1. Introduction
Conformation traits are important in equine breeding as they have been associated with health, longevity and performance [
1,
2,
3,
4,
5,
6,
7,
8]. Conformation is a phenome and encompasses relatively objective traits such as the length of a body segment or an angle between joints, but also highly subjective traits such as the type (breed type, sex type) and the shape of the head, withers, back or croup. Many horse breeding associations perform conformation evaluations at breeding shows by subjective assessment by breeding experts on scoring sheets (e.g., subjective valuating scores, linear profiling) and/or through measurements made on the living animal (e.g., withers height, croup height, limb length). These data commonly provide the basis for the analysis of variance components and subsequent breeding value estimation in European sport horse breeds [
9,
10].
Despite their widespread use, essentially due to their simple implementation, conformation traits based on subjective assessments, including linear profiling, exhibited certain limitations. In many studies, the scale of the scoring scheme was not used in full, leading to poorly distributed data tending towards the optimum, while the lower extreme of the scale was avoided [
11,
12,
13]. Furthermore, the inter-rater reliability between experts on the breed (judges) who routinely assessed conformation traits in horses was highly variable depending on the breed: the repeated assessments of linear profiling traits in the Pura Raza Español were highly correlated based on intra-class correlation coefficients (ICCs; 0.96 < ICC < 0.99) [
14], indicating reliable data. However, the repeatability of conformation traits that were judged on 4306 Finnhorse and 294 Standardbred trotter foals was estimated using a correlation coefficient (r) that only ranged from 0.06 to 0.48 in Standardbred trotters and from 0.24 to 0.38 in Finnhorses [
15], respectively. Furthermore, multiple experts simultaneously assessing the same Lipizzaner horses showed poor inter-rater reliabilities based on the kappa statistic (0.06 < κ < 0.49) [
16,
17]. The reliability of expert scores is seldom reported as many horse breed associations only assess the conformation of horses once in their lifetimes. Hence, specific reliability studies are rare, making it difficult to assess the quality of the scoring data.
The Franches-Montagnes (FM) horse is the last native Swiss breed. In its history that spans over one hundred years, the breed has evolved from a draught horse used for tilling fields to the light draught horse breed used for leisure known today [
18]. To be able to perform these different functions, the conformation of the FM had to adapt over time, and conformation traits were used empirically to move towards modern breeding goals. Since 1990, three-year-old FM horses have been presented in hand and scored for 19 conformation and five locomotor traits on a linear profiling scale by experts of the breed. However, the distribution of the scores does not always follow a normal distribution, with the lowest scores being avoided, and the mean tending towards the perceived optimum (9) instead of the scale median (5) [
19]. The same trend could be observed for gait quality traits [
20,
21]. Furthermore, FM experts scoring the same horses simultaneously, either in hand or on the treadmill over video recordings, had poor agreement (ICC < 0.50) for all scored gait quality traits [
20,
21]. The heritability for conformation traits in the FM ranged from 0.09 (length of shoulder, hind limb muscle) to 0.79 (height at withers, measured) [
22].
The heritability of conformation traits naturally depends on the breed, the sample size used for the calculation, the method of data collection (subjective evaluation, linear profiling score or measurement), and the underlying genetic architecture. However, some tendencies are similar between breeds. Based on a meta-analysis of 30 studies on genetic parameter estimates of conformation traits in horses, height at croup and height at withers had the highest heritability for measured conformation traits (h
2 = 0.61 for height at croup and h
2 = 0.58 for height at withers) and were highly genotypically correlated (r
g = 0.94) [
23]. The size of the horse is routinely measured, with data available from many horses and breeds, and the genetic architecture has also been shown to be highly responsive to selection as most of the variation in the height at withers is essentially determined by four genes [
24]. Furthermore, objective measures are generally more heritable compared to corresponding scored traits [
23]. This may be at least partially due to lower subjectivity in the data collection. However, one key source of error in measuring conformation traits is the landmark definition, i.e., which anatomical structures should be used as reference points, and whether they can be easily identified [
25].
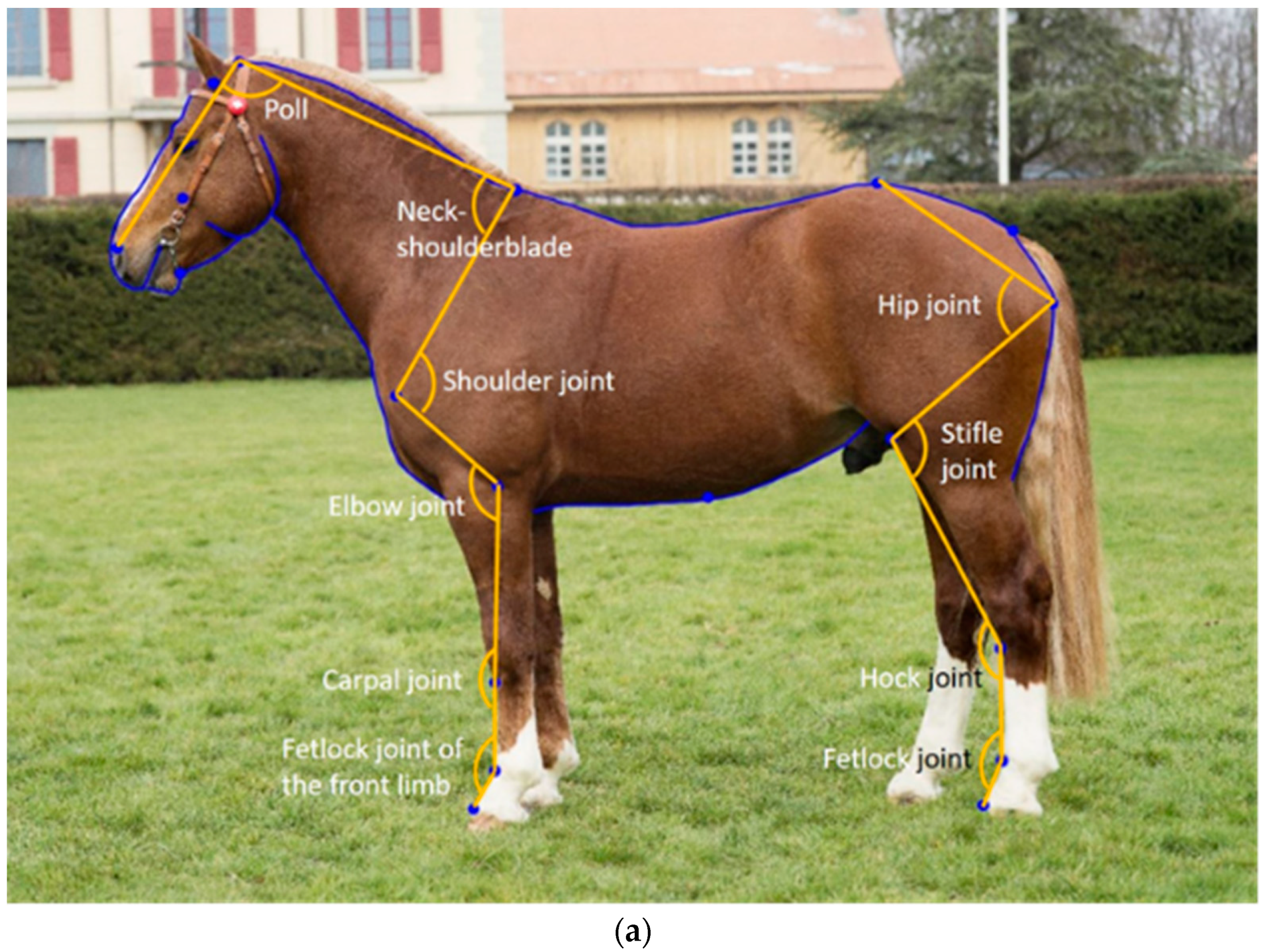
The horse shape space model proposed by Druml et al. [
16] uses standardized photographs to extract shape data from landmarks on fixed anatomical structures, and semi-landmarks equidistantly placed on curves so that they can then be analysed as landmarks. This method was first developed in the Lipizzaner [
16,
17] and then applied to the FM horse [
26]. While only the overall shape was initially analysed, joint angle measurements were later extracted from the initial landmarks. When comparing linear profiling traits describing, e.g., the shoulder or croup incline with objectively measured joint angles from the horse shape space model, the two corresponding traits were not significantly associated, suggesting that the expert scores do not represent the variation that is objectively quantified using the horse shape space model [
26]. In the initial horse shape space model [
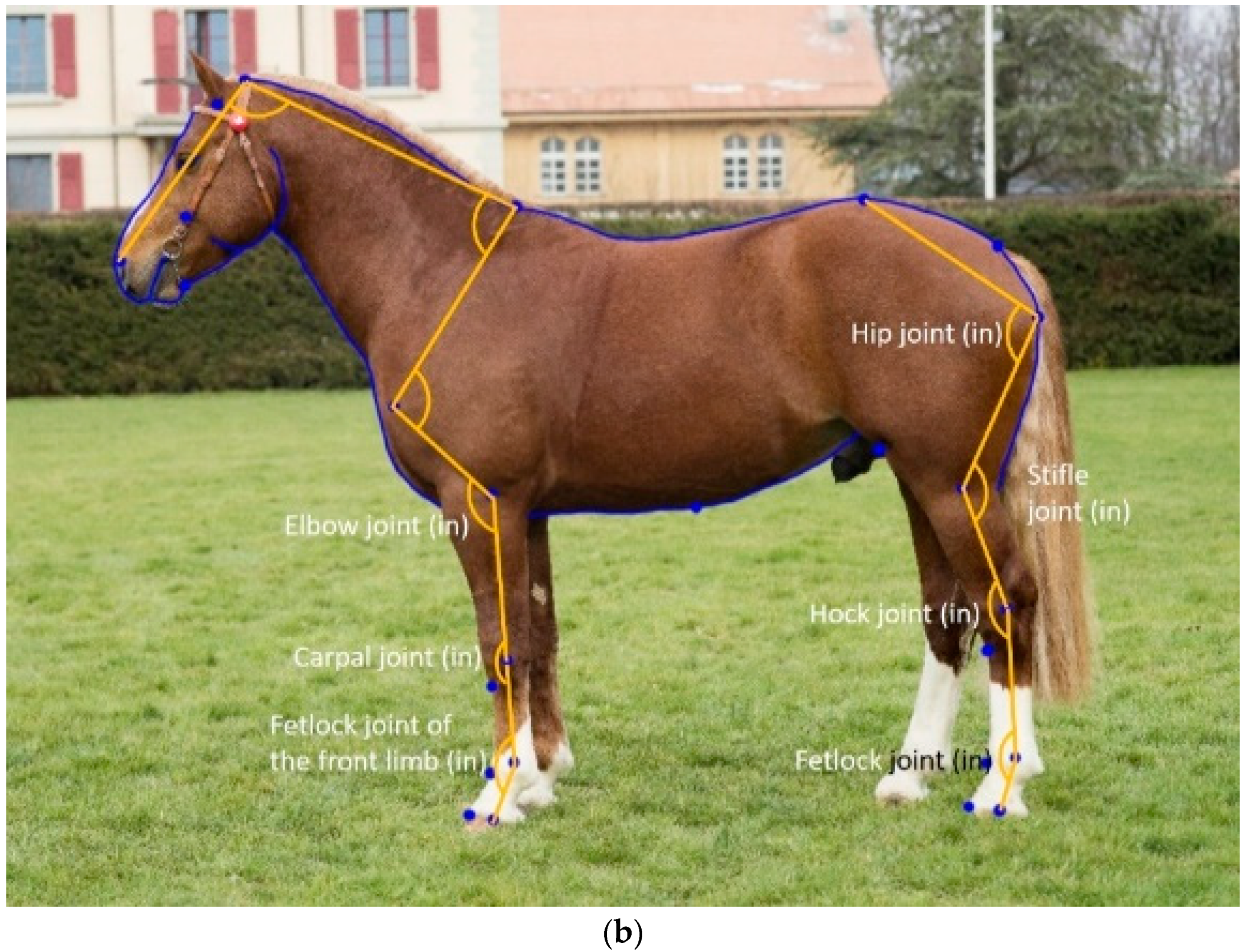
16], the majority of landmarks used to extract joint angle measurements were placed on the surface of the horse (i.e., in front of the joint), as that made them easier to place. However, placing the landmarks in the centre of the joint is expected to improve the predictive relationship between conformation and joint movement, provided they can be placed as accurately as those placed in front of the joints can.
The aims of the study were to estimate variance components and heritability for conformation traits extracted from the horse shape space model in the FM horse breed. Using different landmark settings, joint angles were evaluated both for their heritability and repeatability. Finally, the evolution of the conformation of the FM breed was visualized for the period from 1940 to 2018.
4. Discussion
The joint angles with the highest heritability (h
2) were the poll and the fetlock joint of the front limb (
Table 5). The high h
2 of the poll angle was consistent with results from a multi-breed genome-wide association study of the same joint angles from 300 FM and 224 Lipizzaner horse photographs [
31]. The best-associated quantitative trait locus (QTL) was the poll angle on equine chromosome (ECA) 28, near the gene
ALX1, associated with cranial morphology [
32]. The genome-wide h
2 for the poll angle in the two breeds was h
2 = 0.38, nearly equal to the pedigree-based h
2 estimated here. In contrast, the highest genome-wide h
2 for Lipizzaner and FM was for the fetlock joint of the hind limb (h
2 = 0.58) and a suggestive QTL on ECA 27, whereas this trait had a much lower pedigree-based h
2 = 0.19 in the FM.
The placement of the landmarks for the calculation of the joint angles changed the acuteness of the joint angles. For all pairs of joint angles, the landmark placement inside the joint was significantly larger (less acute) than when the landmarks were placed in front of the joint (old model). However, the phenotypic correlation is more relevant to understand whether the landmark placement affects the anatomical meaning of the measurement. For example, the elbow joint remained virtually identical whether the third landmark was placed in front or within the carpal joint, as shown by near-perfect genetic and phenotypic correlations between the two elbow joint measurements (
Table 6), and a nearly identical repeatability (ICC,
Table 2) and h
2 (
Table 5). The two types of elbow joint angles were also significantly affected by the same combination of posture and other external variables (age, sex and year of birth,
Table 3). For this angle, it makes no difference which landmark placement to use. The two types of carpal joint angles were the least phenotypically correlated of the paired joint angles (
Table 6), the angle from the old model (landmark in front of the joint) was significantly different between mares and stallions, while with the new landmark placement inside the joint, the sex difference was between mares and geldings (
Table 4). The heritability for the new landmark placement was higher (
Table 5), with a nearly identical ICC (
Table 2). In this case, it makes more sense to measure the carpal joint with the new landmark placement. For the fetlock angle of the front limb, h
2 (
Table 5) and ICC (
Table 2) were higher with the new landmark placement, affected by the age of the horse and not by the birth year (
Table 4), as was the case for fetlock angle measured with the old landmark placement. For the joint angles of the front limb (elbow, carpus and fetlock), the new landmark placement increases h
2 (
Table 5) and ICC (or at least does not negatively affect the latter;
Table 2).
The trends were less clear in the hind limbs. For the stifle and hock, ICC (
Table 2) and h
2 (
Table 5) concurred, so that the angle that was measured the most accurately was also the one with the highest h
2 (stifle with landmarks within the joints, hock with landmarks outside the joints). The results were more difficult to interpret for the hip and fetlock joint of the hind limb. For the hip joint, the ICC was only slightly higher, but the h
2 lower, when using the centre of the stifle joint as the third landmark. However, the hip joint angle measured with the landmarks in front of the patella was additionally affected by the head height and the sex (mares were significantly different from geldings;
Table 4) which suggests that the angle is more affected by the posture (i.e., environmental effects). While the ICC was lower for the fetlock joint of the hind limb (
Table 2), the h
2 was higher when using the landmarks in front of the joints (
Table 5). Deciding which measurement is better for a particular joint is complicated in the case when h
2 and ICC are not in lockstep. Another way to choose the most informative landmark placement might be to compare the joint angles with kinematic parameters, to assess whether one set of landmarks is a better predictor for movements associated with gait quality traits such as hind limb protraction [
33], i.e., whether a certain joint angle measurement is more functionally relevant. Considering the low amount of additional effort involved in placing the supplementary landmarks, we currently recommend assessing the ICC and h
2 of both types of joint angle measurements in other breeds using the two landmark settings, in order to optimise the results.
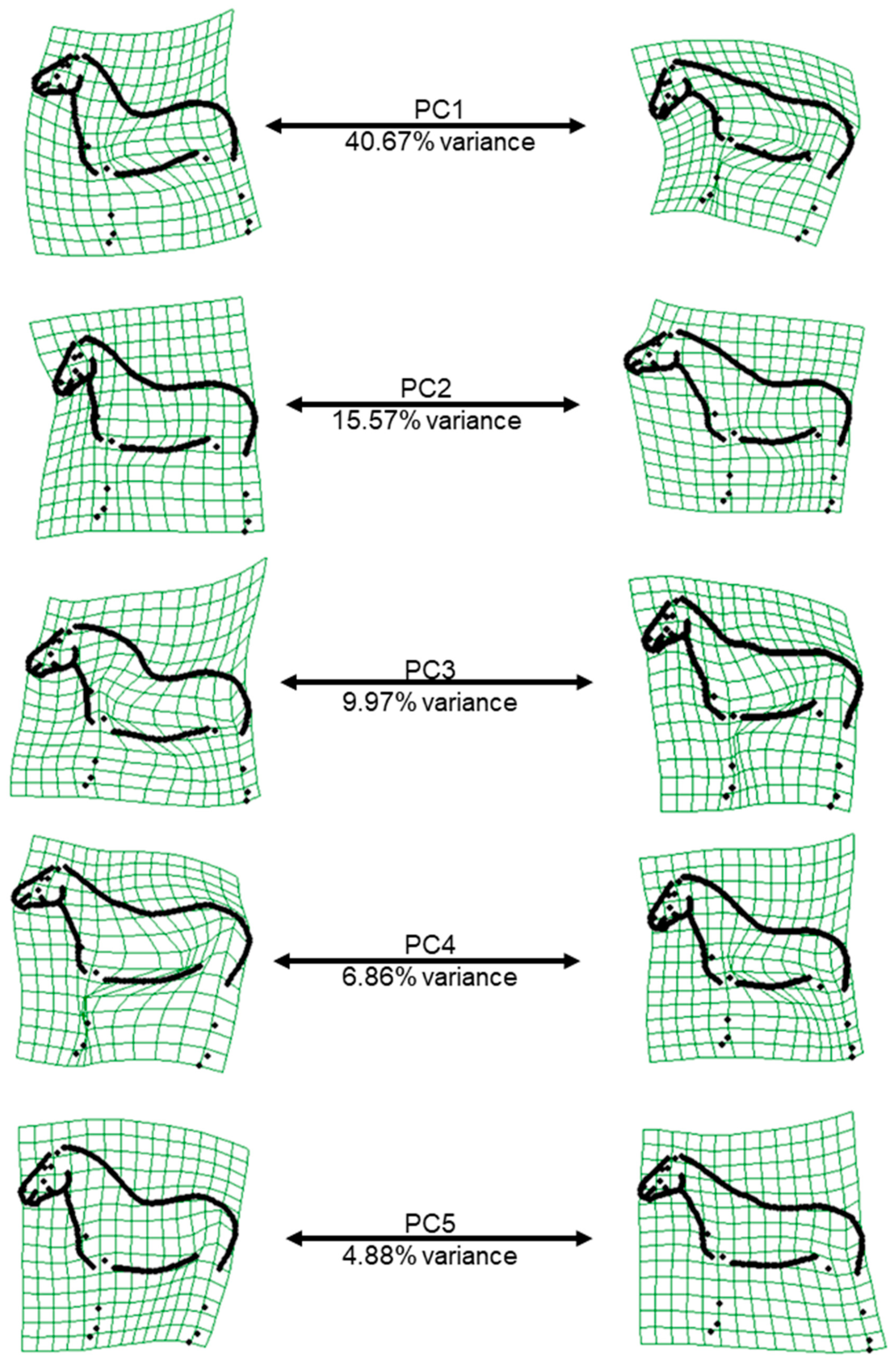
The first and second relative warp scores (PC1 and PC2) represented the shape variation induced by the head-neck position (PC1: head height, PC2: flexion-extension at the poll,
Figure 2), which is why the posture variable head height was so strongly associated with PC1. For PC2 (as well as the poll angle), when the horse has its head turned towards the camera, the angle decreases, which explains the significant association between the variable “head in relation to camera” with PC2 and poll angle. In practice, the variation due to the head and neck position is hard to avoid, and this result was consistent with several previous horse shape space studies on FM and Lipizzaner horses [
16,
17,
26]. While PC1 and PC2 explain most of the shape variation, they are less heritable than the three following PCs, reflecting the fact that the variation in shape mainly originates from the posture, which is an “environmental” effect. The moderate h
2 of PC3, PC4 and PC5 is consistent with previous findings that the body type (heavy–light), quantified by bone thickness up to now, is the second highest source of conformational variation in the horse after height [
34]. However, these PCs are also affected by posture, especially by the position of the front and hind limbs. Therefore, posture variables should always be considered when working with conformational data, although considering all the posture variables as fixed effects in the model of analysis might have caused an over-parameterization of the model, thereby affecting the accuracy of the estimates. The majority of additive genetic, phenotypic and residual variances were large (>1;
Table 5), in contrast to other studies that included several thousand horses to estimate genetic parameters [
12,
13,
14,
15,
23]. Furthermore, the standard errors for the genetic correlations, in particular, were large as well (
Table 6 and
Table 8). Reducing the postural variance in the data when photographing the horses and increasing the sample size should improve the accuracy of the estimates on the long term.
The current accuracy of data extracted from the horse shape space model still shows potential for selection on conformation traits based on this method, especially if a horse breeding association routinely records these traits. Of the 19 linear conformation traits routinely assessed by breeding experts in the FM, five describe joint angles we quantified here. We can therefore compare our h
2 estimated for measured joint angles on 608 horses against the h
2 estimates for 18,297 horses tested between 1994 and 2013 [
22]. The scored trait “shoulder incline” (straight–inclined) had h
2 = 0.09 [
22], while the measured shoulder joint angle’s h
2 was twice as high (
Table 5). The trait “front limb” (back-at-the-knee–over-at-the-knee) had h
2 = 0.14 [
22], compared to the measured carpal joint angles (h
2 = 0.13 measured in front of the joint, h
2 = 0.27 within the joint), suggesting that this trait has the potential for improvement when using the measurement within the joint. As the range of the measurement is limited for both carpal joint angles, any slight error in landmark placement has a disproportionate effect on the accuracy of the measurement, as is shown by the lower ICC compared to, e.g., the hip joint angles (
Table 2). The scored “croup incline” (horizontal–sloping) had h
2 in the same range as the hip joint angles (h
2 = 0.20 [
22]). The measured hock joint angles exhibited a lower h
2 than the “hock angle” trait (straight–angulated, h
2 = 0.19, [
22]). These traits were also not associated with each other in a previous direct comparison between measurements and scores [
26]. In this case, the linear profiling score may be considered more useful for selection than the hock joint measurement. The final linear profiling trait with an equivalent joint angle is the “fetlock angle” (straight–weak). However, whether this score described the front limbs only, or a combination of front and hind limbs, is not specified. Both measurements of the fetlock joint of the front limb had a higher h
2 than the scored “fetlock angle” (h
2 = 0.11, [
22]), while the fetlock joint of the hind limb was less heritable when measured within the joint, and more heritable when measured in front of the joint (
Table 5). At minimum, selection on shoulder and fetlock angles might therefore be improved by applying the horse shape space model.
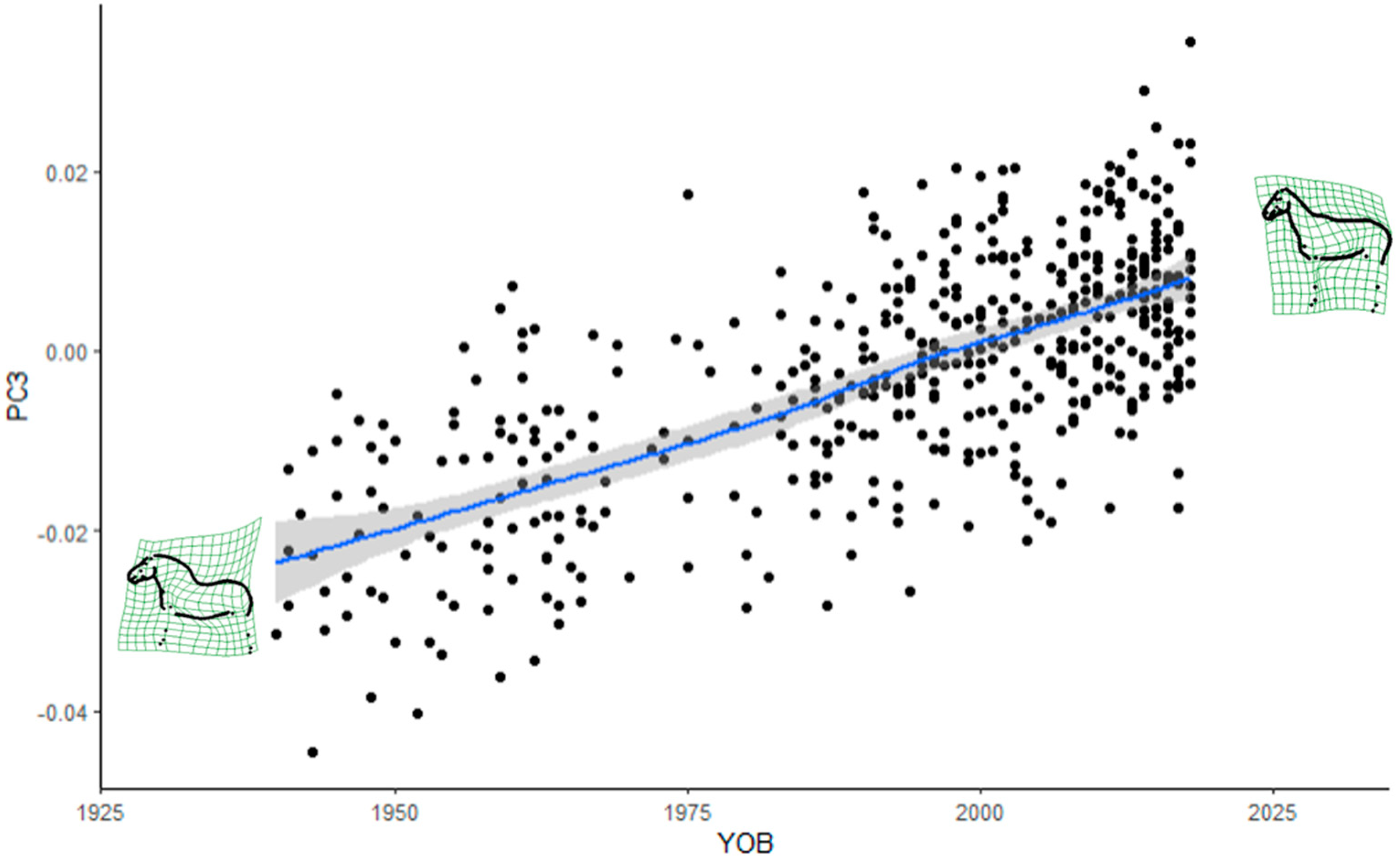
Furthermore, it was demonstrated that horse shape space data can be successfully applied to assess the evolution of a breed over time. The FM stallion population evolved from a heavy to a light draught horse type. The absence of a plateau in more recent years is a cause for concern, as the breed may lose its breed-specific type of a light draught horse. Some changes in the trajectories of the trend lines seemed to coincide with recent introgressions from lighter breeds. More specifically, the changes in the poll angle starting in the 1980s may be a consequence of the Swedish Warmblood introgression in the 1970s, while changes in most of the measurements from the 1990s onward in most of the measurements may be due to the two Swiss Warmblood stallions that were introgressed in 1990. The current favoured use of stallions with high admixture proportions from the last introgressions in FM breeding may accelerate the observed changes in conformation.
Apart from the small sample size, there were some additional limitations to our study. Some stallions from the 1940s and 1950s were only distantly related to the more recent population, which might affect estimates of the variance components due to gaps in the pedigree. Furthermore, all the horses born before 2004 were stallions, due to the low availability of mare photographs in the archives of the Swiss National Stud Farm. However, this allowed us to increase our sample size and to retrace the evolution of the FM conformation traits over time. Whether the FM breeding association will implement the horse shape space model in their selection programme depends on several points: social acceptance by the breeders, practical considerations (time to take the appropriate photographs) and technical feasibility (automation of landmark placement). One further limitation to this study is that we could not provide in-depth analysis of the type of conformation favourable to specific disciplines, as was investigated in other morphometric studies [
35,
36]. This could be the subject of a future study.