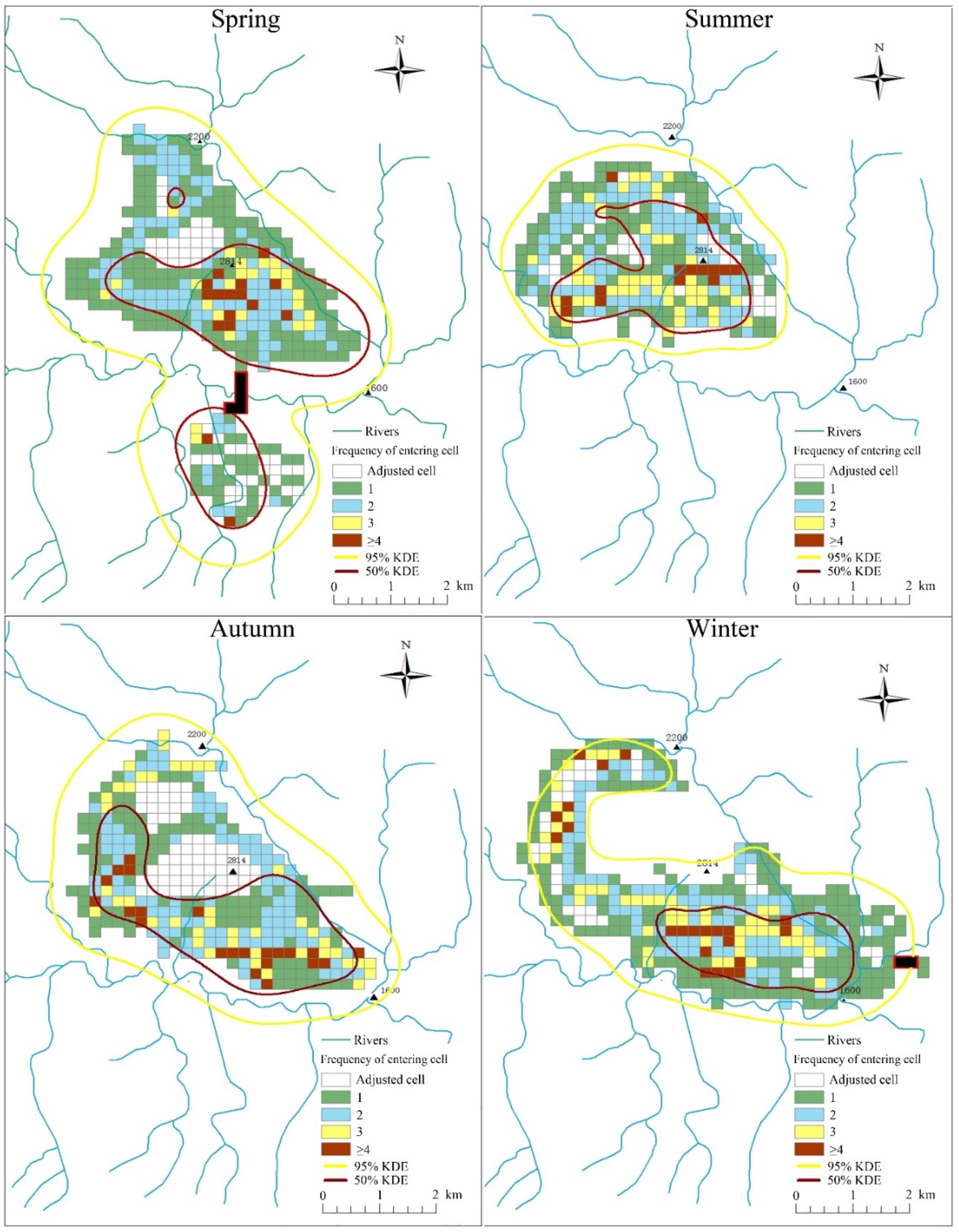
3.1. Seasonal Home Range Size Variation
3.2. Food Species and Food Abundance
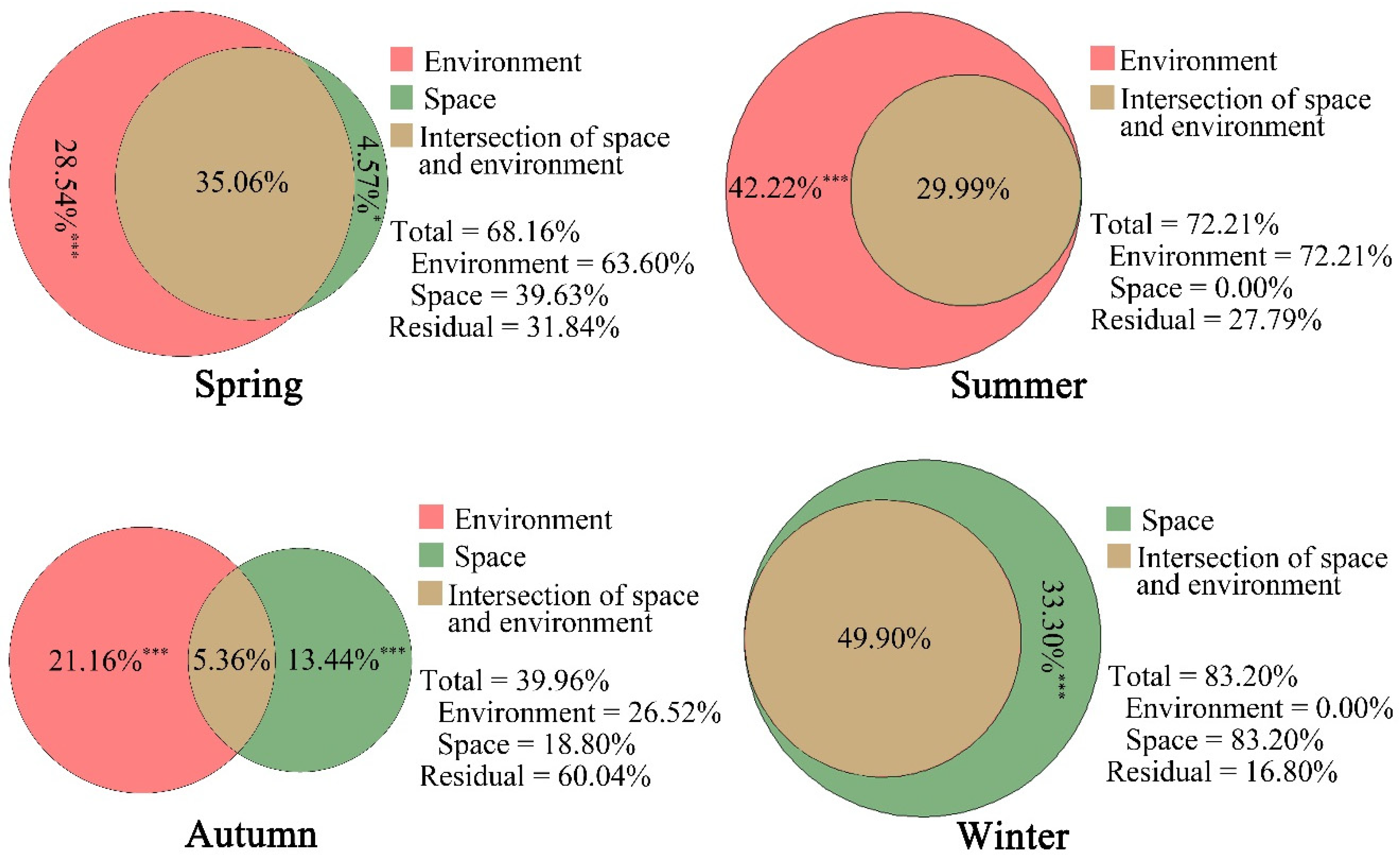
3.3. Relationships between Environmental Variables and Home Range Use Intensity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Powell, R.A.; Mitchell, M.S. What is a home range? J. Mammal. 2012, 93, 948–958. [Google Scholar] [CrossRef]
- Ren, B.; Li, M.; Long, Y.; Wu, R.; Wei, F. Home range and seasonality of Yunnan snub-nosed monkeys. Integr. Zool. 2009, 4, 162–171. [Google Scholar] [CrossRef]
- Wada, K.; Ichiki, Y. Seasonal home range use by Japanese monkeys in the snowy Shiga Heights. Primates 1980, 21, 468–483. [Google Scholar] [CrossRef]
- Li, B.; Chen, C.; Ji, W.; Ren, B. Seasonal home range changes of the Sichuan snub-nosed monkey (Rhinopithecus roxellana) in the Qinling Mountains of China. Folia. Primatol. 2000, 71, 375–386. [Google Scholar] [CrossRef] [PubMed]
- McNab, B.K. Bioenergetics and the determination of home range size. Am. Nat. 1963, 97, 133–140. [Google Scholar] [CrossRef]
- Herfindal, I.; Linnell, J.D.C.; Odden, J.; Nilsen, E.B.; Andersen, R. Prey density, environmental productivity and home-range size in the Eurasian lynx (Lynx lynx). J. Zool. 2005, 265, 63–71. [Google Scholar] [CrossRef]
- Saïd, S.; Servanty, S. The influence of landscape structure on female roe deer home-range size. Landsc. Ecol. 2005, 20, 1003–1012. [Google Scholar] [CrossRef]
- Castillo-Contreras, R.; Carvalho, J.; Serrano, E.; Mentaberre, G.; Fernández-Aguilar, X.; Colom, A.; González-Crespo, C.; Lavín, S.; López-Olvera, J. Urban wild boars prefer fragmented areas with food resources near natural corridors. Sci. Total Environ. 2018, 615, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Mcloughlin, P.D.; Ferguson, S.H. A hierarchical pattern of limiting factors helps explain variation in home range size. Ecoscience 2000, 7, 123–130. [Google Scholar] [CrossRef]
- McKinney, T. A classification system for describing anthropogenic influence on nonhuman primate populations. Am. J. Primatol. 2015, 77, 715–726. [Google Scholar] [CrossRef]
- Volampeno, M.S.N.; Masters, J.C.; Downs, C.T. Home range size in the blue-eyed black lemur (Eulemur flavifrons): A comparison between dry and wet seasons. Mamm. Biol. 2011, 76, 157–164. [Google Scholar] [CrossRef]
- Erinjery, J.J.; Kavana, T.; Singh, M. Food resources, distribution and seasonal variations in ranging in lion-tailed macaques, Macaca silenus in the Western Ghats, India. Primates 2015, 56, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, H.; Li, Y.; Dong, B.; Qiu, C.; Yang, J.; Zong, Y.; Chen, H.; Zhao, Y.; Zhang, Y. Study on habitat suitability and environmental variable thresholds of rare waterbirds. Sci. Total. Environ. 2021, 785, 147316. [Google Scholar] [CrossRef] [PubMed]
- Itonaga, K.; Jones, S.M.; Wapstra, E. Effects of maternal basking and food quantity during gestation provide evidence for the selective advantage of matrotrophy in a viviparous lizard. PLoS ONE 2012, 7, e41835. [Google Scholar] [CrossRef] [PubMed]
- Jacquier, M.; Calenge, C.; Say, L.; Devillard, S.; Ruette, S. Altitude shapes the environmental drivers of large-scale variation in abundance of a widespread mammal species. Ecol. Evol. 2020, 10, 119–130. [Google Scholar] [CrossRef]
- Huang, Z.-P.; Cui, L.-W.; Scott, M.B.; Wang, S.-J.; Xiao, W. Seasonality of reproduction of wild black-and-white snub-nosed monkeys (Rhinopithecus bieti) at Mt. Lasha, Yunnan, China. Primates 2012, 53, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Albert, A.; Huynen, M.C.; Savini, T.; Hambuckers, A. Influence of food resources on the ranging pattern of northern pig-tailed macaques (Macaca leonina). Int. J. Primatol. 2013, 34, 696–713. [Google Scholar] [CrossRef]
- Izumiyama, S.; Mochizuki, T.; Shiraishi, T. Troop size, home range area and seasonal range use of the Japanese macaque in the Northern Japan Alps. Ecol. Res. 2003, 18, 465–474. [Google Scholar] [CrossRef]
- Palacios, E.; Rodriguez, A. Ranging pattern and use of space in a group of red howler monkeys (Alouatta seniculus) in a southeastern Colombian rainforest. Am. J. Primatol. 2001, 55, 233–251. [Google Scholar] [CrossRef]
- Koganezawa, M.; Imaki, H. The effects of food sources on Japanese monkey home range size and location, and population dynamics. Primates 1999, 40, 177–185. [Google Scholar] [CrossRef]
- Grueter, C.C.; Li, D.; van Schaik, C.P.; Ren, B.; Long, Y. Ranging of Rhinopithecus bieti in the Samage Forest, China. I. Characteristics of Range Use. Int. J. Primatol. 2008, 29, 1121–1145. [Google Scholar] [CrossRef]
- Strier, K.B. Ranging behavior of woolly spider monkeys, or muriquis, Brachyteles arachnoides. Int. J. Primatol. 1987, 8, 575–591. [Google Scholar] [CrossRef]
- Grueter, C.C.; Li, D.; Ren, B.; Li, M. Overwintering strategy of Yunnan snub-nosed monkeys: Adjustments in activity scheduling and foraging patterns. Primates 2013, 54, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Ban, S.D.; Boesch, C.; Janmaat, K.R. Taï chimpanzees anticipate revisiting high-valued fruit trees from further distances. Anim. Cogn. 2014, 17, 1353–1364. [Google Scholar] [CrossRef]
- Maruhashi, T.; Saito, C.; Agetsuma, N. Home range structure and inter-group competition for land of Japanese macaques in evergreen and deciduous forests. Primates 1998, 39, 291–301. [Google Scholar] [CrossRef]
- Fedigan, F. Behavioral adaptations to heat stress and water scarcity in white-faced capuchins (Cebus capucinus) in Santa Rosa National Park, Costa Rica. Am. J. Phys. Anthropol. 2009, 138, 101–111. [Google Scholar]
- Campos, F.A.; Bergstrom, M.L.; Childers, A.; Hogan, J.D.; Jack, K.M.; Melin, A.; Mosdossy, K.N.; Myers, M.S.; Parr, N.A.; Sargeant, E. Drivers of home range characteristics across spatiotemporal scales in a Neotropical primate, Cebus capucinus. Anim. Behav. 2014, 91, 93–109. [Google Scholar] [CrossRef]
- Hillebrand, H. On the generality of the latitudinal diversity gradient. Am. Nat. 2004, 163, 192–211. [Google Scholar] [CrossRef]
- Jiang, H.; Liu, Z.; Zhang, Y.; Southwick, C. Population ecology of rhesus monkeys (Macaca mulatta) at Nanwan nature reserve, Hainan, China. Am. J. Primatol. 1991, 25, 207–217. [Google Scholar] [CrossRef]
- Qu, W.; Zhang, Y.; Manry, D.; Southwick, C.H. Rhesus monkeys (Macaca mulatta) in the Taihang mountains, Jiyuan county, Henan, China. Int. J. Primatol. 1993, 14, 607–621. [Google Scholar]
- Glessner, K.D.; Britt, A. Population density and home range size of Indri indri in a protected low altitude rain forest. Int. J. Primatol. 2005, 26, 855–872. [Google Scholar] [CrossRef]
- Merker, S. Habitat-specific ranging patterns of Dian’s tarsiers (Tarsius dianae) as revealed by radiotracking. Am. J. Primatol. 2006, 68, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Bitetti, M. Home-range use by the tufted capuchin monkey (Cebus apella nigritus) in a subtropical rainforest of Argentina. J. Zool. 2010, 253, 33–45. [Google Scholar] [CrossRef]
- Scholz, F.; Kappeler, P.M. Effects of seasonal water scarcity on the ranging behavior of Eulemur fulvus rufus. Int. J. Primatol. 2004, 25, 599–613. [Google Scholar] [CrossRef]
- Coleman, B.T.; Hill, R.A. Living in a landscape of fear: The impact of predation, resource availability and habitat structure on primate range use. Anim. Behav. 2014, 88, 165–173. [Google Scholar] [CrossRef]
- Long, Y.; Richardson, M. Rhinopithecus roxellana (amended version of 2020 assessment). In The IUCN Red List of Threatened Species; IUCN Global Species Programme Red List Unit: Cambridge, UK. [CrossRef]
- Sung, W. China Red Data Book of Endangered Animals: Mammalia; Science Press: Beijing, China, 1998. [Google Scholar]
- Wang, Y.; Jiang, X.; Li, D. Classification and distribution of the extant subspecies of golden snub-nosed monkey (Rhinopithecus [Rhinopithecus] roxellana). In The Natural History of the Doucs and Snub-Nosed Monkeys; World Scientific: Hackensack, NJ, USA, 1998; pp. 53–64. [Google Scholar]
- Mao, Z.; Bai, W.; Fu, L.; Cai, T.; Huang, Y.; Hong, Y.; Hou, J.; Luo, H.; Zhang, J.; Zhou, C. Investigation on beasts of suspicious distribution in Mabian Dafengding nature reserve. J. China West. Norm. Univ. (Nat. Sci.) 2022, 43, 1–8. [Google Scholar]
- Qiao, J.; Jia, G.; Zhou, H.; Gong, L.; Jiang, Y.; Xiao, N.; Gao, X.; Wen, A.; Wang, J. Mammal and bird diversity recorded with camera traps in Gongga Mountain National Nature Reserve, Sichuan, China. Biodivers. Sci. 2022, 30, 20395. [Google Scholar] [CrossRef]
- Guo, S.; Li, B.; Watanabe, K. Diet and activity budget of Rhinopithecus roxellana in the Qinling Mountains, China. Primates 2007, 48, 268–276. [Google Scholar] [CrossRef]
- Hou, R.; He, S.; Wu, F.; Chapman, C.; Pan, R.; Garber, P.; Guo, S.; Li, B. Seasonal variation in diet and nutrition of the northern-most population of Rhinopithecus roxellana. Am. J. Primatol. 2018, 80, e22755. [Google Scholar] [CrossRef]
- Liu, X.; Stanford, C.; Yang, J.; Yao, H.; Li, Y. Foods eaten by the Sichuan snub-nosed monkey (Rhinopithecus roxellana) in Shennongjia National Nature Reserve, China, in relation to nutritional chemistry. Am. J. Primatol. 2013, 75, 860–871. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, Z.; Li, C.; Grueter, C.C. Effects of seasonal folivory and frugivory on ranging patterns in Rhinopithecus roxellana. Int. J. Primatol. 2010, 31, 609–626. [Google Scholar] [CrossRef]
- Ren, B.; Li, B.; Li, M.; Wei, F. Inter-population variation of diets of golden snub-nosed monkeys (Rhinopithecus roxellana) in China. Acta. Theriol. Sin. 2010, 30, 357–364. [Google Scholar]
- Ren, B.; Ming, L.; Long, Y.; Wei, F. Influence of day length, ambient temperature, and seasonality on daily travel distance in the Yunnan snub-nosed monkey at Jinsichang, Yunnan, China. Am. J. Primatol. 2010, 71, 233–241. [Google Scholar]
- van Schaik, C.P.; Assink, P.R.; Salafsky, N.S. Territorial behavior in southeast Asian langurs: Resource defense or mate defense? Am. J. Primatol. 1992, 26, 233–242. [Google Scholar] [CrossRef]
- Harris, T.; Chapman, C. Variation in diet and ranging of black and white colobus monkeys in Kibale National Park, Uganda. Primates 2007, 48, 208–221. [Google Scholar] [CrossRef]
- Guo, Y.; Ren, B.; Dai, Q.; Zhou, J.; Zhou, J. Habitat estimates reveal that there are fewer than 400 Guizhou snub-nosed monkeys, Rhinopithecus brelichi, remaining in the wild. Glob. Ecol. Conserv. 2020, 24, e01181. [Google Scholar]
- Chu, Y.; Sha, J.; Kawazoe, T.; Dong, X. Sleeping site and tree selection by Sichuan snub-nosed monkeys (Rhinopithecus roxellana) in Baihe Nature Reserve, Sichuan, China. Am. J. Primatol. 2018, 80, e22936. [Google Scholar] [CrossRef]
- Tan, C.L.; Guo, S.; Li, B. Population structure and ranging patterns of Rhinopithecus roxellana in Zhouzhi National Nature Reserve, Shaanxi, China. Int. J. Primatol. 2007, 28, 577–591. [Google Scholar] [CrossRef]
- Zhou, X.; Yang, W.; Jin, K. Study on the habitat selection of Sichuan snub-nosed monkey in Baishuijiang Nature Reserve, Gansu. Gansu For. 2020, 5, 35–36. [Google Scholar]
- Wang, W.; Chu, Y.; Hu, G. Habitat selection of golden snub-nosed monkey (Rhinopithecus roxellana) of Baihe Nature Reserve in autumn. J. China West. Norm. Univ. (Nat. Sci.) 2013, 34, 16–21. [Google Scholar]
- Wang, Y.; Zhou, C.; Zhang, J.; Zhou, Y.; Hu, J.; Xiong, Y. Spatial niche of the rodents in summer in Tangjiahe Nature Reserve. Acta. Theriol. Sin. 2005, 25, 39–44. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, J.; Shen, L.; Zhang, H.; Xu, L.; Hu, Z.; Xu, H. Biomass of small mammals in Tangjiahe Nature Reserve. Chin. J. Ecol. 2005, 24, 707–710. [Google Scholar]
- Yao, G.; Li, Y.; Li, D.; Williams, P.; Hu, J. Phylogenetic analysis of the endangered takin in the confluent zone of the Qinling and Minshan Mountains using mtDNA control region. Mitochondrial DNA Part A 2016, 27, 2594–2605. [Google Scholar] [CrossRef]
- Shen, L.; Gao, Z.; Ou, W.; Chen, W.; Ma, W. Investigation of amphibian and reptile in Sichuan Tangjiahe Nature Reserve. Sichuan J. Zool. 1999, 18, 132–134. [Google Scholar] [CrossRef]
- Fan, Y.; Li, D.; Huang, X.; Li, Y.; Chen, L.; Wang, X.; Xia, W.; Hu, J. Social structure of Sichuan snub-nosed monkeys (Rhinopithecus roxellana) in Minshan Mountains: A case study of Sichuan snub-nosed monkey in Tangjiahe National Nature Reserve. Sichuan J. Zool. 2015, 34, 832–836. [Google Scholar] [CrossRef]
- Zhang, B. Season distribution in China. Acta. Geogr. Sin. 1934, 1, 32–77. [Google Scholar]
- Zhang, Z. Research on Biodiversity in Tangjiahe National Nature Reserve, China; Science Press: Beijing, China, 2016. [Google Scholar]
- ESRI. ArcGIS Desktop: Release 10.3; Environmental Systems Research Institute: Redlands, CA, USA, 2011; Available online: https://desktop.arcgis.com/zh-cn/arcmap/10.3/main/get-started/whats-new-in-arcgis.htm (accessed on 1 April 2020).
- Rodfers, A.; Kie, J.; Wright, D.; Beyer, H.; Carr, A. HRT: Home Range Tools for ArcGIS. Version 2.0; Ontario Ministry of Natural Resources, Centre for Northern Forest Ecosystem Research: Thunder Bay, ON, Canada, 2015; Available online: http://flash.lakeheadu.ca/~arodgers/hre/ (accessed on 1 April 2020).
- Seaman, D.E.; Powell, R.A. An evaluation of the accuracy of kernel density estimators for home range analysis. Ecology 1996, 77, 2075–2085. [Google Scholar] [CrossRef]
- Worton, B. Kernel methods for estimating the utilization distribution in home-range studies. Ecology 1989, 70, 164–168. [Google Scholar] [CrossRef]
- Mekonnen, A.; Fashing, P.; Bekele, A.; Hernandez-Aguilar, R.; Rueness, E.; Nguyen, N.; Stenseth, N. Impacts of habitat loss and fragmentation on the activity budget, ranging ecology and habitat use of Bale monkeys (Chlorocebus djamdjamensis) in the southern Ethiopian highlands. Am. J. Primatol. 2017, 79, e22644. [Google Scholar] [CrossRef] [Green Version]
- Altmann, J. Observational study of behavior: Sampling methods. Behaviour 1974, 49, 227–266. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, B.; Qin, S.; Li, S.; Huang, X. Review of research and application of forest canopy closure and its measuring methods. World For. Res. 2008, 21, 40–45. [Google Scholar]
- Wikum, D.A.; Shanholtzer, G.F. Application of the Braun-Blanquet cover-abundance scale for vegetation analysis in land development studies. Environ. Manag. 1978, 2, 323–329. [Google Scholar] [CrossRef]
- Legendre, P.; Legendre, L. Numerical Ecology, 3rd English ed.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 785–906. [Google Scholar]
- R-Core-Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Borcard, D.; Gillet, F.; Legendre, P. Numerical Ecology with R; Springer: New York, NY, USA, 2011. [Google Scholar]
- Dray, S.; Legendre, P.; Peres-Neto, P.R. Spatial modelling: A comprehensive framework for principal coordinate analysis of neighbour matrices (PCNM). Ecol. Modell. 2006, 196, 483–493. [Google Scholar] [CrossRef]
- Kirkpatrick, R.C.; Long, Y.C.; Zhong, T.; Xiao, L. Social organization and range use in the Yunnan snub-nosed monkey Rhinopithecus bieti. Int. J. Primatol. 1998, 19, 13–51. [Google Scholar] [CrossRef]
- Murphy, S.J.; Audino, L.D.; Whitacre, J.; Eck, J.L.; Wenzel, J.W.; Queenborough, S.A.; Comita, L.S. Species associations structured by environment and land-use history promote beta-diversity in a temperate forest. Ecology 2015, 96, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hull, V.; Ouyang, Z.; Li, R.; Connor, T.; Yang, H.; Zhang, Z.; Silet, B.; Zhang, H.; Liu, J. Divergent responses of sympatric species to livestock encroachment at fine spatiotemporal scales. Biol. Conserv. 2017, 209, 119–129. [Google Scholar] [CrossRef]
- Li, Y. Terrestriality and tree stratum use in a group of Sichuan snub-nosed monkeys. Primates 2007, 48, 197–207. [Google Scholar] [CrossRef]

| Environmental Variable | Description |
|---|---|
| Elevation (m) | The elevation at the location of the plant species eaten. |
| Type of forest | Evergreen broadleaf forest, evergreen and deciduous broadleaf mixed forest, deciduous broadleaf forest, coniferous–broadleaf mixed forest, or coniferous forest. |
| Primary forest or secondary forest | Primary forest: Forest that has never been destroyed by human activities. |
| Secondary forest: Forest where large trees were cut down 40 years ago and have since regenerated. | |
| Dead trees | Dead trees in an upright position with DBH ≥ 10 cm. |
| Fallen trees | Fallen trees with DBH ≥ 10 cm when standing upright. |
| Tree stumps | Tree stumps with a diameter ≥ 10 cm. |
| Distance to the nearest water source | 0–50 m, 50–100 m, >100 m. |
| Tree height | Mean height of trees with DBH ≥ 10 cm. |
| Canopy density | 0–25%, 25–50%, 50–75%, and 75–100%. |
| Mean DBH of trees | Mean DBH of trees with DBH ≥ 10 cm. |
| Tree density | Stem density of trees with DBH ≥ 10 cm. |
| Dominant trees species | Tree species with the largest stem density in the quadrat among trees with DBH ≥ 10 cm. |
| Shrub height | Mean height of shrubs. |
| Shrub cover | 0–25%, 25–50%, 50–75%, and 75–100%. |
| No. of Days Observed | No. of Days per Month (Mean ± SE) | No. of GPS Points | Grid Cell Method (km2) | 50% KDE (km2) | 95% KDE (km2) | |
|---|---|---|---|---|---|---|
| Overall | 74 | 6.2 ± 0.8 | 1763 | 24.0 | 6.28 | 26.95 |
| Spring | 15 | 7.5 ± 0.5 | 528 | 15.4 | 9.86 | 31.79 |
| Summer | 19 | 6.3 ± 1.5 | 398 | 11.6 | 5.58 | 15.96 |
| Autumn | 18 | 9.0 ± 2.0 | 344 | 13.7 | 7.20 | 23.19 |
| Winter | 22 | 4.4 ± 1.2 | 493 | 15.6 | 4.23 | 19.37 |
| Seasons | The Ratio of Observed Diet Part (%) | |||||
|---|---|---|---|---|---|---|
| Bark | Bud | Tender Leaf | Mature Leaf | Fruit | ||
| Spring | April | 56.00 | 24.00 | 20.00 | 0.00 | 0.00 |
| may | 63.63 | 32.47 | 3.90 | 0.00 | 0.00 | |
| Summer | June | 69.23 | 26.92 | 3.85 | 0.00 | 0.00 |
| July | 50.00 | 15.38 | 30.77 | 0.00 | 3.85 | |
| August | 29.41 | 47.06 | 5.88 | 17.65 | 0.00 | |
| Autumn | September | 36.58 | 0.00 | 0.00 | 12.20 | 51.22 |
| October | 36.59 | 2.44 | 0.00 | 20.73 | 40.24 | |
| Winter | November | 72.73 | 0.00 | 0.00 | 27.27 | 0.00 |
| December | 60.00 | 0.00 | 0.00 | 40.00 | 0.00 | |
| January | 75.00 | 25.00 | 0.00 | 0.00 | 0.00 | |
| February | 56.25 | 43.75 | 0.00 | 0.00 | 0.00 | |
| March | 60.53 | 23.68 | 10.53 | 5.26 | 0.00 | |
| Seasons | Significant Environmental Variables | Coefficient | Intercept | t | p |
|---|---|---|---|---|---|
| Spring | None | None | −19.00 | None | None |
| Summer | Tree density | 0.91 | −65.70 | 2.26 | 0.045 |
| Autumn | Moderate distance to water source (50–100 m) | 7.96 | 29.40 | 2.12 | 0.039 |
| Dominant trees of Chinese wingnut | 31.65 | 29.40 | 2.14 | 0.038 | |
| Winter | Tree density | 0.66 | −22.30 | 2.12 | 0.043 |
| Canopy density | −26.02 | −22.30 | −2.08 | 0.046 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, G.; Fan, Y.; Li, D.; Hull, V.; Shen, L.; Li, Y.; Hu, J. The Influence of Environmental Variables on Home Range Size and Use in the Golden Snub-Nosed Monkey (Rhinopithecus roxellana) in Tangjiahe National Nature Reserve, China. Animals 2022, 12, 2338. https://doi.org/10.3390/ani12182338
Yao G, Fan Y, Li D, Hull V, Shen L, Li Y, Hu J. The Influence of Environmental Variables on Home Range Size and Use in the Golden Snub-Nosed Monkey (Rhinopithecus roxellana) in Tangjiahe National Nature Reserve, China. Animals. 2022; 12(18):2338. https://doi.org/10.3390/ani12182338
Chicago/Turabian StyleYao, Gang, Yuanying Fan, Dayong Li, Vanessa Hull, Limin Shen, Yanhong Li, and Jie Hu. 2022. "The Influence of Environmental Variables on Home Range Size and Use in the Golden Snub-Nosed Monkey (Rhinopithecus roxellana) in Tangjiahe National Nature Reserve, China" Animals 12, no. 18: 2338. https://doi.org/10.3390/ani12182338
APA StyleYao, G., Fan, Y., Li, D., Hull, V., Shen, L., Li, Y., & Hu, J. (2022). The Influence of Environmental Variables on Home Range Size and Use in the Golden Snub-Nosed Monkey (Rhinopithecus roxellana) in Tangjiahe National Nature Reserve, China. Animals, 12(18), 2338. https://doi.org/10.3390/ani12182338







