Exo-Erythrocytic Development of Avian Haemosporidian Parasites in European Owls
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Molecular Diagnostics
2.2.1. DNA Extraction
2.2.2. Polymerase Chain Reaction
2.2.3. Sequencing and Sequence Analysis
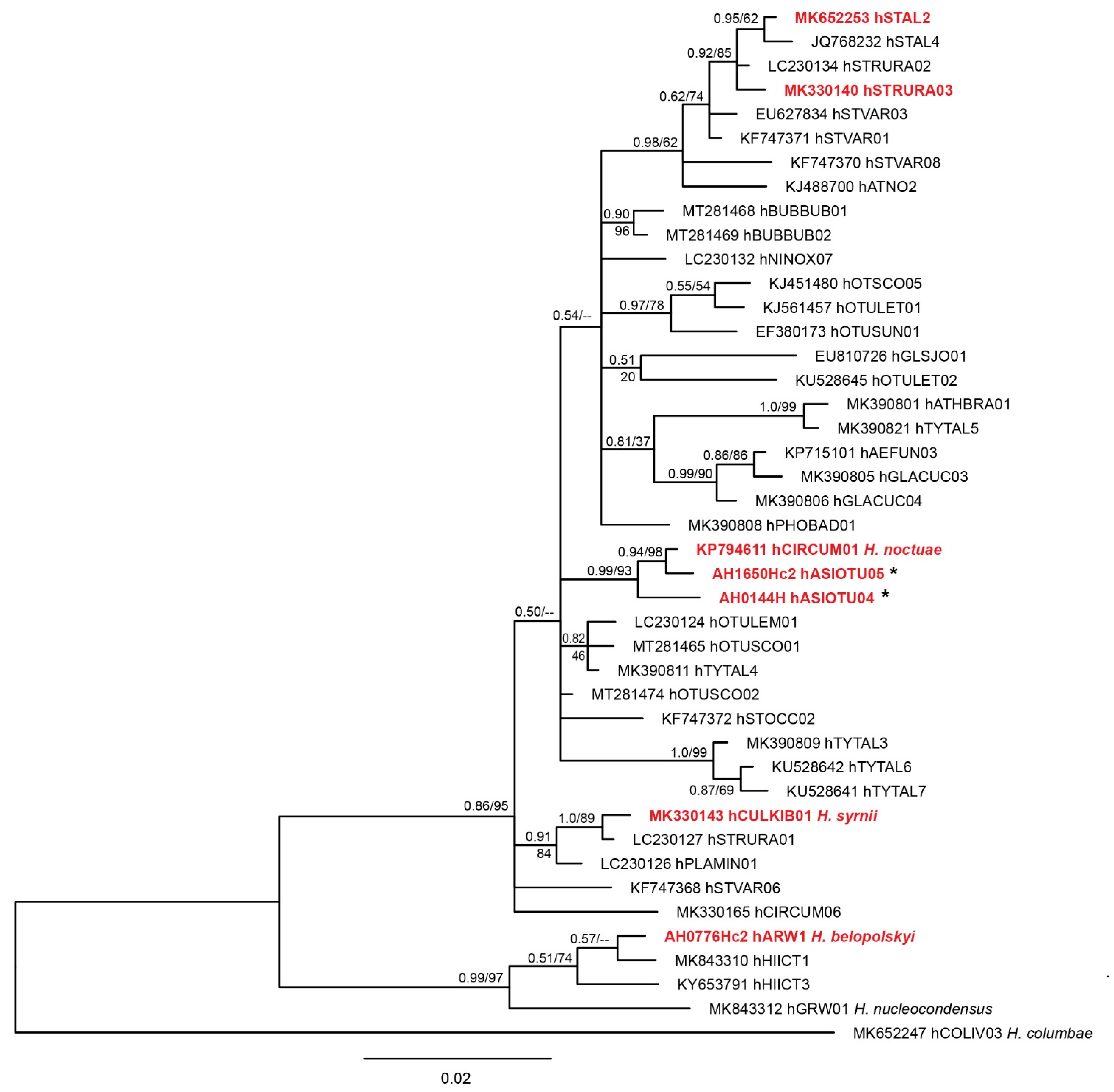
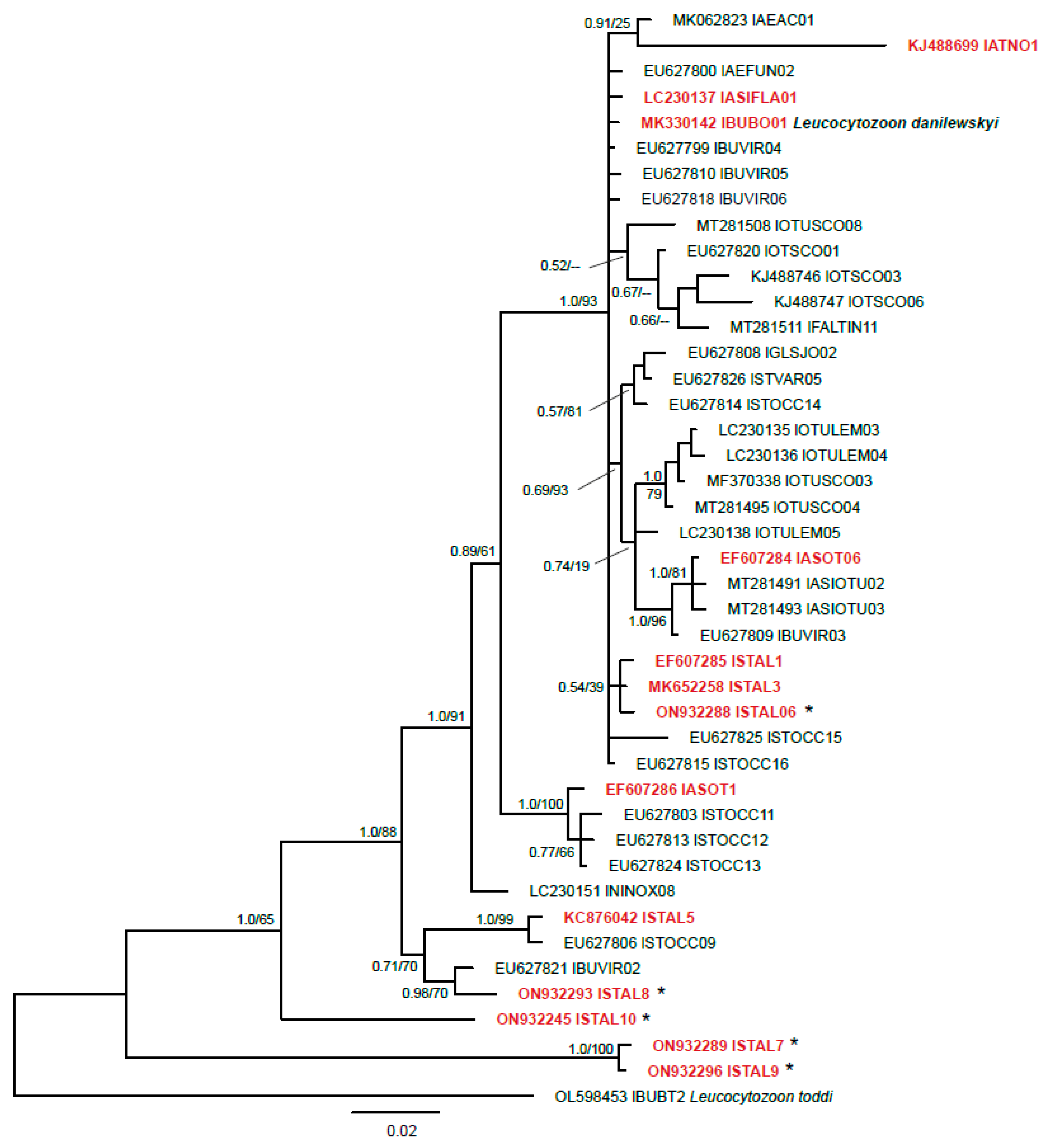
2.2.4. Phylogenetic Analysis
2.3. Histological Examination
2.4. Chromogenic In Situ Hybridization
2.5. Immunohistochemistry
2.6. Ethical Statements
3. Results
3.1. Molecular Diagnostics
3.2. Histological Diagnostics
3.2.1. Description of Leucocytozoon Exo-Erythrocytic Stages
3.2.2. Description of Haemoproteus Exo-Erythrocytic Stages
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clark, N.J.; Clegg, S.M.; Lima, M.R. A review of global diversity in avian haemosporidians (Plasmodium and Haemoproteus: Haemosporida): New insights from molecular data. Int. J. Parasitol. 2014, 44, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Valkiūnas, G.; Atkinson, C.T. Introduction to life cycles, taxonomy, distribution, and basic research techniques. In Avian Malaria and Related Parasites in the Tropics: Ecology, Evolution and Systematics; Santiago-Alarcon, D., Marzal, A., Eds.; Springer Nature: Cham, Switzerland, 2020; pp. 45–80. [Google Scholar] [CrossRef]
- Valkiūnas, G. Avian Malaria Parasites and Other Haemosporidia; CRC Press: Boca Raton, FL, USA, 2005; ISBN 0415300975. [Google Scholar]
- Valkiūnas, G.; Iezhova, T.A. Keys to the avian malaria parasites. Malar. J. 2018, 17, 212. [Google Scholar] [CrossRef] [PubMed]
- Bensch, S.; Hellgren, O.; Pérez-Tris, J. MalAvi: A public database of malaria parasites and related haemosporidians in avian hosts based on mitochondrial cytochrome b lineages. Mol. Ecol. Resour. 2009, 9, 1353–1358. [Google Scholar] [CrossRef] [PubMed]
- Bensch, S.; Canbäck, B.; DeBarry, J.D.; Johansson, T.; Hellgren, O.; Kissinger, J.C.; Palinauskas, V.; Videvall, E.; Valkiūnas, G. The genome of Haemoproteus tartakovskyi and its relationship to human malaria parasites. Genome Biol. Evol. 2016, 8, 1361–1373. [Google Scholar] [CrossRef] [PubMed]
- Ricklefs, R.E.; Medeiros, M.; Ellis, V.A.; Svensson-Coelho, M.; Blake, J.G.; Loiselle, B.A.; Soares, L.; Fecchio, A.; Outlaw, D.; Marra, P.P.; et al. Avian migration and the distribution of malaria parasites in New World passerine birds. J. Biogeogr. 2017, 44, 1113–1123. [Google Scholar] [CrossRef]
- Perkins, S.L. Malaria’s many mates: Past, present and future of the systematics of the order Haemosporida. J. Parasitol. 2014, 100, 11–25. [Google Scholar] [CrossRef]
- Sehgal, R.N. Manifold habitat effects on the prevalence and diversity of avian blood parasites. Int. J. Parasitol. Parasites Wildl. 2015, 4, 421–430. [Google Scholar] [CrossRef]
- Videvall, E.; Cornwallis, C.K.; Ahrén, D.; Palinauskas, V.; Valkiūnas, G.; Hellgren, O. The transcriptome of the avian malaria parasite Plasmodium ashfordi displays host-specific gene expression. Mol. Ecol. 2017, 26, 2939–2958. [Google Scholar] [CrossRef]
- Ellis, V.A.; Sari, E.H.R.; Rubenstein, D.R.; Dickerson, R.C.; Bensch, S.; Ricklefs, R.E. The global biogeography of avian haemosporidian parasites is characterized by local diversification and intercontinental dispersal. Parasitology 2019, 146, 213–219. [Google Scholar] [CrossRef] [Green Version]
- Valkiūnas, G.; Iezhova, T.A. Exo-erythrocytic development of avian malaria and related haemosporidian parasites. Malar. J. 2017, 16, 101. [Google Scholar] [CrossRef]
- Bennett, G.F.; Peirce, M.A.; Ashford, R.W. Avian haematozoa: Mortality and pathogenicity. J. Nat. Hist. 1993, 27, 993–1001. [Google Scholar] [CrossRef]
- Dinhopl, N.; Mostegl, M.M.; Richter, B.; Nedorost, N.; Maderner, A.; Fragner, K.; Weissenböck, H. Application of in-situ hybridization for the detection and identification of avian malaria parasites in paraffin wax-embedded tissues from captive penguins. Avian Pathol. 2011, 40, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Himmel, T.; Harl, J.; Kübber-Heiss, A.; Konicek, C.; Fernández, N.; Juan-Sallés, C.; Ilgūnas, M.; Valkiūnas, G.; Weissenböck, H. Molecular probes for the identification of avian Haemoproteus and Leucocytozoon parasites in tissue sections by chromogenic in situ hybridization. Parasites Vectors 2019, 12, 282. [Google Scholar] [CrossRef] [PubMed]
- Ilgūnas, M.; Romeiro Fernandes Chagas, C.; Bukauskaitė, D.; Bernotienė, R.; Iezhova, T.; Valkiūnas, G. The life-cycle of the avian haemosporidian parasite Haemoproteus majoris, with emphasis on the exoerythrocytic and sporogonic development. Parasites Vectors 2019, 12, 516. [Google Scholar] [CrossRef]
- Ferrell, S.T.; Snowden, K.; Marlar, A.B.; Garner, M.; Lung, N.P. Fatal hemoprotozoal infections in multiple avian species in a zoological park. J. Zoo Wildl. Med. 2007, 38, 309–316. [Google Scholar] [CrossRef]
- Donovan, T.A.; Schrenzel, M.; Tucker, T.A.; Pessier, A.P.; Stalis, I.H. Hepatic hemorrhage, hemocoelom, and sudden death due to Haemoproteus infection in passerine birds: Eleven cases. J. Vet. Diagn. Investig. 2008, 20, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Cardona, C.J.; Ihejirika, A.; McClellan, L. Haemoproteus lophortyx infection in bobwhite quail. Avian Dis. 2002, 46, 249–255. [Google Scholar] [CrossRef]
- Cannell, B.L.; Krasnec, K.V.; Campbell, K.; Jones, H.I.; Miller, R.D.; Stephens, N. The pathology and pathogenicity of a novel Haemoproteus spp. infection in wild little penguins (Eudyptula minor). Vet. Parasitol. 2013, 197, 74–84. [Google Scholar] [CrossRef]
- Palinauskas, V.; Iezhova, T.A.; Križanauskienė, A.; Markovets, M.Y.; Bensch, S.; Valkiūnas, G. Molecular characterization and distribution of Haemoproteus minutus (Haemosporida, Haemoproteidae): A pathogenic avian parasite. Parasitol. Int. 2013, 62, 358–363. [Google Scholar] [CrossRef]
- Groff, T.C.; Lorenz, T.J.; Crespo, R.; Iezhova, T.; Valkiūnas, G.; Sehgal, R.N.M. Haemoproteosis lethality in a woodpecker, with molecular and morphological characterization of Haemoproteus velans (Haemosporida, Haemoproteidae). Int. J. Parasitol. Parasites Wildl. 2019, 10, 93–100. [Google Scholar] [CrossRef]
- Ortiz-Catedral, L.; Brunton, D.; Stidworthy, M.F.; Elsheikha, H.M.; Pennycott, T.; Schulze, C.; Braun, M.; Wink, M.; Gerlach, H.; Pendl, H.; et al. Haemoproteus minutus is highly virulent for Australasian and South American parrots. Parasites Vectors 2019, 12, 40. [Google Scholar] [CrossRef] [PubMed]
- Duc, M.; Ilgūnas, M.; Valkiūnas, G. Patterns of Haemoproteus majoris (Haemosporida, Haemoproteidae) megalomeront development. Acta Trop. 2020, 212, 105706. [Google Scholar] [CrossRef] [PubMed]
- Duc, M.; Ilgūnas, M.; Kubiliūnaitė, M.; Valkiūnas, G. First report of Haemoproteus (Haemosporida, Haemoproteidae) megalomeronts in the brain of an avian host, with description of megalomerogony of Haemoproteus pastoris, the blood parasite of the Common starling. Animals 2021, 11, 2824. [Google Scholar] [CrossRef]
- Shokrani, H.; Norouzian, H.; Dezfoulian, O. Exo-Erythrocytic stages of Haemoproteus sp. in Common buzzard (Buteo buteo): A histopathological and molecular study. Int. J. Parasitol. Parasites Wildl. 2021, 16, 64–69. [Google Scholar] [CrossRef]
- Winkler, D.W.; Billerman, S.M.; Lovette, I.J. Owls (Strigidae), version 1.0. In Birds of the World; Billerman, S.M., Keeney, B.K., Rodewald, P.G., Schulenberg, T.S., Eds.; Cornell Lab of Ornithology: Ithaca, NY, USA, 2020. [Google Scholar] [CrossRef]
- Greiner, E.C.; Bennett, G.F.; White, E.M.; Coombs, R.F. Distribution of the avian hematozoa of north America. Can. J. Zool. 1975, 53, 1762–1787. [Google Scholar] [CrossRef] [PubMed]
- Bennett, G.F.; Whiteway, M.; Woodworth-Lynas, C. A host-parasite catalogue of the avian haematozoa. Mem. Univ. Nfld./Occ. Pap. Biol. 1982, 5, 1–243. [Google Scholar]
- Bishop, M.A.; Bennett, G.F. Host-parasite catalogue of the avian haematozoa: Supplement 1, and Bibliography of the avian blood-inhabiting haematozoa. Mem. Univ. Nfld./Occ. Pap. Biol. 1992, 15, 1–244. [Google Scholar]
- Murata, K. Prevalence of blood parasites in Japanese wild birds. J. Vet. Med. Sci. 2002, 64, 785–790. [Google Scholar] [CrossRef]
- Ishak, H.D.; Dumbacher, J.P.; Anderson, N.L.; Keane, J.J.; Valkiūnas, G.; Haig, S.M.; Tell, L.A.; Sehgal, R.N. Blood parasites in owls with conservation implications for the Spotted Owl (Strix occidentalis). PLoS ONE 2008, 3, e2304. [Google Scholar] [CrossRef] [PubMed]
- Krone, O.; Waldenström, J.; Valkiūnas, G.; Lessow, O.; Müller, K.; Iezhova, T.A.; Fickel, J.; Bensch, S. Haemosporidian blood parasites in European birds of prey and owls. J. Parasitol. 2008, 94, 709–715. [Google Scholar] [CrossRef]
- Leppert, L.L.; Dufty, A.M., Jr.; Stock, S.; Oleyar, M.D.; Kaltenecker, G.S. Survey of blood parasites in two forest owls, Northern Saw-whet Owls and Flammulated Owls, of western North America. J. Wildl. Dis. 2008, 44, 475–479. [Google Scholar] [CrossRef]
- Ortego, J.; Cordero, P.J. PCR-based detection and genotyping of haematozoa (Protozoa) parasitizing eagle owls, Bubo bubo. Parasitol. Res. 2009, 104, 467–470. [Google Scholar] [CrossRef]
- Karadjian, G.; Puech, M.P.; Duval, L.; Chavatte, J.M.; Snounou, G.; Landau, I. Haemoproteus syrnii in Strix aluco from France: Morphology, stages of sporogony in a hippoboscid fly, molecular characterization and discussion on the identification of Haemoproteus species. Parasite 2013, 20, 32. [Google Scholar] [CrossRef]
- Drovetski, S.V.; Aghayan, S.A.; Mata, V.A.; Lopes, R.J.; Mode, N.A.; Harvey, J.A.; Voelker, G. Does the niche breadth or trade–off hypothesis explain the abundance–occupancy relationship in avian Haemosporidia? Mol. Ecol. 2014, 23, 3322–3329. [Google Scholar] [CrossRef]
- Ciloglu, A.; Yildirim, A.; Duzlu, O.; Onder, Z.; Dogan, Z.; Inci, A. Investigation of avian haemosporidian parasites from raptor birds in Turkey, with molecular characterisation and microscopic confirmation. Folia Parasitol. 2016, 63, 23. [Google Scholar] [CrossRef]
- Giorgiadis, M.; Guillot, J.; Duval, L.; Landau, I.; Quintard, B. Haemosporidian parasites from captive Strigiformes in France. Parasitol. Res. 2020, 119, 2975–2981. [Google Scholar] [CrossRef]
- Niedringhaus, K.D.; Fenton, H.M.A.; Cleveland, C.A.; Anderson, A.N.; Schwartz, D.; Alex, C.E.; Rogers, K.H.; Mete, A.; Yabsley, M.J. Case Series: Virulent hemosporidiosis infections in juvenile great horned owls (Bubo virginianus) from Louisiana and California, USA. Vet. Parasitol. Reg. Stud. Rep. 2018, 12, 49–54. [Google Scholar] [CrossRef]
- Lee, S.H.; Kwak, D.; Kim, K.T. The first clinical cases of Haemoproteus infection in a snowy owl (Bubo scandiacus) and a goshawk (Accipiter gentilis) at a zoo in the Republic of Korea. J. Vet. Med. Sci. 2018, 80, 1255–1258. [Google Scholar] [CrossRef]
- Barino, G.T.M.; Rossi, M.F.; de Oliveira, L.; Reis Junior, J.L.; D’Agosto, M.; Dias, R.J.P. Haemoproteus syrnii (Haemosporida: Haemoproteidae) in owls from Brazil: Morphological and molecular characterization, potential cryptic species, and exo- erythrocytic stages. Parasitol. Res. 2021, 120, 243–255. [Google Scholar] [CrossRef]
- Yoshimoto, M.; Ozawa, K.; Kondo, H.; Echigoya, Y.; Shibuya, H.; Sato, Y.; Sehgal, R.N.M. A fatal case of a captive snowy owl (Bubo scandiacus) with Haemoproteus infection in Japan. Parasitol. Res. 2021, 120, 277–288. [Google Scholar] [CrossRef]
- Khan, R.A. Development of Leucocytozoon ziemanni (Laveran). J. Parasitol. 1975, 61, 449–457. [Google Scholar] [CrossRef]
- Laird, P.W.; Zijderveld, A.; Linders, K.; Rudnicki, M.A.; Jaenisch, R.; Berns, A. Simplified mammalian DNA isolation procedure. Nucleic Acids Res. 1991, 19, 4293. [Google Scholar] [CrossRef]
- Bensch, S.; Stjernman, M.; Hasselquist, D.; Ostman, O.; Hansson, B.; Westerdahl, H.; Pinheiro, R.T. Host specificity in avian blood parasites: A study of Plasmodium and Haemoproteus mitochondrial DNA amplified from birds. Proc. R. Soc. B. Biol. Sci. 2000, 267, 1583–1589. [Google Scholar] [CrossRef]
- Hellgren, O.; Waldenström, J.; Bensch, S. A new PCR assay for simultaneous studies of Leucocytozoon, Plasmodium, and Haemoproteus from avian blood. J. Parasitol. 2004, 90, 797–802. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, A.; de la Puente, J.; Onrubia, A.; Pérez-Tris, J. Molecular characterization of haemosporidian parasites from kites of the genus Milvus (Aves: Accipitridae). Int. J. Parasitol. 2013, 43, 381–387. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequences alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Rozas, J.; Ferrer Mata, A.; Sánchez DelBarrio, J.C.; Guirao Rico, S.; Librado, P.; Ramos Onsins, S.E.; Sánchez-Gracia, A. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; Von Haeseler, A.; Lanfear, R. IQ-TREE 2: New models and efficient methods for phylogenetic inference in the genomic era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Lillie, R.D. Histopathologic Technic and Practical Histochemistry, 3rd ed.; Blakiston Division; McGraw-Hill: New York, NY, USA, 1965. [Google Scholar]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Scmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harl, J.; Himmel, T.; Valkiūnas, G.; Weissenböck, H. The nuclear 18S ribosomal DNAs of avian haemosporidian parasites. Malar. J. 2019, 18, 305. [Google Scholar] [CrossRef] [PubMed]
- Skalli, O.; Pelte, M.F.; Peclet, M.C.; Gabbiani, G.; Gugliotta, P.; Bussolati, G.; Ravazzola, M.; Orci, L. Alpha-smooth muscle actin, a differentiation marker of smooth muscle cells, is present in microfilamentous bundles of pericytes. J. Histochem. Cytochem. 1989, 37, 315–321. [Google Scholar] [CrossRef]
- Alarcon-Martinez, L.; Yilmaz-Ozcan, S.; Yemisci, M.; Schallek, J.; Kılıç, K.; Can, A.; Di Polo, A.; Dalkara, T. Capillary pericytes express α-smooth muscle actin, which requires prevention of filamentous-actin depolymerization for detection. Elife 2018, 7, e34861. [Google Scholar] [CrossRef] [PubMed]
- McClure, H.E.; Poonswad, P.; Greiner, E.C.; Laird, M. Haematozoa in the Birds of Eastern and Southern Asia. Memorial University of Newfoundland: St. John’s, NL, Canada, 1978. [Google Scholar]
- White, E.M.; Greiner, E.C.; Bennett, G.F.; Herman, C.M. Distribution of the hematozoa of Neotropical birds. Rev. Biol. Trop. 1978, 26, 43–102. [Google Scholar] [PubMed]
- Peirce, M.A. Distribution and host-parasite check-list of the haematozoa of birds in Western Europe. J. Nat. Hist. 1981, 15, 419–458. [Google Scholar] [CrossRef]
- Bennett, G.F.; Earlé, R.A.; Du Toit, H.; Huchzermeyer, F.W. A host-parasite catalogue of the haematozoa of the sub-Saharan birds. Onderstepoort J. Vet. Res. 1992, 59, 1–73. [Google Scholar]
- Salakij, C.; Pornpanom, P.; Lertwatcharasarakul, P.; Kasorndorkbua, C.; Salakij, J. Haemoproteus in barn and collared scops owls from Thailand. J. Vet. Sci. 2018, 19, 280–289. [Google Scholar] [CrossRef]
- Palinauskas, V.; Žiegytė, R.; Šengaut, J.; Bernotienė, R. Different paths—the same virulence: Experimental study on avian single and co-infections with Plasmodium relictum and Plasmodium elongatum. Int. J. Parasitol. 2018, 48, 1089–1096. [Google Scholar] [CrossRef]
- Evans, M.; Otter, A. Fatal combined infection with Haemoproteus noctuae and Leucocytozoon ziemanni in juvenile snowy owls (Nyctea scandiaca). Vet. Rec. 1998, 143, 72–76. [Google Scholar] [CrossRef]
- Pornpanom, P.; Chagas, C.R.F.; Lertwatcharasarakul, P.; Kasorndorkbua, C.; Valkiūnas, G.; Salakij, C. Molecular prevalence and phylogenetic relationship of Haemoproteus and Plasmodium parasites of owls in Thailand: Data from a rehabilitation centre. Int. J. Parasitol. Parasites Wildl. 2019, 9, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Lotta, I.A.; Valkiūnas, G.; Pacheco, M.A.; Escalante, A.A.; Hernández, S.R.; Matta, N.E. Disentangling Leucocytozoon parasite diversity in the neotropics: Descriptions of two new species and shortcomings of molecular diagnostics for leucocytozoids. Int. J. Parasitol. Parasites Wildl. 2019, 9, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Harl, J.; Himmel, T.; Valkiūnas, G.; Ilgūnas, M.; Nedorost, N.; Matt, J.; Kübber-Heiss, A.; Alic, A.; Konicek, C.; Weissenböck, H. Avian haemosporidian parasites of accipitriform raptors. Malar. J. 2022, 21, 14. [Google Scholar] [CrossRef] [PubMed]
- Desser, S.S. Schizogony and gametogony of Leucocytozoon simondi and associated reactions in the avian host. J. Protozool. 1967, 14, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Mayer, M. Über sein Entwicklung von Halteridium. Arch. Fur Schiffs Und Trop. 1910, 14, 197–202. [Google Scholar]
- Olias, P.; Wegelin, M.; Zenker, W.; Freter, S.; Gruber, A.D.; Klopfleisch, R. Avian malaria deaths in parrots, Europe. Emerg. Infect. Dis. 2011, 17, 950–952. [Google Scholar] [CrossRef]
- García-Del-Río, M.; Sancho, R.; Martínez, J.; Merino, S. Blood parasite infections in Strigiformes and Psittaciformes species in captivity with a new record of potential fatal blood parasite transmission to parrots. J. Zoo. Wildl. Med. 2021, 51, 799–813. [Google Scholar] [CrossRef]
- Krotoski, W.A. The hypnozoite and malarial relapse. Prog. Clin. Parasitol. 1989, 1, 1–19. [Google Scholar]
- Valkiūnas, G.; Iezhova, T.A.; Loiseau, C.; Sehgal, R.N. Nested cytochrome B polymerase chain reaction diagnostics detect sporozoites of hemosporidian parasites in peripheral blood of naturally infected birds. J. Parasitol. 2009, 95, 1512–1515. [Google Scholar] [CrossRef]
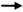 )—meront, short black arrow (
)—meront, short black arrow (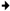 )—cytomere, black arrowhead (
)—cytomere, black arrowhead ( )—merozoite, open arrowhead (
)—merozoite, open arrowhead ( )—intracellular young parasite stages, open arrow (
)—intracellular young parasite stages, open arrow ( )—meront containing capillary, long white arrow (
)—meront containing capillary, long white arrow ( )—erythrocytes, short white arrow (
)—erythrocytes, short white arrow ( )—host cell nucleus, white arrowhead (
)—host cell nucleus, white arrowhead ( )—wall. All scale bars are 25 µm.
)—wall. All scale bars are 25 µm.
 )—meront, short black arrow (
)—meront, short black arrow ( )—cytomere, black arrowhead (
)—cytomere, black arrowhead ( )—merozoite, open arrowhead (
)—merozoite, open arrowhead ( )—intracellular young parasite stages, open arrow (
)—intracellular young parasite stages, open arrow ( )—meront containing capillary, long white arrow (
)—meront containing capillary, long white arrow ( )—erythrocytes, short white arrow (
)—erythrocytes, short white arrow ( )—host cell nucleus, white arrowhead (
)—host cell nucleus, white arrowhead ( )—wall. All scale bars are 25 µm.
)—wall. All scale bars are 25 µm.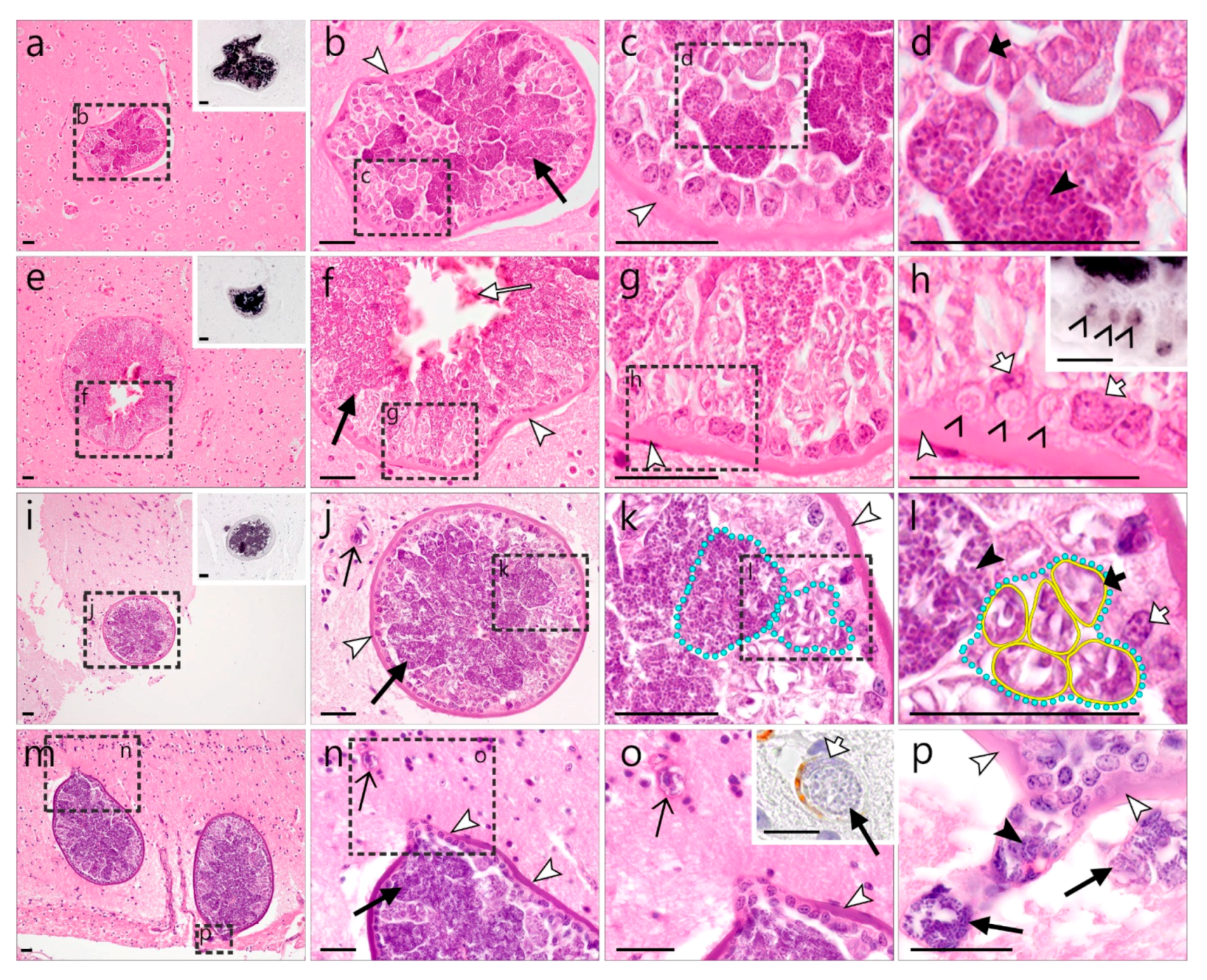
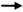 )—meront, short black arrow (
)—meront, short black arrow (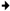 )—cytomere, black arrowhead (
)—cytomere, black arrowhead ( )—merozoite, open arrowhead (
)—merozoite, open arrowhead (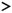 )—intracellular young parasite stages, long white arrow (
)—intracellular young parasite stages, long white arrow ( )—erythrocytes, short white arrow (
)—erythrocytes, short white arrow ( )—host cell nucleus, white arrowhead (
)—host cell nucleus, white arrowhead ( )—wall, asterisk (
)—wall, asterisk ( )—smooth muscle layer. Scale bars are 25 µm.
)—smooth muscle layer. Scale bars are 25 µm.
 )—meront, short black arrow (
)—meront, short black arrow ( )—cytomere, black arrowhead (
)—cytomere, black arrowhead ( )—merozoite, open arrowhead (
)—merozoite, open arrowhead ( )—intracellular young parasite stages, long white arrow (
)—intracellular young parasite stages, long white arrow ( )—erythrocytes, short white arrow (
)—erythrocytes, short white arrow ( )—host cell nucleus, white arrowhead (
)—host cell nucleus, white arrowhead ( )—wall, asterisk (
)—wall, asterisk ( )—smooth muscle layer. Scale bars are 25 µm.
)—smooth muscle layer. Scale bars are 25 µm.
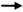 )—meront, white arrowhead (
)—meront, white arrowhead ( )—wall. Scale bars are 25 µm.
)—wall. Scale bars are 25 µm.
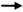 )—meront, white arrowhead (
)—meront, white arrowhead ( )—wall. Scale bars are 25 µm.
)—wall. Scale bars are 25 µm.
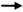 )—megalomeront, short black arrow (
)—megalomeront, short black arrow ( )—cytomere, white arrowhead (
)—cytomere, white arrowhead ( )—wall, asterisk (
)—wall, asterisk ( )—necrosis. Scale bars are 50 µm.
)—necrosis. Scale bars are 50 µm.
 )—megalomeront, short black arrow (
)—megalomeront, short black arrow ( )—cytomere, white arrowhead (
)—cytomere, white arrowhead ( )—wall, asterisk (
)—wall, asterisk ( )—necrosis. Scale bars are 50 µm.
)—necrosis. Scale bars are 50 µm.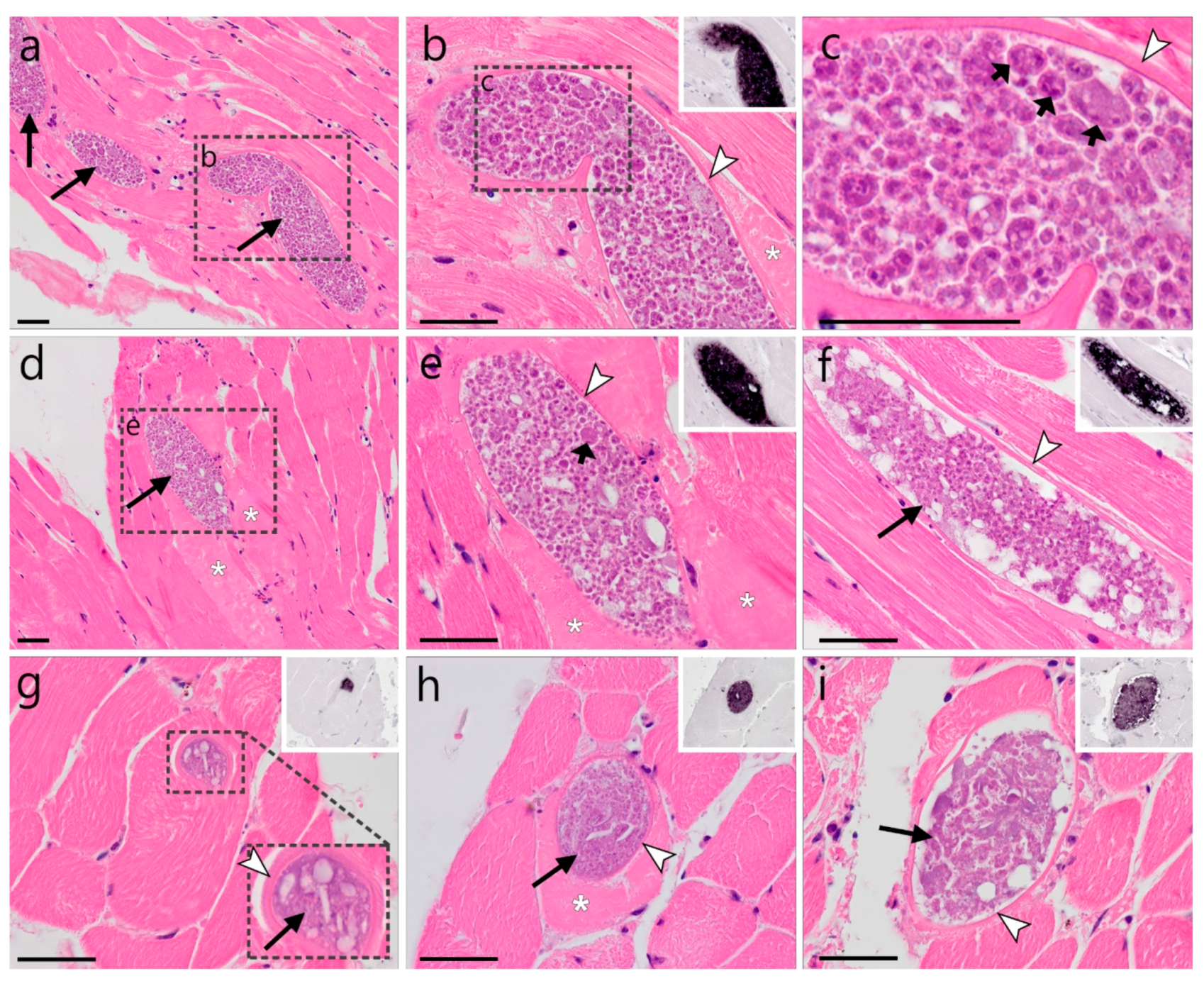
| Host Family and Species | n Examined | Sample Origin (n) | n Positive | Identified cytB Lineages a | |||||
|---|---|---|---|---|---|---|---|---|---|
| Plas | Haem | Leuc | Total (%) | Plasmodium | Haemoproteus | Leucocytozoon | |||
| Strigidae | |||||||||
| Asio flammeus | 1 | AT | 0 | 0 | 1 | 1 (100%) | - | - | L. sp. lASIFLA01 (1) |
| A. otus | 30 | AT (24), LT (6) | 0 | 9 | 18 | 21 (70%) | - | H. noctuae hCIRCUM01 (5); H. sp. hASIOTU04 (2), hASIOTU05 (1) | L. sp. lASOT1 (1); lASOT06 (17), |
| Athene noctua | 3 | AT | 1 | 0 | 1 | 1 (33.3%) | P. relictum pSGS1 (1) | - | L. sp. lATNO1 (1) |
| Bubo bubo | 10 | AT | 0 | 4 | 3 | 5 (50%) | - | H. sp. hSTRURA03 (4) | L. danilewskyi lBUBO01 (2); L. sp. lSTAL3 (1) |
| Glaucidium passerinum | 6 | AT (1), LT (5) | 0 | 1 | 2 | 3 (50%) | - | - | L. sp. lASOT06 (1), lSTAL3 (1) |
| Strix aluco | 39 | AT (17), LT (22) | 0 | 23 | 21 | 34 (87.2%) | - | H. syrnii hSTAL2 (15), hCULKIB01 (11) | L. sp. lSTAL1 (1), lSTAL3 (4), lSTAL5 (4), lSTAL6 (1), lSTAL7 (3), lSTAL8 (1), lSTAL9 (3), lSTAL10 (1) |
| Strix sp. | 1 | AT | 0 | 0 | 0 | 0 (0%) | - | - | - |
| S. uralensis | 29 | AT (26), LT (3) | 1 | 20 | 7 | 24 (82.8%) | P. circumflexum pTURDUS1 (1) | H. syrnii hCULKIB01 (9), hSTAL2 (14); H. belopolskyi hARW1 (1); H. sp. hSTRURA03 (1) | L. cf. californicus lCIAE02 (1); L. sp. lSTAL3 (5), lSTAL7 (1), lSTAL10 (1), |
| Tytonidae | |||||||||
| Tyto alba | 2 | AT (1), LT (1) | 0 | 0 | 0 | 0 (0%) | - | - | - |
| Total | 121 | 2 | 57 | 53 | 89 (73.6) | ||||
| Host Species | Individual | Parasite Species and cytB Lineage | Infected Organs and Distribution of Meronts a,b |
|---|---|---|---|
| Asio otus | AH1539 | L. sp. lASOT06 | heart muscle: meronts (L)—multifocal |
| Strix aluco | AH0145 | L. spp. lSTAL5, lSTAL9 | brain: meronts (L)—oligofocal |
| AH0221 | H. syrnii hCULKIB01, L. sp. lSTAL5 | brain: meronts (L, S)—multifocal kidney: meronts (S)—focal heart muscle: meronts (L, S)—oligofocal | |
| AH0410 | H. syrnii hCULKIB01, hSTAL2, L. sp. lSTAL5, L. sp. (unresolved) | heart muscle: meronts (L)—diffuse | |
| AH1957 | L. sp. lSTAL5, L. sp. (unresolved) | heart muscle: meronts (L)—diffuse | |
| ZA21/018 | H. spp., L. spp. (unresolved co-infection) | heart muscle: meronts (L)—diffuse | |
| S. uralensis | ZA21/071 | H. syrnii hSTAL2 | skeletal muscle: megalomeronts (H)—multifocal |
| ZA21/105 | L. sp. lSTAL7 | kidney: meronts (L)—oligofocal |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilgūnas, M.; Himmel, T.; Harl, J.; Dagys, M.; Valkiūnas, G.; Weissenböck, H. Exo-Erythrocytic Development of Avian Haemosporidian Parasites in European Owls. Animals 2022, 12, 2212. https://doi.org/10.3390/ani12172212
Ilgūnas M, Himmel T, Harl J, Dagys M, Valkiūnas G, Weissenböck H. Exo-Erythrocytic Development of Avian Haemosporidian Parasites in European Owls. Animals. 2022; 12(17):2212. https://doi.org/10.3390/ani12172212
Chicago/Turabian StyleIlgūnas, Mikas, Tanja Himmel, Josef Harl, Mindaugas Dagys, Gediminas Valkiūnas, and Herbert Weissenböck. 2022. "Exo-Erythrocytic Development of Avian Haemosporidian Parasites in European Owls" Animals 12, no. 17: 2212. https://doi.org/10.3390/ani12172212
APA StyleIlgūnas, M., Himmel, T., Harl, J., Dagys, M., Valkiūnas, G., & Weissenböck, H. (2022). Exo-Erythrocytic Development of Avian Haemosporidian Parasites in European Owls. Animals, 12(17), 2212. https://doi.org/10.3390/ani12172212







