Dosage Compensation of the X Chromosome during Sheep Testis Development Revealed by Single-Cell RNA Sequencing
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Care
2.2. Reagents
2.3. Animals and Sample Collection
2.4. Preparation of Single Testicular Cell Suspension
2.5. Single-Cell RNA-Sequencing Performance, Library Preparation and Sequencing
2.6. Mapping, Cell Identification and Clustering Analysis
2.7. Calculating Relative RNA Content from Each Cell Type
2.8. Comparing RNA Output by Chromosome and Spermatogenic Stage
2.9. Immunofluorescence
3. Results
3.1. Mapping, Cell Identification and Clustering Analysis
3.2. X and Autosomal Total Transcription
3.3. X Chromosome and Autosomal Relative RNA Production
3.4. MSL Expression in Germ Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, K.; Zeng, Q.; Feng, Y.; Hu, Y.; An, Q.; Li, T.; Qin, L.J.; Liu, J.Y.; Xue, Z.; Fan, G. X-chromosome dosage compensation dynamics in human early embryos. bioRxiv 2020. [Google Scholar] [CrossRef]
- Ohno, S. Sex Chromosomes and Sex-Linked Genes; Springer: Berlin/Heidelberg, Germany, 1966. [Google Scholar]
- Julien, P.; Brawand, D.; Soumillon, M.; Necsulea, A.; Liechti, A.; Schütz, F.; Daish, T.; Grützner, F.; Kaessmann, H.; Barton, N.H. Mechanisms and Evolutionary Patterns of Mammalian and Avian Dosage Compensation. PLoS Biol. 2012, 10, e1001328. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.E.; Flock, K.; Jue, N.; Zhang, M.; Jones, A.; Al Seesi, S.; Mandoiu, I.; Pillai, S.; Hoffman, M.; O’Neill, R. Dosage Compensation and Gene Expression of the X Chromosome in Sheep. G3 Genesgenetics 2018, 9, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Di, K.N.; Disteche, C.M. Dosage compensation of the active X chromosome in mammals. Nat. Genet. 2006, 38, 47–53. [Google Scholar]
- Duan, J.E.; Jue, N.K.; Jiang, Z.; O’Neill, R.; Tian, X.C. 144 Dosage Compensation and X-Linked Gene Expression in Bovine In Vivo and In Vitro Embryos. Reprod. Fertil. Dev. 2015, 28, 202. [Google Scholar] [CrossRef]
- Gupta, V.; Parisi, M.; Sturgill, D.; Nuttall, R.; Doctolero, M.; Dudko, O.K.; Malley, J.D.; Eastman, P.S.; Oliver, B. Global analysis of X-chromosome dosage compensation. J. Biol. 2006, 5, 3. [Google Scholar] [CrossRef]
- Deng, X.; Hiatt, J.B.; Nguyen, D.K.; Ercan, S.; Sturgill, D.; Hillier, L.W.; Schlesinger, F.; Davis, C.A.; Reinke, V.J.; Gingeras, T.R.; et al. Evidence for compensatory upregulation of expressed X-linked genes in mammals, Caenorhabditis elegans and Drosophila melanogaster. Nat. Genet. 2011, 43, 1179–1185. [Google Scholar] [CrossRef]
- Kharchenko, P.V.; Xi, R.; Park, P.J. Evidence for dosage compensation between the X chromosome and autosomes in mammals. Nat. Genet. 2011, 43, 1167–1169. [Google Scholar] [CrossRef]
- Goto, T.; Monk, M. Regulation of X-Chromosome Inactivation in Development in Mice and Humans. Microbiol. Mol. Biol. Rev. 1998, 62, 362. [Google Scholar] [CrossRef]
- Shi, Z.; Lim, C.; Tran, V.; Cui, K.; Zhao, K.; Chen, X. Single-cyst transcriptome analysis of Drosophila male germline stem cell lineage. Development 2020, 147, dev.184259. [Google Scholar] [CrossRef]
- Witt, E.; Shao, Z.; Hu, C.; Krause, H.M.; Zhao, L. Single-cell RNA-sequencing reveals pre-meiotic X-chromosome dosage compensation in Drosophila testis. PLoS Genet. 2021, 17, e1009728. [Google Scholar] [CrossRef] [PubMed]
- Argyridou, E.; Parsch, J. Regulation of the X Chromosome in the Germline and Soma of Drosophila melanogaster Males. Genes 2018, 9, 242. [Google Scholar] [CrossRef] [PubMed]
- Meiklejohn, C.D.; Landeen, E.L.; Cook, J.M.; Kingan, S.B.; Presgraves, D.C. Sex Chromosome-Specific Regulation in the Drosophila Male Germline But Little Evidence for Chromosomal Dosage Compensation or Meiotic Inactivation. PLoS Biol. 2011, 9, e1001126. [Google Scholar] [CrossRef] [PubMed]
- Chow, J.; Heard, E. X inactivation and the complexities of silencing a sex chromosome. Curr. Opin. Cell Biol. 2009, 21, 359–366. [Google Scholar] [CrossRef]
- Dmitriev, R.I.; Pestov, N.B.; Korneenko, T.V.; Gerasimova, A.V.; Zhao, H.; Modyanov, N.N.; Kostina, M.B.; Shakhparonov, M.I. Tissue Specificity of Alternative Splicing of Transcripts Encoding Hampin, a New Mouse Protein Homologous to the Drosophila MSL-1 Protein. Russ. J. Bioorganic Chem. 2005, 31, 325–331. [Google Scholar] [CrossRef]
- Taipale, M.; Rea, S.; Richter, K.; Vilar, A.; Lichter, P.; Imhof, A.; Akhtar, A. hMOF Histone Acetyltransferase Is Required for Histone H4 Lysine 16 Acetylation in Mammalian Cells. Mol. Cell. Biol. 2005, 25, 6798–6810. [Google Scholar] [CrossRef]
- Peng, Z.; Du, J.; Sun, B.; Dong, X.; Xu, G.; Zhou, J.; Huang, Q.; Liu, Q.; Quan, H.; Ding, J. Structure of human MRG15 chromo domain and its binding to Lys36-methylated histone H3. Nucleic Acids Res. 2006, 34, 6621–6628. [Google Scholar]
- Tominaga, K.; Kirtane, B.; Jackson, J.G.; Ikeno, Y.; Ikeda, T.; Hawks, C.; Smith, J.R.; Matzuk, M.M.; Pereira-Smith, O.M. MRG15 Regulates Embryonic Development and Cell Proliferation. Mol. Cell. Biol. 2005, 25, 2924–2937. [Google Scholar] [CrossRef]
- Dmitriev, R.I.; Korneenko, T.V.; Bessonov, A.A.; Shakhparonov, M.I.; Modyanov, N.N.; Pestov, N.B. Characterization of hampin/MSL1 as a node in the nuclear interactome. Biochem. Biophys. Res. Commun. 2007, 355, 1051–1057. [Google Scholar] [CrossRef]
- Wu, L.; Zee, B.M.; Wang, Y.; Garcia, B.A.; Dou, Y. The RING Finger Protein MSL2 in the MOF Complex Is an E3Ubiquitin Ligase for H2B K34 and Is Involved in Crosstalk with H3 K4 and K79 Methylation. Mol. Cell 2011, 43, 132–144. [Google Scholar] [CrossRef]
- Deng, X.; Berletch, J.B.; Ma, W.; Nguyen, D.K.; Hiatt, J.B.; Noble, W.S.; Shendure, J.; Disteche, C.M. Mammalian X upregulation is associated with enhanced transcription initiation, RNA half-life, and MOF-mediated H4K16 acetylation. Dev. Cell 2013, 25, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Sangrithi, M.N.; Royo, H.; Mahadevaiah, S.K.; Ojarikre, O.; Bhaw, L.; Sesay, A.; Peters, A.H.; Stadler, M.; Turner, J.M. Non-Canonical and Sexually Dimorphic X Dosage Compensation States in the Mouse and Human Germline. Dev. Cell 2017, 40, 289–301.e283. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Ma, J.; Wan, Z.; Wang, Q.; Zhang, Y. Characterization of sheep spermatogenesis through single cell RNA sequencing. FASEB J. 2020, 35, e21187. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Sosa, E.; Chitiashvili, T.; Nie, X.; Rojas, E.J.; Oliver, E.; DonorConnect; Plath, K.; Hotaling, J.M.; Stukenborg, J.B. Single-cell analysis of the developing human testis reveals somatic niche cell specification and fetal germline stem cell establishment. Cell Stem Cell 2021, 28, 764–778. [Google Scholar] [CrossRef] [PubMed]
- The adult human testis transcriptional cell atlas. Cell Res. 2018, 28, 1141–1157. [CrossRef] [PubMed]
- Daniel, G.C.; Ma, Q.; Manske, G.L.; Niederriter, S.A.; Xianing, Z.; Simone, M.; Lindsay, M.; Caleb, S.; Gurczynski, S.J.; Moore, B.B. A Comprehensive Roadmap of Murine Spermatogenesis Defined by Single-Cell RNA-Seq. Dev. Cell 2018, 46, 651–667. [Google Scholar]
- Lukassen, S.; Bosch, E.; Ekici, A.B.; Winterpacht, A. Characterization of germ cell differentiation in the male mouse through single-cell RNA sequencing. Sci. Rep. 2018, 8, 6521. [Google Scholar] [CrossRef] [PubMed]
- Turner, J. Meiotic Silencing in Mammals. Annu. Rev. Genet. 2015, 49, 395–412. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, R.; Mckinnell, C.; Kivlin, C.; Fisher, J. Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood. Reproduction 2003, 125, 769–784. [Google Scholar] [CrossRef]
- Meyts, E.R.D.; Almstrup, K.; Nielsen, J.E.; Skakkebæk, N.E. The Testis in Childhood Between Birth and Puberty; Springer: London, UK, 2013; pp. 69–75. [Google Scholar] [CrossRef]
- Skandalakis, J.; Gray, S.; Rowe, J.J. Testis and epididymis. J. Reprod. Fertil. 1983, 58, 395–399. [Google Scholar]
- Gu, L.; Walters, J.R. Evolution of Sex Chromosome Dosage Compensation in Animals: A Beautiful Theory, Undermined by Facts and Bedeviled by Details. Genome Biol. Evol. 2017, 9, 2461–2476. [Google Scholar] [CrossRef] [PubMed]
- Rastelli, L.; Kuroda, M.I. An analysis of maleless and histone H4 acetylation in Drosophila melanogaster spermatogenesis. Mech. Dev. 1998, 71, 107–117. [Google Scholar] [CrossRef]
- Bone, J.R.; Lavender, J.; Richman, R.; Palmer, M.J.; Turner, B.M.; Kuroda, M.I. Acetylated histone H4 on the male X chromosome is associated with dosage compensation in Drosophila. Genes Dev. 1994, 8, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Hamada, F.N.; Park, P.J.; Gordadze, P.R.; Kuroda, M.I. Global regulation of X chromosomal genes by the MSL complex in Drosophila melanogaster. Genes Dev. 2005, 19, 2289–2294. [Google Scholar] [CrossRef] [PubMed]
- Keller, C.I.; Akhtar, A. The MSL complex: Juggling RNA-protein interactions for dosage compensation and beyond. Curr. Opin. Genet. Dev. 2015, 31, 1–11. [Google Scholar] [CrossRef]
- Raja, S.J.; Charapitsa, I.; Conrad, T.; Vaquerizas, J.M.; Gebhardt, P.; Holz, H.; Kadlec, J.; Fraterman, S.; Luscombe, N.M.; Akhtar, A. The nonspecific lethal complex is a transcriptional regulator in Drosophila. Mol. Cell 2010, 38, 827–841. [Google Scholar] [CrossRef] [PubMed]


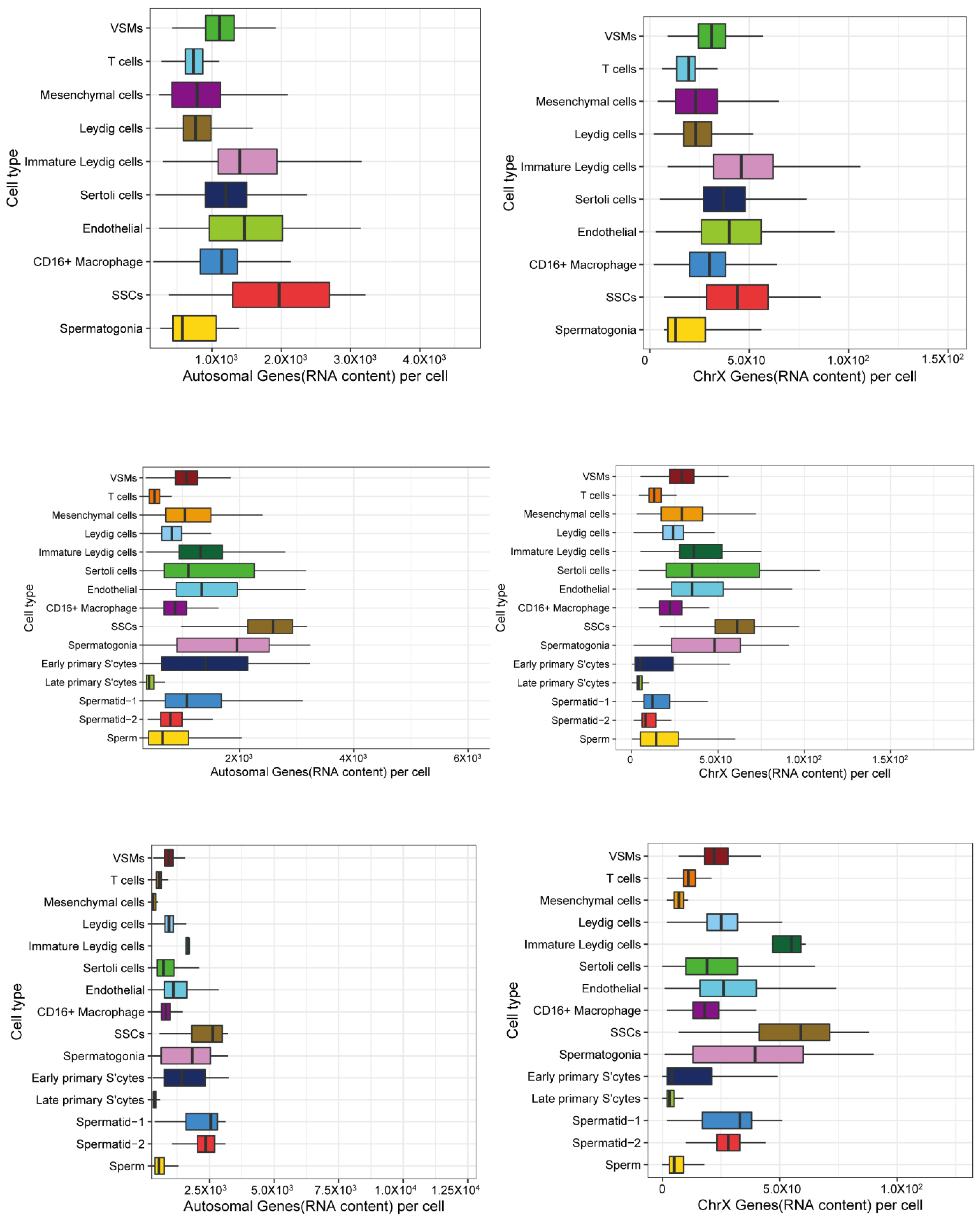
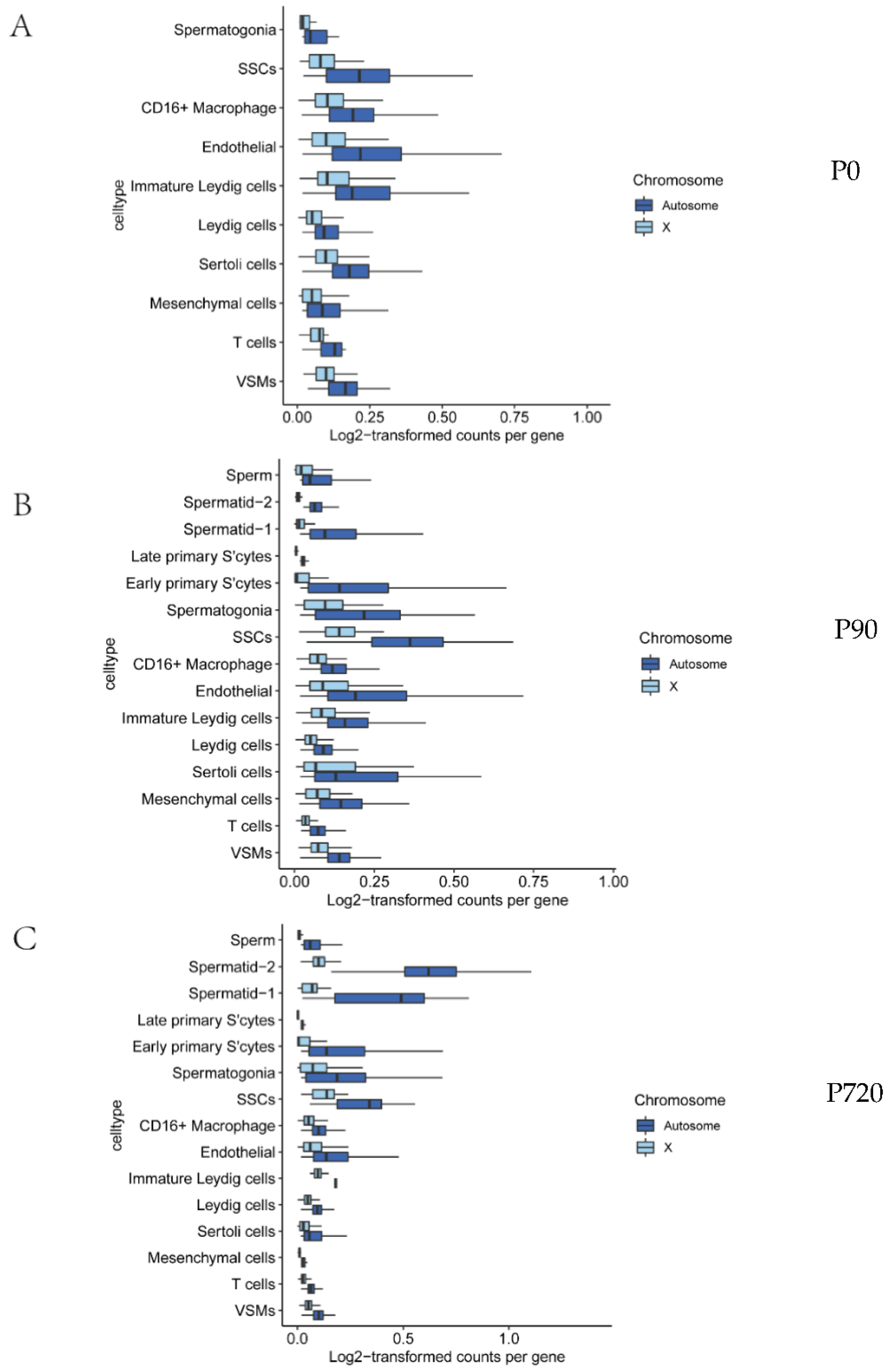
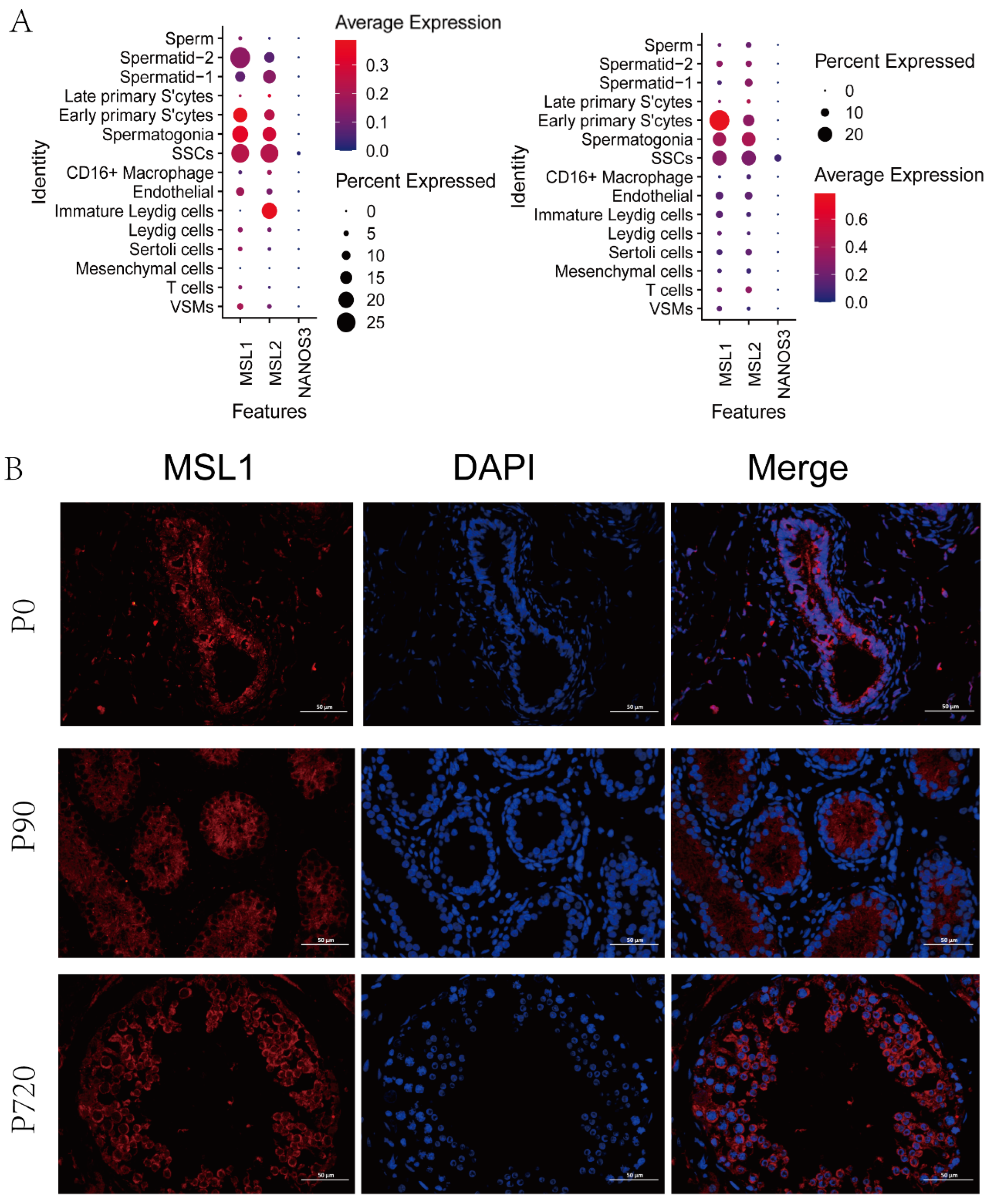
| Cell Type | X Counts | Autosome Counts | X/Autosome Ratio |
|---|---|---|---|
| P0 | |||
| CD16+ Macrophage | 128.5353 | 4641.2119 | 0.0277 |
| Endothelial | 130.2266 | 6034.0977 | 0.0216 |
| Immature Leydig cells | 142.4536 | 5328.6118 | 0.0267 |
| Leydig cells | 75.9112 | 2764.3817 | 0.0275 |
| Mesenchymal | 70.1564 | 2510.0474 | 0.0280 |
| T cells | 75.7143 | 2641.5000 | 0.0287 |
| VSMs | 110.2424 | 3778.6869 | 0.0292 |
| Sertoli cells | 114.5922 | 4240.6668 | 0.0270 |
| SSC | 108.9451 | 5488.4945 | 0.0198 |
| Spermatogonia | 34.9231 | 1683.3077 | 0.0207 |
| P30 | |||
| CD16+ Macrophage | 82.3359 | 2801.8828 | 0.0294 |
| Endothelial | 132.4890 | 5846.1923 | 0.0227 |
| Immature Leydig cells | 101.3784 | 3810.3851 | 0.0266 |
| Leydig cells | 57.7191 | 2056.5287 | 0.0281 |
| Mesenchymal | 88.0381 | 3548.5905 | 0.0248 |
| T cells | 43.5422 | 1807.6024 | 0.0241 |
| VSMs | 85.2736 | 3195.1857 | 0.0267 |
| Sertoli cells | 124.1719 | 4505.4355 | 0.0276 |
| SSC | 157.9563 | 8479.7961 | 0.0186 |
| Spermatogonia | 107.1697 | 5025.1150 | 0.0213 |
| Early primary S’cytes | 36.0818 | 5056.9308 | 0.0071 |
| Late primary S’cytes | 5.5809 | 594.5732 | 0.0094 |
| Spermatid-1 | 31.2000 | 4163.8228 | 0.0075 |
| Spermatid-2 | 20.1000 | 1843.7667 | 0.0109 |
| Sperm | 34.9865 | 1622.8475 | 0.0216 |
| P90 | |||
| CD16+ Macrophage | 62.8087 | 2341.6242 | 0.0268 |
| Endothelial | 90.9568 | 4067.5760 | 0.0224 |
| Immature Leydig cells | 107.8000 | 3975.8000 | 0.0271 |
| Leydig cells | 54.3794 | 2121.6286 | 0.0256 |
| Mesenchymal cells | 17.0870 | 791.7826 | 0.0216 |
| T cells | 34.3602 | 1462.0158 | 0.0235 |
| VSMs | 58.2791 | 2217.8428 | 0.0263 |
| Sertoli cells | 56.2993 | 2160.6361 | 0.0261 |
| SSC | 140.0526 | 7333.1184 | 0.0191 |
| Spermatogonia | 92.8073 | 4542.2374 | 0.0204 |
| Early primary S’cytes | 37.2829 | 5132.5854 | 0.0073 |
| Late primary S’cytes | 4.0830 | 531.4857 | 0.0077 |
| Spermatid-1 | 66.8285 | 10,416.3366 | 0.0064 |
| Spermatid-2 | 113.7132 | 16,945.3000 | 0.0067 |
| Sperm | 14.2388 | 2233.5853 | 0.0064 |
| Cell Type | X/Autosome Ratio of Raw Counts | RAW p Value | Adjusted p Value |
|---|---|---|---|
| P0 | |||
| CD16+ Macrophage | 0.58 | 7.22 × 10−46 | 5.1 × 10−45 |
| Endothelial | 0.57 | 9.69 × 10−44 | 5.8 × 10−43 |
| Immature Leydig cells | 0.55 | 2.23 × 10−79 | 1.8 × 10-78 |
| Leydig cells | 0.54 | 0 | 0 |
| Mesenchymal cells | 0.54 | 2.27 × 10−36 | 1.1 × 10−35 |
| T cells | 0.53 | 0.00012207 | 0.00024 |
| VSMs | 0.43 | 5.78 × 10−18 | 2.3 × 10−17 |
| Sertoli cells | 0.55 | 0 | 0 |
| SSCs | 0.39 | 1.21 × 10−16 | 3.6 × 10−16 |
| Spermatogonia | 0.41 | 0.000244141 | 0.00024 |
| P30 | |||
| CD16+ Macrophage | 0.53 | 9.69 × 10−44 | 7.8 × 10−43 |
| Endothelial | 0.48 | 6.88 × 10−232 | 9.6 × 10−231 |
| Immature Leydig cells | 0.49 | 4.95 × 10−26 | 2 × 10−25 |
| Leydig cells | 0.55 | 0 | 0 |
| Mesenchymal cells | 0.56 | 5.92 × 10−19 | 1.8 × 10−18 |
| T cells | 0.53 | 2.54 × 10−15 | 5.1 × 10−15 |
| VSMs | 0.45 | 4.37 × 10−52 | 3.9 × 10−51 |
| Sertoli cells | 0.58 | 1.39 × 10−85 | 1.4 × 10−84 |
| SSCs | 0.37 | 1.50 × 10−35 | 9 × 10−35 |
| Spermatogonia | 0.42 | 2.62 × 10−145 | 3.4 × 10−144 |
| Early primary S’cytes | 0.14 | 7.70 × 10−28 | 3.9 × 10−27 |
| Late primary S’cytes | 0.19 | 6.02 × 10−108 | 7.2 × 10−107 |
| Spermatid-1 | 0.15 | 4.72 × 10−95 | 5.2 × 10−94 |
| Spermatid-2 | 0.22 | 1.67 × 10−11 | 1.7 × 10−11 |
| Sperm | 0.43 | 2.46 × 10−38 | 1.7 × 10−37 |
| P720 | |||
| CD16+ Macrophage | 0.52 | 1.29863 × 10−50 | 6.5 × 10−50 |
| Endothelial | 0.47 | 5.74 × 10−191 | 7.5 × 10−190 |
| Immature Leydig cells | 0.43 | 0.0625 | 0.062 |
| Leydig cells | 0.52 | 0 | 0 |
| Mesenchymal cells | 0.51 | 2.38419 × 10−07 | 0.00000048 |
| T cells | 0.54 | 2.4637 × 10−105 | 2.7 × 10−104 |
| VSMs | 0.44 | 3.1914 × 10−62 | 2.9 × 10−61 |
| Sertoli cells | 0.53 | 5.85977 × 10−50 | 2.3 × 10−49 |
| SSCs | 0.38 | 3.67866 × 10−14 | 1.1 × 10−13 |
| Spermatogonia | 0.41 | 2.00458 × 10−60 | 1.6 × 10−59 |
| Early primary S’cytes | 0.14 | 2.77632 × 10−60 | 1.9 × 10−59 |
| Late primary S’cytes | 0.15 | 4.5374 × 10−224 | 6.4 × 10−223 |
| Spermatid-1 | 0.13 | 2.0614 × 10−52 | 1.2 × 10−51 |
| Spermatid-2 | 0.13 | 1.6026 × 10−88 | 1.6 × 10−87 |
| Sperm | 0.13 | 2.199 × 10−126 | 2.6 × 10−125 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, J.; Zhang, Y.; Su, H.; Wang, C.; Wang, D.; Yang, Y.; Li, X.; Qi, W.; Li, H.; Li, X.; et al. Dosage Compensation of the X Chromosome during Sheep Testis Development Revealed by Single-Cell RNA Sequencing. Animals 2022, 12, 2169. https://doi.org/10.3390/ani12172169
Su J, Zhang Y, Su H, Wang C, Wang D, Yang Y, Li X, Qi W, Li H, Li X, et al. Dosage Compensation of the X Chromosome during Sheep Testis Development Revealed by Single-Cell RNA Sequencing. Animals. 2022; 12(17):2169. https://doi.org/10.3390/ani12172169
Chicago/Turabian StyleSu, Jie, Yue Zhang, Hong Su, Caiyun Wang, Daqing Wang, Yanyan Yang, Xiunan Li, Wangmei Qi, Haijun Li, Xihe Li, and et al. 2022. "Dosage Compensation of the X Chromosome during Sheep Testis Development Revealed by Single-Cell RNA Sequencing" Animals 12, no. 17: 2169. https://doi.org/10.3390/ani12172169
APA StyleSu, J., Zhang, Y., Su, H., Wang, C., Wang, D., Yang, Y., Li, X., Qi, W., Li, H., Li, X., Song, Y., & Cao, G. (2022). Dosage Compensation of the X Chromosome during Sheep Testis Development Revealed by Single-Cell RNA Sequencing. Animals, 12(17), 2169. https://doi.org/10.3390/ani12172169






