Simple Summary
Follistatin involves in the regulation of ovarian follicular development in mammals; however, the role of follistatin in goose ovarian follicular development has not been investigated. In this study, following immuno-neutralization of follistatin bioactivity in geese, the number of ovarian pre-ovulatory follicles significantly increased, and mRNA levels of genes involved in ovarian steroidogenesis and yolk deposition were upregulated in the granulosa layer of pre-hierarchical follicles. These results suggest that follistatin plays a limiting role in the development of ovarian pre-hierarchical follicles into pre-ovulatory follicles. These results also expand our understanding of the mechanism of follistatin on ovarian follicular development in geese.
Abstract
In order to explore the role of follistatin (FST) in ovarian follicular development and egg production in Yangzhou geese, sixty-four egg laying geese of the same genetic origin were selected and divided into two groups with equal numbers. One group was immunized against the recombinant goose FST protein by intramuscular injection, whereas the control group received bovine serum albumin (BSA) injection. Immunization against FST significantly increased the number of pre-ovulatory follicles. Furthermore, immunization against FST upregulated Lhr, Star, Vldlr, Smad3, and Smad4 mRNA levels in the granulosa layer of pre-hierarchical follicles. The results suggest that FST plays a limiting role in the development of ovarian pre-hierarchical follicles into pre-ovulatory follicles by decreasing follicular sensitivity to activin in geese. The mechanism may be achieved by regulating the SMAD3 signaling pathway, which affects progesterone synthesis and yolk deposition in pre-hierarchical follicles.
1. Introduction
Follistatin (FST), a glycosylated single-chain protein, was first identified in the follicular fluid of cattle and pigs by its ability to suppress FSH secretion in pituitary cell cultures [1,2]. Subsequently, FST has been discovered to be an activin-binding protein [3], and the activin–FST complex consists of one activin dimer and two FST molecules [4,5]. Therefore, the biological actions of FST are thought to be mediated mainly by neutralizing the bioactivity of activin. An increasing body of evidence shows that, in addition to activins, FST interacts with other TGF-β family members in the ovary, presumably through a similar binding mechanism [6].
In mammalian species, Fst mRNA abundance increases as follicles develop from small antral to pre-ovulatory follicles and declines during the atretic process [7,8,9,10]. However, primordial follicles do not express Fst mRNA [11,12]. Ovarian granulosa cells are the main site responsible for FST production and secretion. Fst-null mice survive through birth but die within hours of delivery [13]. Conversely, transgenic mice overexpressing FST survive to adulthood; however, defects were found in the gonad [14]. In male transgenic mice, the testes are generally smaller and show variable degrees of Leydig cell hyperplasia and seminiferous tubule degeneration. In female transgenic mice, uteri are thin and ovaries are small because folliculogenesis is blocked at the early primary or secondary (antral) follicle stages. Moreover, FST immunization in Hereford-cross heifers resulted in an increase in the number of small follicles at the time of wave emergence [15]. These results suggest that FST plays a limiting regulatory role in the development of ovarian follicles.
In birds, Fst mRNA abundance was found to be greater in smaller pre-hierarchical follicles than in larger pre-ovulatory follicles, and the greatest expression occurred in small yellow follicles [16,17], which is incongruous with that in mammals. Follicles in avian ovaries are arranged in a hierarchical order and consist of pre-hierarchical large white follicles (LWFs), small yellow follicles (SYFs), and hierarchical large yellow follicles (LYFs, also called pre-ovulatory follicles). The highest abundance of Fst mRNA was observed in SYFs, suggesting that FST may play a critical role in early follicular development, especially during follicle selection by regulating activin availability. In addition, FST may also play a significant role in the regulation of egg production, since egg production predominantly depends on the number of pre-hierarchical follicles selected into the hierarchical developmental stage [18]. Domestic geese have lower egg laying performance than chickens and ducks and possess a unique ovarian follicle development mode [19]. In the present study, we investigated the effects of immunization against FST on the recruitment of pre-hierarchical follicles, growth of pre-ovulatory follicles, and egg production in Yangzhou geese, aiming to explore a method to improve egg laying performance.
2. Materials and Methods
2.1. Ethics
Animal experiments were approved by the Jiangsu Academy of Agricultural Sciences Experimental Animal Ethics Committee and carried out according to the Regulations for the Administration of Affairs Concerning Experimental Animals (Decree No. 63 of the Jiangsu Academy of Agricultural Sciences, 8 July 2014).
2.2. Immunogen
The coding sequence of a 148-amino-acid peptide of goose FST (residues 93–240, NCBI Reference Sequence: XP_013036067.1) was cloned into the pRSET-A vector between BamHI and KpnI sites (Invitrogen, Carlsbad, CA, USA). The recombinant protein was expressed and purified as described previously [18]. SDS-PAGE and Western blot were performed to analyze the protein purity. The immunogen (1 mg/mL) was prepared by emulsifying recombinant proteins with mineral oil (Solarbio, Beijing, China). Bovine serum albumin (BSA) was emulsified in mineral oil as well and used as control. Both recombinant FST protein and BSA were dissolved in physiological saline.
2.3. Animal Experiments
The experiments were conducted from September to November at Anhui Tianzhijiao Goose Industry Co., Ltd (117°99′ E, 32°07′ N), Chuzhou, Anhui Province, China. A flock of approximately 1-year-old Yangzhou geese in the late stage of out-of-season laying were of the same genetic origin and were exposed to a 12 h daily photoperiod (12 L: 12 D) as described previously [20]. Of these, 64 individuals were randomly divided into two groups with equal numbers. All individuals were kept in the same goose barn. For each group, 32 individuals were kept together in a pen (6 m × 4 m). After 1 week of adaptation, the experimental group was intramuscularly administered FST antigen (1 mg) on day 1, whereas the control group was similarly administered BSA antigen at the same dose. Booster immunization was administrated on day 21.
Geese were fed ad libitum with a mixed feed of 12.5% crude protein, supplemented with green grass whenever possible, as described previously [20]. Blood samples were randomly collected from 12 geese per group by venipuncture (wing vein) on days 1, 11, 21, 31, and 42. Plasma was obtained by centrifugation and stored at –20 °C until analysis of antibody titers. Antibody titers were measured by ELISA, as described previously [18].
2.4. Tissue Collection
On day 42 of the experiment, six individuals with hard-shell eggs in the uterus were slaughtered for tissue sample collection from each group. Ovarian follicles were classified as LWFs (white follicles, 4 mm < diameter < 8 mm), SYFs (yolky follicles, 8 mm ≤ diameter ≤ 10 mm), and LYFs (yolky follicles, diameter > 10 mm). As described previously [18], granulosa layers of ovarian follicles were separated from the five largest LYFs (F1: 48.5 ± 0.6 mm; F2: 42.4 ± 0.8 mm; F3: 33.7 ± 0.5 mm; F4: 23.8 ± 0.7 mm; F5: 14.4 ± 0.3 mm), SYFs, and LWFs. In addition, hypothalamus and pituitary gland tissues were collected. All tissue samples were snap-frozen in liquid nitrogen, and then stored at –80 °C until used.
2.5. RNA Isolation, cDNA Synthesis, and Quantitative Real-Time PCR
Total RNA was isolated using the RNAprep Pure Tissue Kit (Tiangen, Beijing, China) and then reverse-transcribed using the PrimeScript™ RT Master Mix (Takara, Kusatsu, Japan). The resulting cDNA was used for quantitative real-time PCR (qRT-PCR) using TB GreenTM Premix Ex TaqTM II (Takara, Kusatsu, Japan). Some primers were obtained from our previous study, including Gapdh, Gnrh1, Gnih, Fshb, Lhb, Lhr, Fshr, Star, Cyp11a1, Hsd3b, Ocln, and Smad4 [18]. Other primers were designed using Oligo 7 software, as shown in Table 1. Relative mRNA levels were calculated by the 2−ΔΔCt method [21]. mRNA levels of target genes were normalized to the housekeeping gene Gapdh. Data were presented as mean ± standard error of mean (SEM).

Table 1.
Primers used in the real-time PCR assay of genes.
2.6. Statistical Analysis
The data of antibody titers and mRNA levels of Lhr, Star, Cyp11a1, Hsd3b, Ocln, Vldlr, Smad2, Smad3, Smad4, and Fshr genes were transformed into logarithms before analysis because of great variation. Other data of mRNA levels of Fst, Gnrh1, Gnih, Fshb, and Lhb genes, follicle counts, and egg production were not transformed before analysis. Differences in mRNA levels of genes were analyzed using a two-way ANOVA with the classification of ovarian follicles and immunization treatment as the fixed factors. The two-way ANOVA was performed using Univariate GLM. For each group, differences in mRNA levels of each gene were analyzed using a one-way ANOVA followed by the Tukey’s multiple comparison test. An independent sample t-test was used for comparison between the two groups. A value of p < 0.05 was considered significant. All statistical analyses were performed using the IBM SPSS Statistics version 22 (IBM Corp., Armonk, NY, USA).
3. Results
3.1. Expression Pattern of Goose Fst Gene in the Granulosa Layer of Ovarian Follicles
The abundance of Fst mRNA declines as follicles mature in geese (Figure 1). Fst mRNA was the most abundant in SYFs, which was significantly higher than that in other follicles. The abundance of Fst mRNA in LWFs did not differ from amounts in F4 and F5 but differed from those in F1 to F3 (p < 0.05). No significant differences were observed among F1 to F5.
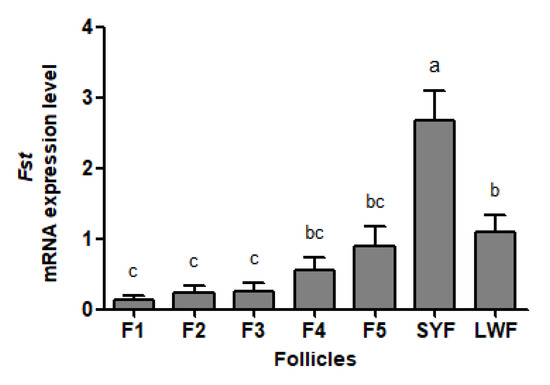
Figure 1.
Fst mRNA abundance in the granulosa layers from ovarian pre-hierarchical follicles (SYFs and LWFs) and pre-ovulatory follicles (F1 to F5) in Yangzhou geese (n = 4). a,b,c represents a significant difference among the means (p < 0.05). Data represent mean ± SEM.
3.2. Expression of Recombinant Goose FST Protein
As an His-tag fused protein, recombinant goose FST protein was expressed with a predicted molecular mass of ~20 kDa. A main band of the recombinant goose FST protein was detected at the expected size of 20 kDa by SDS-PAGE (Figure 2A). The additional higher band with a molecular mass of 40 kDa was caused by dimer formation. Moreover, two immuno-reactive bands were observed by Western blot (Figure 2B).
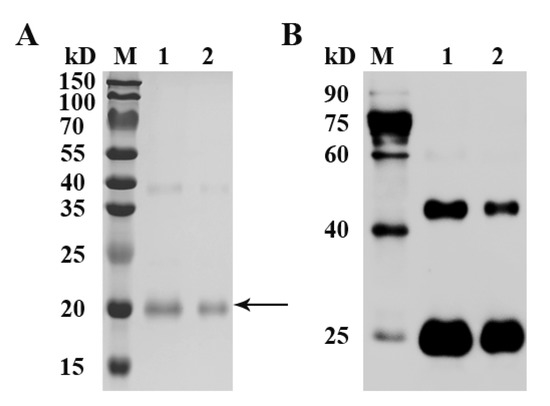
Figure 2.
SDS-PAGE (A) and Western blot (B) analysis of recombinant goose FST protein. SDS-PAGE gel was stained with Coomassie blue. Arrow indicates the target protein. After incubation with an anti-His tag primary antibody followed by an HRP-conjugated secondary antibody, immuno-reactive bands were visualized with ECL reagent. M, protein standards; 1 and 2, purified proteins.
3.3. Antibody Titer, Egg Production and Ovarian Follicular Development
After primary immunization, the anti-FST antibody titer level increased gradually in FST-immunized geese and was higher than that in BSA-immunized control geese on day 11 (p ˂ 0.05) (Figure 3A). The anti-FST antibody titer level continued to increase in FST-immunized geese after booster immunization and reached the peak on day 31 (Figure 3A). The egg-laying rate in FST-immunized geese was higher than that in BSA-immunized control geese after primary immunization (Figure 3B). During the entire experimental period, the cumulative number of eggs laid per goose was 10.6 for the FST-immunized group, whereas it was 7.7 for the BSA-immunized group (Figure 3C). On day 42 of the experiment, the ovarian follicles of both groups of geese were collected and classified (Figure 3D). Compared with those in BSA-immunized control geese, the number of LYFs was significantly higher in FST-immunized geese. No significant differences were observed in the number of SYFs and LWFs.
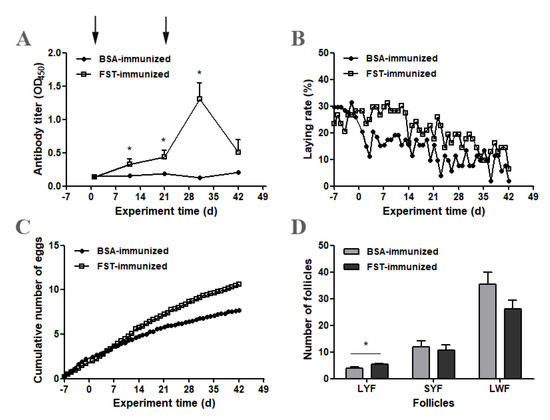
Figure 3.
Changes in antibody titers, egg production and ovarian follicular development in FST-immunized or BSA-immunized control geese. (A) Anti-FST antibody titers (n = 12). (B) The laying rate represents the average laying rates over two consecutive days. (C) The cumulative number of eggs laid per goose equals the total number of eggs divided by the number of geese. (D) The number of ovarian follicles. * represents a significant difference between the two groups at one time point (p < 0.05). Data represent mean ± SEM.
3.4. Gene Expression of Gnrh1, Gnih, Fshb, and Lhb in Hypothalamus and Pituitary Gland Tissues
Compared with those in BSA-immunized control geese, both Gnrh1 and Gnih mRNA levels from the hypothalamus tissue were significantly higher in FST-immunized geese (Figure 4A,B). In the pituitary gland tissue, both Fshb and Lhb mRNA levels in FST-immunized geese were not significantly different from those in BSA-immunized control geese (Figure 4C,D).
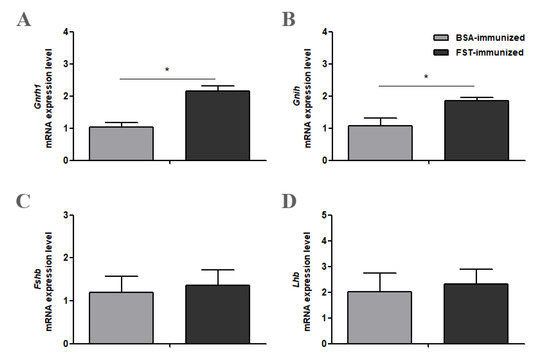
Figure 4.
Gnrh1 (A), Gnih (B), Fshb (C) and Lhb (D) mRNA levels in the hypothalamus or pituitary gland in FST-immunized or the BSA-immunized control geese (n = 6). * represents a significant difference between the two groups (p < 0.05). Data represent mean ± SEM.
3.5. Gene Expression of Lhr, Star, Cyp11a1, Hsd3b, Ocln, and Vldlr in the Granulosa Layer of Ovarian Follicles
The abundance of Lhr, Star, Cyp11a1, and Hsd3b mRNA increased as follicles matured in both groups (Figure 5A–D). Compared with those in BSA-immunized control geese, Lhr mRNA levels in F5 and LWFs (p < 0.05, Figure 5A) and Star mRNA levels in F3, F5, SYFs, and LWFs (p < 0.05, Figure 5B) were higher in FST-immunized geese. Both the classification of ovarian follicles and immunization treatment influenced Lhr and Star mRNA levels. There was an interaction (p < 0.05) between these two variables for the Star mRNA level. Only the classification of ovarian follicles influenced Cyp11a1 and Hsd3b mRNA levels (Figure 5C,D).
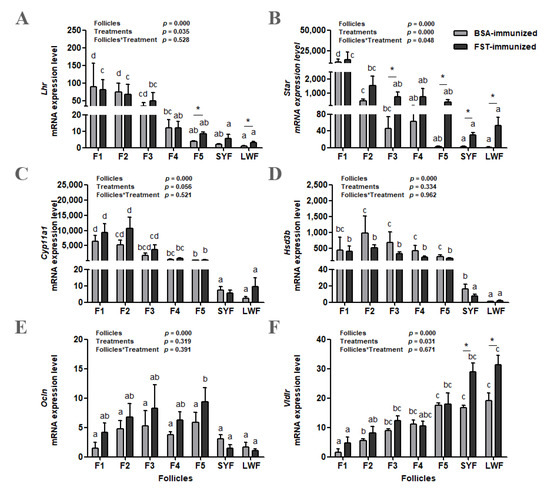
Figure 5.
Lhr (A), Star (B), Cyp11a1 (C), Hsd3b (D), Ocln (E), and Vldlr (F) mRNA levels in granulosa layers from ovarian pre-hierarchical follicles (SYFs and LWFs) and pre-ovulatory follicles (F1 to F5) in FST-immunized or BSA-immunized control geese (n = 6). For each group, a,b,c,d represents a significant difference among the means (p < 0.05). * represents a significant difference between the two groups in one type of follicle (p < 0.05). Data represent mean ± SEM.
The abundance of Ocln mRNA increased with follicular maturation but declined in F1 in both groups (Figure 5E). Only the classification of ovarian follicles influenced the Ocln mRNA abundance. The abundance of Vldlr mRNA decreased with follicular maturation in both groups (Figure 5F). Both the classification of ovarian follicles and immunization treatment had significant effects on Vldlr mRNA level; however, there was no interaction (p = 0.671) between these two variables. Compared with those in BSA-immunized control geese, Vldlr mRNA levels in SYFs and LWFs were significantly higher in FST-immunized geese (Figure 5F).
3.6. Gene Expression of Smad and Fshr in the Granulosa Layer of Ovarian Follicles
The abundance of the Smad2, Smad3, and Smad4 mRNA increased as follicles matured in both groups, and then decreased in F1 (Figure 6A–C). Both the classification of ovarian follicles and immunization treatment influenced Smad3 and Smad4 mRNA levels; however, there was no interaction between these two variables. Only the classification of ovarian follicles influenced the Smad2 mRNA level. Compared with those in BSA-immunized control geese, Smad2 mRNA levels in F2 and F5 (p < 0.05, Figure 6A) and Smad3 mRNA levels in F5, SYFs, and LWFs (p < 0.05, Figure 6B) were higher in FST-immunized geese. There were significant differences in Smad4 mRNA levels in F2, F3, F4, F5, and LWFs between the two groups (Figure 6C). Compared with that in LWFs, the Fshr mRNA level increased in SYFs and then remained high in F1 to F5 in both groups (Figure 6D). Only the classification of ovarian follicles influenced the Fshr mRNA level. There was a significant difference in Fshr mRNA levels from F5 between the two groups.
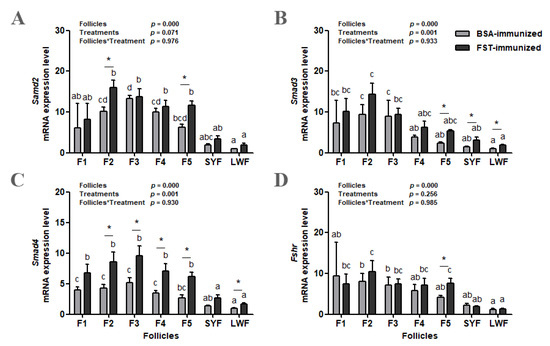
Figure 6.
Smad2 (A), Smad3 (B), Smad4 (C), and Fshr (D) mRNA levels in granulosa layers from ovarian pre-hierarchical follicles (SYFs and LWFs) and pre-ovulatory follicles (F1 to F5) in FST-immunized or BSA-immunized control geese (n = 6). For each group, a, b, c represents a significant difference among the means (p < 0.05). * represents a significant difference between the two groups (p < 0.05) in one type of follicle. Data represent mean ± SEM.
4. Discussion
FST plays an important part in the regulation of ovarian follicular development by neutralizing effects of activin in mammals; however, only a few studies have explored the same in fowls. In this study, the abundance of Fst mRNA in ovarian follicles of Yangzhou geese were investigated first. This was found to be consistent with previous studies on other domestic fowls in which Fst mRNA abundance peaked in SYFs and then declined with ovarian follicular maturation. Following immuno-neutralization of FST bioactivity in geese, the number of LYFs significantly increased, indicating that the development potential of ovarian pre-hierarchical follicles into pre-ovulatory follicles had been enhanced. We further demonstrated that enhanced ovarian pre-hierarchical follicle development was associated with increased mRNA levels of genes involved in ovarian steroidogenesis and yolk deposition in FST-immunized geese.
Anti-FST antibodies gradually appeared in the blood circulation of geese as the titers increased after primary immunization and peaked following booster immunization. Corresponding to the appearance of anti-FST antibodies, both the egg-laying rate and the cumulative number of eggs laid per goose were higher in the FST-immunized group than those in the BSA-immunized group. It was noted that the egg-laying rates decreased in the BSA-immunized group at the early stage of the experiment, which might be due to the stresses caused by the experiment operation and antigen administration, as geese very easily suffered from stresses. However, the anti-FST antibody resisted the detrimental effect of such stresses and maintained egg production by prompting the maturation of LYFs in the FST-immunized group. An important observation was that there was an increase in the number of LYFs in FST-immunized geese. Considering that avian ovarian follicles develop in a size hierarchy, this result suggests that more LWFs were recruited and developed into LYFs through the SYFs. Pre-hierarchical follicles, including LWFs and SYFs, are gonadotropin-independent and mainly regulated by local ovarian growth factors, such as members of the TGF-β family [22]. Activin, a member of the TGF-β family, plays an important role in the promotion and maintenance of follicular growth during the early developmental stage [23]. In hen pre-hierarchical follicles, activin A induces SMAD signaling through its own membrane receptors type IIB and type I and stimulates the differentiation of granulosa cells [24,25]. Considering that FST inhibits activin bioactivity by forming an inactive complex with it, we hypothesized that immuno-neutralization of FST bioactivity enhances the developmental potential of LWFs and SYFs by increasing activin availability. This result was in line with previous findings in which the number of smaller follicles at the time of wave emergence increased in FST-immunized heifers resulting from the reduced neutralization of activin [15].
In hens, follicular activin A was mainly confined to the theca layer and was low in pre-hierarchical follicles; however, it increased sharply as follicles entered the pre-ovulatory hierarchy [26]. Similarly to activin A, FST predominated in the theca layer, although significant amounts were also present in the granulosa layer of pre-hierarchical follicles [26]. In vitro studies in hens have shown that activin A significantly increased mRNA levels of gonadotropin receptors Fshr and Lhr in granulosa cells of pre-ovulatory follicles [27]; however, it inhibited the proliferation of granulosa cells of pre-ovulatory follicles [27,28]. In this study, Lhr and Fshr mRNA levels were significantly upregulated in the granulosa layer of pre-ovulatory follicle F5 in FST-immunized geese, and the Lhr mRNA level was also upregulated in the granulosa layer of pre-hierarchical follicle LWFs. These results were similar to those of a previous study, in which activin A increased the expression level of Lhr mRNA, but not Fshr mRNA, in granulosa layer cells of pre-hierarchical follicle SYFs in hens [24]. These results suggest that FST plays a limiting role in the regulation of granulosa cell differentiation by neutralizing activin A, which promotes gonadotropin receptor expression.
For the pre-hierarchical follicles in the hen, the granulosa layer cells are undifferentiated and steroidogenically incompetent, and mRNA levels of Lhr, Star, Cyp11a1, and Hsd3b, the genes that are involved in progesterone synthesis, are low [29,30,31,32]. Once follicles enter the pre-ovulatory hierarchy, the Star mRNA level increases, and follicles begin to produce progesterone [29]. In this study, early granulosa cell differentiation and steroidogenic competency in pre-hierarchical follicles from FST-immunized geese could be seen from Star mRNA levels, which were found to increase significantly in SYFs and LWFs. A previous study in hens found that activin A directly induces Lhr mRNA expression and indirectly promotes Star mRNA expression and progesterone production following Lhr mRNA induction in SYFs [24]. Moreover, upregulated Vldlr mRNA levels were observed in SYFs and LWFs, indicating enhanced yolk deposition [33]. During the rapid growth phase, VLDLR migrates to the follicular wall, enabling endocytosis of yolk precursors into the oocyte, followed by follicular differentiation [34]. However, the abundance of Vldlr mRNA decreased with follicular maturation, which may allow the yolk precursor to reach the oocyte membrane directly by passing through intercellular gaps between granulosa cells for receptor-mediated endocytosis [35,36,37]. Until now, there has been no research on Vldlr gene regulated by activin A or FST. It was found that activin A induced Ocln mRNA expression in the granulosa layer of chicken ovarian follicles, resulting in functional tight junctions to prevent access of yolk into the oocyte [38]. However, immunization against FST had no effect on Ocln mRNA expression in this study. These changes in gene expression suggest that immuno-neutralization of FST bioactivity enhances the development potential of ovarian pre-hierarchical follicles, manifested as early granulosa cell differentiation, steroidogenic competency, and raised yolk deposition.
Activins perform their biological functions by forming heterodimeric complexes with type I and type II receptors, in which the type I receptor is trans-phosphorylated by the type II receptor [23]. The activated type I receptor then phosphorylates receptor (R)-SMAD2 and/or 3, which form complexes with Co-SMAD4. These activated SMAD complexes translocate to the nucleus to alter gene transcription. Immunization against FST significantly increased Smad3 mRNA levels in SYFs and LWFs, which was consistent with the pattern of Star mRNA level. This result indicates that the effect of nullifying FST bioactivity to promote the growth and differentiation of pre-hierarchical follicles in geese is mediated by SMAD3 in the enhanced activin receptor signaling pathway. It is worth noting that the Smad4 mRNA level was upregulated in both pre-hierarchical and pre-ovulatory follicles in FST-immunized geese. There is some evidence that FST can regulate ovarian function by forming an inactive complex with other members of the TGF-β superfamily, BMPs [6,39]. Studies in chicken ovaries have demonstrated that BMPs signaling via SMAD molecules promote granulosa cell differentiation and progesterone production [40,41,42]. Therefore, immunization against FST might enhance BMP signaling and result in an increase in progesterone synthesis through SMAD4, thereby accelerating the development of pre-ovulatory follicles in geese.
Unexpectedly, immunization against FST did not significantly increase the Fshb mRNA level from the pituitary gland, which is inconsistent with FST’s known involvement in the inhibition of FSH secretion in birds. Although plasma FSH concentration was not measured in this study, previous studies in heifers have found no change in plasma FSH concentration after FST immunization [15]. However, immunization against FST significantly upregulated Gnrh1 and Gnih mRNA levels from the hypothalamus. In vitro studies had shown that activin A might act at the hypothalamus in mice and activate the secretion of GnRH by modulating Kiss1 gene expression, whereas FST reduced Kiss1 gene expression and abolished activin’s effect on the Kiss1 gene [43]. More studies are needed to understand these findings in birds.
5. Conclusions
Immuno-neutralization of FST bioactivity enhanced the developmental potential of ovarian pre-hierarchical follicles by augmenting follicular sensitivity to activin, which resulted in increased numbers of pre-ovulatory follicles in Yangzhou geese. However, immuno-neutralization of FST bioactivity did not affect the pituitary FSH beta mRNA levels.
Author Contributions
Conceptualization, Z.S.; methodology, R.C.; formal analysis, R.C. and P.Y.; investigation, R.C., P.Y., Z.D., J.L., H.Z. and M.L.; writing—original draft preparation, R.C.; writing—review and editing, Z.S.; visualization, R.C. and P.Y.; project administration, Z.S.; funding acquisition, R.C. and Z.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the China Agriculture Research System of MOF and MARA (grant number CARS-42-20), Exploring and Overturning Innovation Program of Jiangsu Academy of Agricultural Sciences (grant number ZX(21)1215), and the National Natural Science Foundation of China (grant number 32102551).
Institutional Review Board Statement
Animal experiments were approved by the Jiangsu Academy of Agricultural Sciences Experimental Animal Ethics Committee and carried out according to the Regulations for the Administration of Affairs Concerning Experimental Animals (Decree No. 63 of the Jiangsu Academy of Agricultural Sciences, 8 July 2014).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest. There is no conflict of interest with Anhui Tianzhijiao Goose Industry Co., Ltd. (Chuzhou, China).
References
- Ueno, N.; Ling, N.; Ying, S.Y.; Esch, F.; Shimasaki, S.; Guillemin, R. Isolation and partial characterization of follistatin: A single-chain Mr 35,000 monomeric protein that inhibits the release of follicle-stimulating hormone. Proc. Natl. Acad. Sci. USA 1987, 84, 8282–8286. [Google Scholar] [CrossRef]
- Robertson, D.M.; Klein, R.; de Vos, F.L.; McLachlan, R.I.; Wettenhall, R.E.; Hearn, M.T.; Burger, H.G.; de Kretser, D.M. The isolation of polypeptides with FSH suppressing activity from bovine follicular fluid which are structurally different to inhibin. Biochem. Biophys. Res. Commun. 1987, 149, 744–749. [Google Scholar] [CrossRef]
- Nakamura, T.; Takio, K.; Eto, Y.; Shibai, H.; Titani, K.; Sugino, H. Activin-Binding Protein from Rat Ovary Is Follistatin. Science 1990, 247, 836–838. [Google Scholar] [CrossRef] [PubMed]
- de Winter, J.P.; ten Dijke, P.; de Vries, C.J.; van Achterberg, T.A.; Sugino, H.; de Waele, P.; Huylebroeck, D.; Verschueren, K.; van den Eijnden-van Raaij, A.J. Follistatins neutralize activin bioactivity by inhibition of activin binding to its type II receptors. Mol. Cell. Endocrinol. 1996, 116, 105–114. [Google Scholar] [CrossRef]
- Thompson, T.B.; Lerch, T.F.; Cook, R.W.; Woodruff, T.K.; Jardetzky, T.S. The Structure of the Follistatin:Activin Complex Reveals Antagonism of Both Type I and Type II Receptor Binding. Dev. Cell 2005, 9, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-Y.; Morrison, J.R.; Phillips, D.J.; De Kretser, D.M. Regulation of ovarian function by the TGF-beta superfamily and follistatin. Reproduction 2003, 126, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, A.; Shimasaki, S.; DePaolo, L.V.; Erickson, G.F.; Ling, N. Cyclic Changes in Follistatin Messenger Ribonucleic Acid and its Protein in the Rat Ovary During the Estrous Cycle. Endocrinology 1991, 129, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Shukovski, L.; Zhang, Z.W.; Michel, U.; Findlay, J.K. Expression of mRNA for follicle-stimulating hormone suppressing protein in ovarian tissues of cows. Reproduction 1992, 95, 861–867. [Google Scholar] [CrossRef][Green Version]
- Roberts, V.J.; Barth, S.; El-Roeiy, A.; Yen, S.S. Expression of inhibin/activin subunits and follistatin messenger ribonucleic acids and proteins in ovarian follicles and the corpus luteum during the human menstrual cycle. J. Clin. Endocrinol. Metab. 1993, 77, 1402–1410. [Google Scholar] [CrossRef] [PubMed]
- Lindsell, C.E.; Misra, V.; Murphy, B.D. Regulation of follistatin gene expression in the ovary and in primary cultures of porcine granulosa cells. Reproduction 1994, 100, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Tisdall, D.J.; Hudson, N.; Smith, P.; McNatty, K.P. Localization of ovine follistatin and α and βA inhibin mRNA in the sheep ovary during the oestrous cycle. J. Mol. Endocrinol. 1994, 12, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Braw-Tal, R. Expression of mRNA for follistatin and inhibin/activin subunits during follicular growth and atresia. J. Mol. Endocrinol. 1994, 13, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Matzuk, M.M.; Lu, N.; Vogel, H.; Sellheyer, K.; Roop, D.R.; Bradley, A. Multiple defects and perinatal death in mice deficient in follistatin. Nature 1995, 374, 360–363. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Kumar, T.R.; Woodruff, T.; Hadsell, L.A.; DeMayo, F.J.; Matzuk, M.M. Overexpression of Mouse Follistatin Causes Reproductive Defects in Transgenic Mice. Mol. Endocrinol. 1998, 12, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Brogliattil, G.M.; Christensen, C.R.; Adams, G.P. Active immunization against follistatin and its effect on FSH, follicle development and superovulation in heifers. Theriogenology 1999, 52, 49–66. [Google Scholar] [CrossRef]
- Davis, A.J.; Johnson, P.A. Expression Pattern of Messenger Ribonucleic Acid for Follistatin and the Inhibin/Activin Subunits during Follicular and Testicular Development in Gallus domesticus1. Biol. Reprod. 1998, 59, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Niu, D.; Ruan, H.; Yu, X.P.; Chen, G.; He, G.Q.; Yang, P.X. Expression pattern of mRNA for follistatin and in-hibin/activin beta B-subunit during follicular and testicular development in duck. Acta Genet. Sin. 2001, 28, 808–815. (In Chinese) [Google Scholar] [PubMed]
- Chen, R.; Dai, Z.C.; Zhu, H.X.; Lei, M.M.; Li, Y.; Shi, Z.D. Active immunization against AMH reveals its inhibitory role in the development of pre-ovulatory follicles in Zhedong White geese. Theriogenology 2020, 144, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.M.; Shi, Z.D.; Liu, Z.; Liu, Y.; Li, X.W. Endocrine regulations of reproductive seasonality, follicular development and incubation in Magang geese. Anim. Reprod. Sci. 2008, 104, 344–358. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Shao, X.; Chen, Z.; Wei, C.; Lei, M.; Ying, S.; Yu, J.; Shi, Z. Induction of out-of-season egg laying by artificial photoperiod in Yangzhou geese and the associated endocrine and molecular regulation mechanisms. Anim. Reprod. Sci. 2017, 180, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Onagbesan, O.; Bruggeman, V.; Decuypere, E. Intra-ovarian growth factors regulating ovarian function in avian species: A review. Anim. Reprod. Sci. 2009, 111, 121–140. [Google Scholar] [CrossRef] [PubMed]
- Knight, P.G.; Glister, C. Local roles of TGF-β superfamily members in the control of ovarian follicle development. Anim. Reprod. Sci. 2003, 78, 165–183. [Google Scholar] [CrossRef]
- Johnson, A.L.; Bridgham, J.T.; Woods, D.C. Cellular Mechanisms and Modulation of Activin A- and Transforming Growth Factor β-Mediated Differentiation in Cultured Hen Granulosa Cells1. Biol. Reprod. 2004, 71, 1844–1851. [Google Scholar] [CrossRef]
- Lovell, T.M.; Al-Musawi, S.L.; Gladwell, R.T.; Knight, P.G. Gonadotrophins modulate hormone secretion and steady-state mRNA levels for activin receptors (type I, IIA, IIB) and inhibin co-receptor (betaglycan) in granulosa and theca cells from chicken prehierarchical and preovulatory follicles. Reproduction 2007, 133, 1159–1168. [Google Scholar] [CrossRef] [PubMed]
- Lovell, T.M.; Gladwell, R.T.; Groome, N.P.; Knight, P.G. Ovarian follicle development in the laying hen is accompanied by divergent changes in inhibin A, inhibin B, activin A and follistatin production in granulosa and theca layers. J. Endocrinol. 2003, 177, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.A.; Woodcock, J.R.; Kent, T.R. Effect of activin A and inhibin A on expression of the inhibin/activin β-B-subunit and gonadotropin receptors in granulosa cells of the hen. Gen. Comp. Endocrinol. 2006, 147, 102–107. [Google Scholar] [CrossRef]
- Davis, A.J.; Brooks, C.F.; Johnson, P.A. Activin A and gonadotropin regulation of follicle-stimulating hormone and luteinizing hormone receptor messenger RNA in avian granulosa cells. Biol. Reprod. 2001, 65, 1352–1358. [Google Scholar] [CrossRef] [PubMed]
- Nitta, H.; Mason, J.I.; Bahr, J.M. Localization of 3β-Hydroxysteroid Dehydrogenase in the Chicken Ovarian Follicle Shifts from the Theca Layer to Granulosa Layer with Follicular Maturation. Biol. Reprod. 1993, 48, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Johnson, A.L. Regulation of P450 Cholesterol Side-Chain Cleavage Messenger Ribonucleic Acid Expression and Progesterone Production in Hen Granulosa Cells. Biol. Reprod. 1993, 49, 463–469. [Google Scholar] [CrossRef]
- Johnson, A.L.; Bridgham, J.T.; Wagner, B. Characterization of a Chicken Luteinizing Hormone Receptor (cLH-R) Complementary Deoxyribonucleic Acid, and Expression of cLH-R Messenger Ribonucleic Acid in the Ovary. Biol. Reprod. 1996, 55, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.P.; Bridgham, J.T.; Langenau, D.M.; Johnson, A.L.; Goetz, F.W. Conservation of steroidogenic acute regulatory (StAR) protein structure and expression in vertebrates. Mol. Cell. Endocrinol. 2000, 168, 119–125. [Google Scholar] [CrossRef]
- Schneider, W.J. Lipid transport to avian oocytes and to the developing embryo. J. Biomed. Res. 2016, 30, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Steyrer, E.; Retzek, H.; Sanders, E.J.; Schneider, W.J. Chicken oocyte growth: Receptor-mediated yolk deposition. Cell Tissue Res. 1993, 272, 459–471. [Google Scholar] [CrossRef]
- Hu, S.; Liu, H.; Pan, Z.; Xia, L.; Dong, X.; Li, L.; Xu, F.; He, H.; Wang, J. Molecular cloning, expression profile and transcriptional modulation of two splice variants of very low density lipoprotein receptor during ovarian follicle development in geese (Anser cygnoide). Anim. Reprod. Sci. 2014, 149, 281–296. [Google Scholar] [CrossRef]
- Lin, X.; Ma, Y.; Qian, T.; Yao, J.; Mi, Y.; Zhang, C. Basic fibroblast growth factor promotes prehierarchical follicle growth and yolk deposition in the chicken. Theriogenology 2019, 139, 90–97. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, Y.; Liu, Z.; Guo, X.; Sun, Y.; Kang, L.; Jiang, Y. Transcriptomic and proteomic analyses of ovarian follicles reveal the role of VLDLR in chicken follicle selection. BMC Genom. 2020, 21, 486. [Google Scholar] [CrossRef]
- Schuster, M.K.; Schmierer, B.; Shkumatava, A.; Kuchler, K. Activin A and Follicle-Stimulating Hormone Control Tight Junctions in Avian Granulosa Cells by Regulating Occludin Expression. Biol. Reprod. 2004, 70, 1493–1499. [Google Scholar] [CrossRef]
- Otsuka, F.; Moore, R.K.; Iemura, S.-I.; Ueno, N.; Shimasaki, S. Follistatin Inhibits the Function of the Oocyte-Derived Factor BMP-15. Biochem. Biophys. Res. Commun. 2001, 289, 961–966. [Google Scholar] [CrossRef]
- Onagbesan, O.M.; Bruggeman, V.; Van As, P.; Tona, K.; Williams, J.; Decuypere, E. BMPs and BMPRs in chicken ovary and effects of BMP-4 and -7 on granulosa cell proliferation and progesterone production in vitro. Am. J. Physiol. Metab. 2003, 285, E973–E983. [Google Scholar] [CrossRef]
- Kim, D.; Ocón-Grove, O.; Johnson, A.L. Bone Morphogenetic Protein 4 Supports the Initial Differentiation of Hen (Gallus gallus) Granulosa Cells. Biol. Reprod. 2013, 88, 161. [Google Scholar] [CrossRef] [PubMed]
- Stephens, C.S.; Johnson, P.A. Bone morphogenetic protein 15 may promote follicle selection in the hen. Gen. Comp. Endocrinol. 2016, 235, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Tumurgan, Z.; Kanasaki, H.; Tumurbaatar, T.; Oride, A.; Okada, H.; Hara, T.; Kyo, S. Role of activin, follistatin, and inhibin in the regulation of Kiss-1 gene expression in hypothalamic cell models. Biol. Reprod. 2019, 101, 405–415. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).