Impact of Refinements to Handling and Restraint Methods in Mice
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Housing
2.2. Experiment 1: Technician Evaluation of Different Handling Methods
2.3. Experiment 2: Evaluation of a Modified Non-Tail Restraint Method
2.4. Voluntary Interaction Test
2.5. Elevated Zero Maze (EZM)
2.6. Enzyme Immunoassay of Serum Corticosterone (CORT)
2.7. Conditioned Place Preference (CPP)
2.8. Cotton Bud Biting Test
2.9. Data Analysis
3. Results
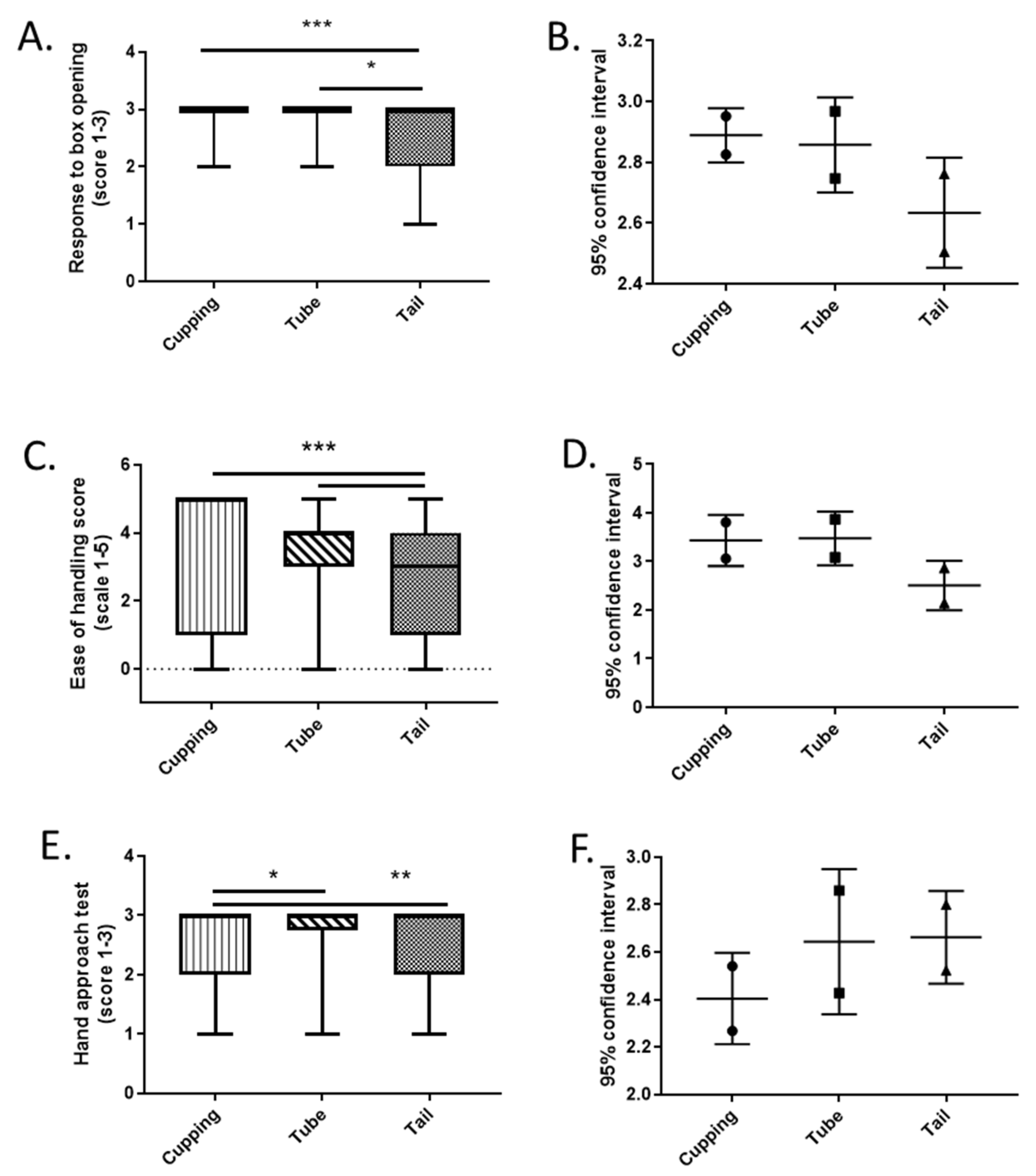
3.1. Experiment 1: Effect of Off-Site Breeding Facility Handling Methods on Overt Behaviours at Destination Unit
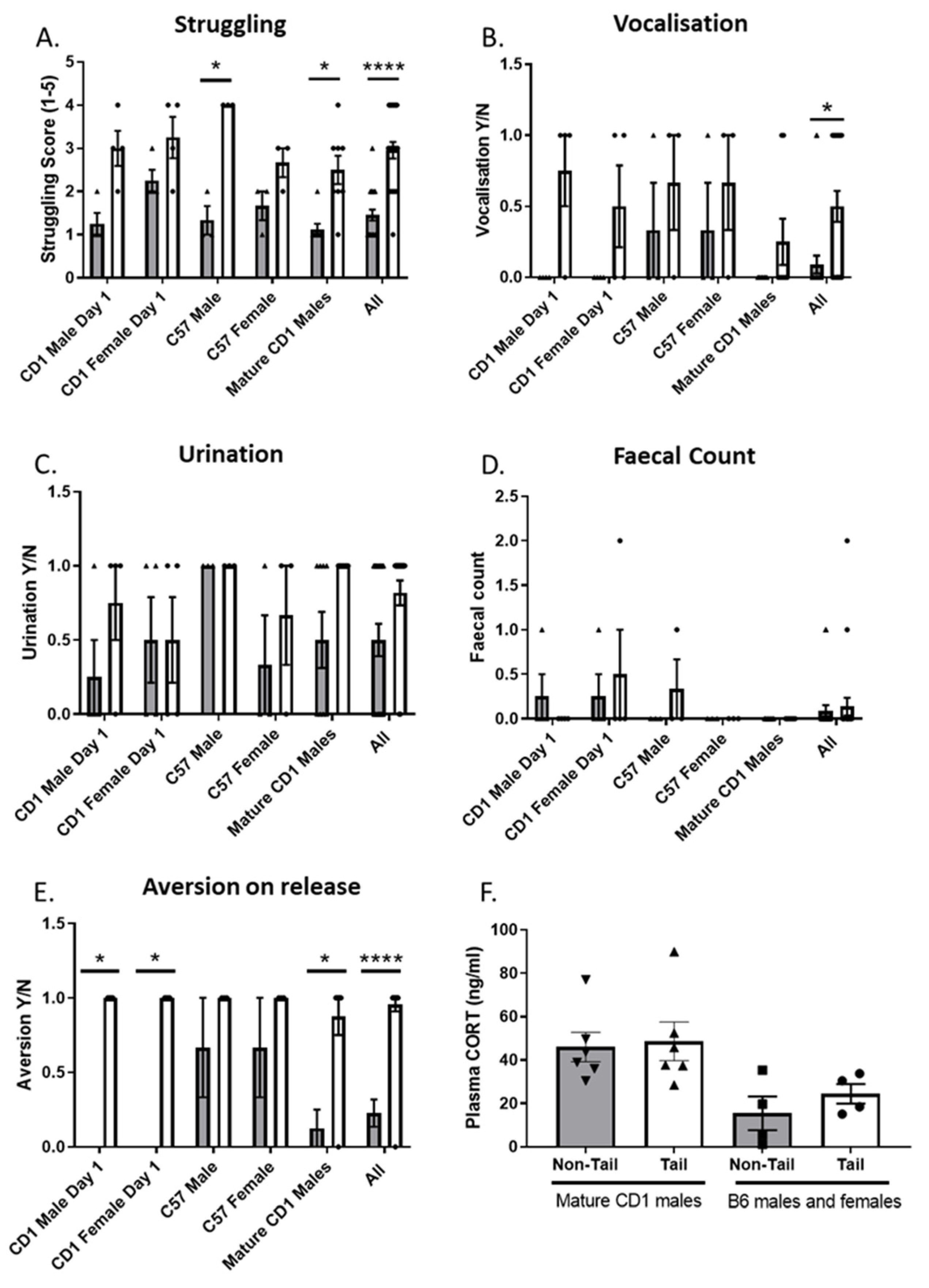
3.2. Experiment 2: Modified Restraint Method Reduces Overt Behaviours Associated with Aversion and Stress
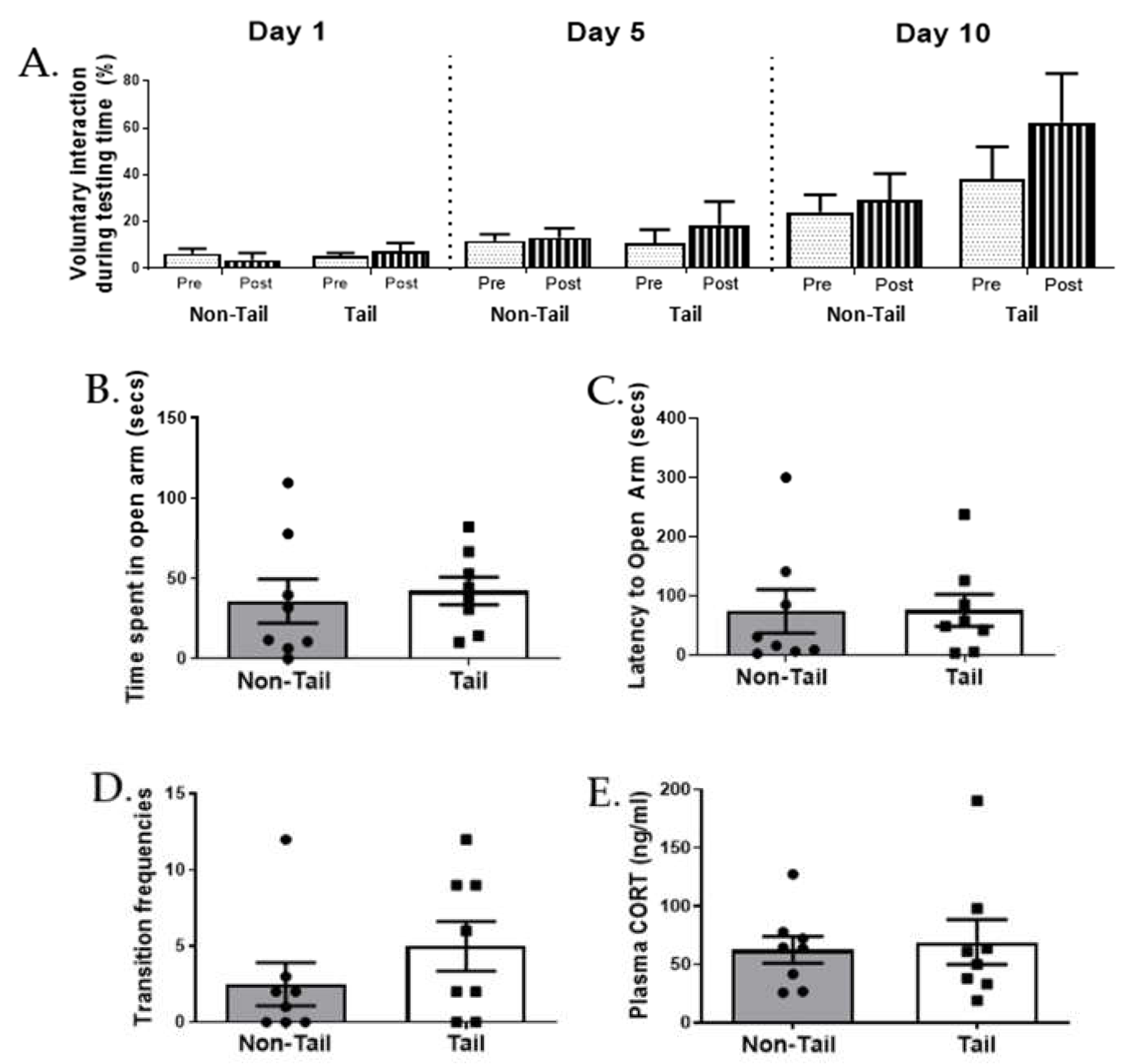
3.3. Tail versus Non-Tail Restraint Had No Effect on Voluntary Interaction
3.4. Different Restraint Methods Have No Effect on Behavioural Measures of Anxiety or Aggression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stuart, S.A.; Robinson, E.S.J. Reducing the stress of drug administration: Implications for the 3Rs. Sci. Rep. 2015, 5, 14288. [Google Scholar] [CrossRef] [PubMed]
- Keim, K.L.; Sigg, E.B. Physiological and biochemical concomitants of restraint stress in rats. Pharmacol. Biochem. Behav. 1976, 4, 289–297. [Google Scholar] [CrossRef]
- Chiba, S.; Numakawa, T.; Ninomiya, M.; Richards, M.C.; Wakabayashi, C.; Kunugi, H. Chronic restraint stress causes anxiety- and depression-like behaviors, downregulates glucocorticoid receptor expression, and attenuates glutamate release induced by brain-derived neurotrophic factor in the prefrontal cortex. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2012, 39, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Harbuz, M.S.; Jessop, D.S.; Lightman, S.L.; Chowdrey, H.S. The effects of restraint or hypertonic saline stress on corticotrophin-releasing factor, arginine vasopressin and proenkephalin A mRNAs in the CFY, Sprague-Dawley and Wistar strains of rat. Brain Res. 1994, 667, 6–12. [Google Scholar] [CrossRef]
- Hurst, J.L.; West, R.S. Taming anxiety in laboratory mice. Nat. Methods 2010, 7, 825–826. [Google Scholar] [CrossRef]
- Heinrichs, S.C. Neurobehavioral consequences of stressor exposure in rodent models of epilepsy. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2010, 34, 808–815. [Google Scholar] [CrossRef]
- Gouveia, K.; Hurst, J.L. Improving the practicality of using non-aversive handling methods to reduce background stress and anxiety in laboratory mice. Sci. Rep. 2019, 9, 20305. [Google Scholar] [CrossRef]
- Nakamura, Y.; Suzuki, K. Tunnel use facilitates handling of ICR mice and decreases experimental variation. J. Vet. Med. Sci. 2018, 80, 886–892. [Google Scholar] [CrossRef]
- Ghosal, S.; Nunley, A.; Mahbod, P.; Lewis, A.G.; Smith, E.P.; Tong, J.; D’Alessio, D.A.; Herman, J.P. Mouse handling limits the impact of stress on metabolic endpoints. Physiol. Behav. 2015, 150, 31–37. [Google Scholar] [CrossRef]
- Clarkson, J.M.; Dwyer, D.M.; Flecknell, P.A.; Leach, M.C.; Rowe, C. Handling method alters the hedonic value of reward in laboratory mice. Sci. Rep. 2018, 8, 2448. [Google Scholar] [CrossRef]
- Gouveia, K.; Hurst, J.L. Reducing mouse anxiety during handling: Effect of experience with handling tunnels. PLoS ONE 2013, 8, e66401. [Google Scholar]
- Gouveia, K.; Hurst, J.L. Optimising reliability of mouse performance in behavioural testing: The major role of non-aversive handling. Sci. Rep. 2017, 7, 44999. [Google Scholar] [CrossRef] [PubMed]
- Mertens, S.; Vogt, M.A.; Gass, P.; Palme, R.; Hiebl, B.; Chourbaji, S. Effect of three different forms of handling on the variation of aggression-associated parameters in individually and group-housed male C57BL/6NCrl mice. PLoS ONE 2019, 14, e0215367. [Google Scholar] [CrossRef] [PubMed]
- Spink, A.J.; Tegelenbosch, R.A.J.; Buma, M.O.S.; Noldus, L.P.J.J. The EthoVision video tracking system—A tool for behavioral phenotyping of transgenic mice. Physiol. Behav. 2001, 73, 731–744. [Google Scholar] [CrossRef]
- Friard, O.; Gamba, M. BORIS: A free, versatile open-source event-logging software for video/audio coding and live observations. Methods Ecol. Evol. 2016, 7, 1325–1330. [Google Scholar] [CrossRef]
- Spiga, F.; Harrison, L.R.; Wood, S.A. Effect of the glucocorticoid receptor antagonist Org 34850 on basal and stress-induced corticosterone secretion. J. Neuroendocrinol. 2007, 19, 891–900. [Google Scholar] [CrossRef]
- Park, S.J.; Lee, J.Y.; Kim, S.J.; Choi, S.; Yune, T.Y.; Ryu, J.H. Toll-like receptor-2 deficiency induces schizophrenia-like behaviors in mice. Sci. Rep. 2015, 5, 8502. [Google Scholar] [CrossRef]
- Litvin, Y.; Blanchard, D.C.; Pentkowski, N.S.; Blanchard, R.J. A pinch or a lesion: A reconceptualization of biting consequences in mice. Aggress. Behav. 2007, 33, 545–551. [Google Scholar] [CrossRef]
- Gould, T.D.; Dao, D.T.; Kovacsics, C.E. Mood and Anxiety-related Phenotypes in Mice: Characterization Using Behavioral Tests; Gould, T.D., Ed.; Humana Press: Totowa, NJ, USA, 2009; pp. 1–20. [Google Scholar]
- Bourin, M.; Petit-Demouliere, B.; Dhonnchadha, B.N.; Hascoet, M. Animal models of anxiety in mice. Fundam. Clin. Pharmacol. 2007, 21, 567–574. [Google Scholar] [CrossRef]
- Augustsson, H.; Dahlborn, K.; Meyerson, B.J. Exploration and risk assessment in female wild house mice (Mus musculus musculus) and two laboratory strains. Physiol. Behav. 2005, 84, 265–277. [Google Scholar] [CrossRef]
- Roy, V.; Merali, Z.; Poulter, M.O.; Anisman, H. Anxiety responses, plasma corticosterone and central monoamine variations elicited by stressors in reactive and nonreactive mice and their reciprocal F1 hybrids. Behav. Brain Res. 2007, 185, 49–58. [Google Scholar] [CrossRef]
- Paul, E.S.; Harding, E.J.; Mendl, M. Measuring emotional processes in animals: The utility of a cognitive approach. Neurosci. Behav. Rev. 2005, 29, 469–491. [Google Scholar] [CrossRef] [PubMed]
- Buwalda, B.; Scholte, J.; de Boer, S.F.; Coppens, C.; Koolhass, J.M. The acute glucocorticoid stress response does not differentiate between rewarding and aversive social stimuli in rats. Horm. Behav. 2012, 61, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Tuli, J.S.; Smith, J.A.; Morton, D.B. Stress measurements in mice after transportation. Lab. Anim. 1995, 29, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Michalikova, S.; van Rensburg, R.; Chazot, P.L.; Ennaceur, A. Anxiety responses in Balb/c, c57 and CD-1 mice exposed to a novel open space test. Behav. Brain Res. 2010, 207, 402–417. [Google Scholar] [CrossRef] [PubMed]
- Parmigiani, S.; Palanza, P.; Rodgers, J.; Ferrari, P.F. Selection, evolution of behavior and animal models in behavioral neuroscience. Neurosci. Biobehav. Rev. 1999, 23, 957–970. [Google Scholar] [CrossRef]
- Robinson, E.S.J. Translational new approaches for investigating mood disorders in rodents and what they may reveal about the underlying neurobiology of major depressive disorder. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018, 373, 1742. [Google Scholar] [CrossRef]
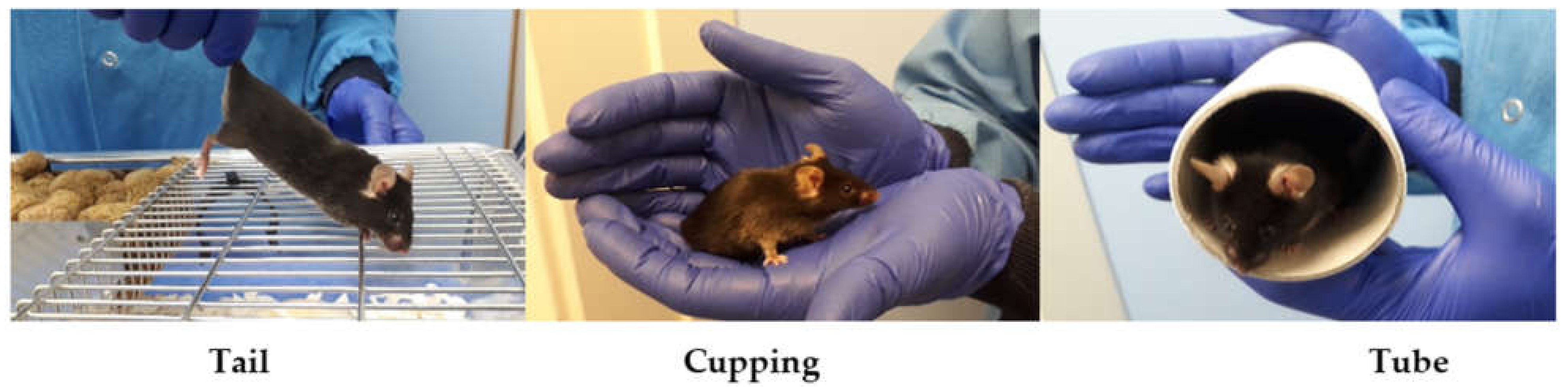
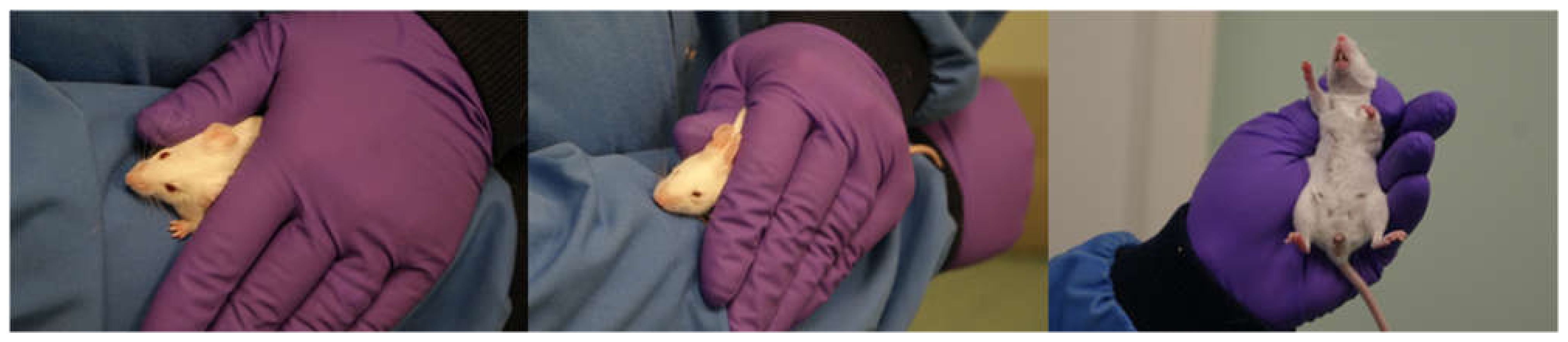

| Cohort | Test (in Order) | Strain | Age | Male | Female | Housing |
|---|---|---|---|---|---|---|
| CD1 | 8 months | 16 | 16 | group (2) | ||
| Modified Restraint Method | mature CD1 | 11 months | 8 | 0 | singly | |
| A | B6 | 8 months | 6 | 6 | singly | |
| Modified Restraint CORT | B6 | 8 months | 6 | 6 | singly | |
| mature CD1 | 11 months | 8 | 0 | singly | ||
| subset A | Voluntary interaction test | CD1 | 8 months | 8 | 8 | group (2) |
| Elevated zero maze | ||||||
| Elevated zero maze CORT | ||||||
| B | Conditioned place preference | mature CD1 | 11 months | 12 | 0 | singly |
| C | Cotton bud test | CD1 | mixed age | 62 | 0 | singly/group |
| B6 | mixed age | 16 | 16 |
| Initial Assessment Upon Box Opening | Hand Approach Test | Ease of Handling |
|---|---|---|
| 3 = calm, minimal response 2 = some agitation 1 = obvious escape attempts | 3 = one or more animals approach hand 2 = no obvious response 1 = active avoidance | 5 = easy 4 = mild escape attempts 3 = moderate escape attempts 2 = escaped1 = jumped from hand 0 = not possible to cup |
| Visual Observations | |
|---|---|
| Struggling effort to be released from grip | |
| (1) No struggling once restrained | |
| (2) Slight struggling for a short period of time | |
| (3) Slight struggling throughout/moderate struggling for a short period | |
| (4) Moderate struggling throughout/severe struggling for a short period | |
| (5) Severe struggling throughout | |
| Vocalisations made during grip | Yes = 1, No = 0 |
| Urination during or after grip | Yes = 1, No = 0 |
| Escape behaviour (indicated by running or avoiding hands) | Yes = 1, No = 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davies, J.R.; Purawijaya, D.A.; Bartlett, J.M.; Robinson, E.S.J. Impact of Refinements to Handling and Restraint Methods in Mice. Animals 2022, 12, 2173. https://doi.org/10.3390/ani12172173
Davies JR, Purawijaya DA, Bartlett JM, Robinson ESJ. Impact of Refinements to Handling and Restraint Methods in Mice. Animals. 2022; 12(17):2173. https://doi.org/10.3390/ani12172173
Chicago/Turabian StyleDavies, Jennifer R., Dandri A. Purawijaya, Julia M. Bartlett, and Emma S. J. Robinson. 2022. "Impact of Refinements to Handling and Restraint Methods in Mice" Animals 12, no. 17: 2173. https://doi.org/10.3390/ani12172173
APA StyleDavies, J. R., Purawijaya, D. A., Bartlett, J. M., & Robinson, E. S. J. (2022). Impact of Refinements to Handling and Restraint Methods in Mice. Animals, 12(17), 2173. https://doi.org/10.3390/ani12172173






