Genomic Prediction for Abortion in Lactating Holstein Dairy Cows
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources for Genetic Evaluation
2.2. Data Editing and Trait Definition for Genetic Evaluation
2.3. Statistical Model for Genetic Evaluation
2.4. Inclusion of Abort Prediction in a Multi-Trait Selection Index
2.5. Demonstration of Evaluation Efficacy
3. Results
3.1. Data Characteristics for Genetic Evaluation
3.2. Variance Components and Summary Statistics for Genetic Evaluation
3.3. Correlations of Abortion with Other Traits
3.4. Demonstration of Evaluation Efficacy
3.4.1. Cow Abortion for the Genetic Groups
3.4.2. Holstein Haplotypes Frequencies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Trait | Expected Response to Selection |
|---|---|
| Fat (Kg) | 9.1 |
| Protein (Kg) | 5 |
| Milk (Kg) | 99.3 |
| Productive Life (mo.) | 1.42 |
| Cow Livability (%) | 0.66 |
| Somatic Cell Score (log) | −0.07 |
| Residual feed Intake | −5.17 |
| Body Size Composite (pts) | −0.21 |
| Udder Composite (pts) | 0.27 |
| Feet and Leg Composite (pts) | 0.08 |
| Daughter Pregnancy Rate (%) | 0.16 |
| Heifer Conception Rate (%) | 0.31 |
| Early First Calving | 0.68 |
| Cow Conception Rate (%) | 0.43 |
| Calving Ability ($) | 5.10 |
| Zoetis Mastitis (STA) | 1.54 |
| Zoetis Metritis (STA) | 1.90 |
| Zoetis Retained Placenta (STA) | 0.56 |
| Zoetis Displaced Abomasum (STA) | 1.04 |
| Zoetis Ketosis (STA) | 1.73 |
| Zoetis Lameness (STA) | 1.19 |
| Zoetis Calf Respiratory (STA) | 0.27 |
| Zoetis Calf Scours (STA) | −0.07 |
| Zoetis Calf Livability (STA) | 0.38 |
| Zoetis Cow Respiratory (STA) | 0.76 |
| Zoetis Cystic Ovary (STA) | 0.22 |
| Zoetis Twinning (STA) | 0.61 |
| Zoetis Cow Abortion (STA) | 0.19 |
References
- Santos, J.; Thatcher, W.; Chebel, R.; Cerri, R.; Galvao, K. The effect of embryonic death rates in cattle on the efficacy of estrus synchronization programs. Anim. Reprod. Sci. 2004, 82, 513–535. [Google Scholar] [CrossRef]
- Hubbert, W. Committee on bovine reproductive nomenclature. Recommendations for standardizing bovine reproductive terms. Cornell Vet. 1972, 62, 216–237. [Google Scholar]
- De Vries, A. Economic value of pregnancy in dairy cattle. J. Dairy Sci. 2006, 89, 3876–3885. [Google Scholar] [CrossRef]
- Cabrera, V. A simple formulation and solution to the replacement problem: A practical tool to assess the economic cow value, the value of a new pregnancy, and the cost of a pregnancy loss. J. Dairy Sci. 2012, 95, 4683–4698. [Google Scholar] [CrossRef] [PubMed]
- Wijma, R.; Stangaferro, M.; Giordano, J. 1268 Reproductive performance and culling dynamics of lactating dairy cows with detected pregnancy loss. J. Anim. Sci. 2016, 94, 611–612. [Google Scholar] [CrossRef]
- Joosten, I.; van Eldik, P.; Elving, L.; van der Mey, G. Factors related to the etiology of retained placenta in dairy cattle. Anim. Reprod. Sci. 1987, 14, 251–262. [Google Scholar] [CrossRef]
- Kaneene, J.; Miller, R. Risk factors for metritis in Michigan dairy cattle using herd-and cow-based modelling approaches. Prev. Vet. Med. 1995, 23, 183–200. [Google Scholar] [CrossRef]
- Ghavi Hossein-Zadeh, N.; Ardalan, M. Cow-specific risk factors for retained placenta, metritis and clinical mastitis in Holstein cows. Vet. Res. Commun. 2011, 35, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Galvao, K. Uterine diseases in dairy cows: Understanding the causes and seeking solutions. Anim. Reprod. (AR) 2018, 10, 228–238. [Google Scholar]
- Giordano, J.; Stangaferro, M.; Wijma, R.; Chandler, W.; Watters, R. Reproductive performance of dairy cows managed with a program aimed at increasing insemination of cows in estrus based on increased physical activity and fertility of timed artificial inseminations. J. Dairy Sci. 2015, 98, 2488–2501. [Google Scholar] [CrossRef] [PubMed]
- Wijma, R.; Pérez, M.; Masello, M.; Stangaferro, M.; Giordano, J. A resynchronization of ovulation program based on ovarian structures present at nonpregnancy diagnosis reduced time to pregnancy in lactating dairy cows. J. Dairy Sci. 2018, 101, 1697–1707. [Google Scholar] [CrossRef] [PubMed]
- Pérez, M.; Wijma, R.; Scarbolo, M.; Cabrera, E.; Sosa, F.; Sitko, E.; Giordano, J. Lactating dairy cows managed for second and greater artificial insemination services with the Short-Resynch or Day 25 Resynch program had similar reproductive performance. J. Dairy Sci. 2020, 103, 10769–10783. [Google Scholar] [CrossRef]
- Vasconcelos, J.; Silcox, R.; Lacerda, J.; Pursley, J.; Wiltbank, M. Pregnancy rate, pregnancy loss, and response to head stress after AI at 2 different times from ovulation in dairy cows. Biol. Reprod. 1997, 56, 230. [Google Scholar]
- López-Gatius, F.; Santolaria, P.; Yániz, J.; Garbayo, J.; Hunter, R. Timing of early foetal loss for single and twin pregnancies in dairy cattle. Reprod. Domest. Anim. 2004, 39, 429–433. [Google Scholar] [CrossRef]
- Romano, J.E.; Fahning, M.L. Effects of early pregnancy diagnosis by per rectal palpation of the amniotic sac on pregnancy loss in dairy cattle. J. Am. Vet. Med. Assoc. 2013, 243, 1462–1467. [Google Scholar] [CrossRef] [PubMed]
- Hovingh, E. Abortions in Dairy Cattle I: Common Causes of Abortions; Virginia Tech: Blacksburg, VA, USA, 2009. [Google Scholar]
- Wiltbank, M.C.; Pursley, J.R. The cow as an induced ovulator: Timed AI after synchronization of ovulation. Theriogenology 2014, 81, 170–185. [Google Scholar] [CrossRef]
- VanRaden, P.; Olson, K.; Null, D.; Hutchison, J. Harmful recessive effects on fertility detected by absence of homozygous haplotypes. J. Dairy Sci. 2011, 94, 6153–6161. [Google Scholar] [CrossRef] [PubMed]
- Fritz, S.; Capitan, A.; Djari, A.; Rodriguez, S.C.; Barbat, A.; Baur, A.; Grohs, C.; Weiss, B.; Boussaha, M.; Esquerre, D. Detection of haplotypes associated with prenatal death in dairy cattle and identification of deleterious mutations in GART, SHBG and SLC37A2. PLoS ONE 2013, 8, e65550. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.; VanRaden, P.; Null, D.; Hutchison, J.; Cooper, T.; Hubbard, S. Haplotype tests for recessive disorders that affect fertility and other traits. In USDA Animal Improvement Program Research Report Genomics; 09-13; USDA: Beltsville, MD, USA, 2015. [Google Scholar]
- Cole, J.; Null, D.; VanRaden, P. Phenotypic and genetic effects of recessive haplotypes on yield, longevity, and fertility. J. Dairy Sci. 2016, 99, 7274–7288. [Google Scholar] [CrossRef]
- Sigdel, A.; Bisinotto, R.S.; Peñagaricano, F. Genes and pathways associated with pregnancy loss in dairy cattle. Sci. Rep. 2021, 11, 1–11. [Google Scholar] [CrossRef]
- Gershoni, M.; Ezra, E.; Weller, J.I. Genetic and genomic analysis of long insemination interval in Israeli dairy cattle as an indicator of early abortions. J. Dairy Sci. 2020, 103, 4495–4509. [Google Scholar] [CrossRef] [PubMed]
- VanRaden, P.; Sanders, A.; Tooker, M.; Miller, R.; Norman, H.; Kuhn, M.; Wiggans, G. Development of a national genetic evaluation for cow fertility. J. Dairy Sci. 2004, 87, 2285–2292. [Google Scholar] [CrossRef]
- McNeel, A.K.; Reiter, B.C.; Weigel, D.; Osterstock, J.; Di Croce, F.A. Validation of genomic predictions for wellness traits in US Holstein cows. J. Dairy Sci. 2017, 100, 9115–9124. [Google Scholar] [CrossRef] [PubMed]
- Vukasinovic, N.; Bacciu, N.; Przybyla, C.; Boddhireddy, P.; DeNise, S. Development of genetic and genomic evaluation for wellness traits in US Holstein cows. J. Dairy Sci. 2017, 100, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Fessenden, B.; Weigel, D.J.; Osterstock, J.; Galligan, D.T.; Di Croce, F. Validation of genomic predictions for a lifetime merit selection index for the US dairy industry. J. Dairy Sci. 2020, 103, 10414–10428. [Google Scholar] [CrossRef] [PubMed]
- McGovern, S.P.; Weigel, D.J.; Fessenden, B.C.; Gonzalez-Peña, D.; Vukasinovic, N.; McNeel, A.K.; Di Croce, F.A. Genomic prediction for twin pregnancies. Animals 2021, 11, 843. [Google Scholar] [CrossRef] [PubMed]
- Sargolzaei, M.; Chesnais, J.; Schenkel, F. FImpute-An efficient imputation algorithm for dairy cattle populations. J. Dairy Sci. 2011, 94, 421. [Google Scholar]
- Norman, H.; Waite, L.; Wiggans, G.; Walton, L. Improving accuracy of the United States genetics database with a new editing system for dairy records. J. Dairy Sci. 1994, 77, 3198–3208. [Google Scholar] [CrossRef]
- Gonzalez-Peña, D.; Vukasinovic, N.; Brooker, J.; Przybyla, C.; Baktula, A.; DeNise, S. Genomic evaluation for wellness traits in US Jersey cattle. J. Dairy Sci. 2020, 103, 1735–1748. [Google Scholar] [CrossRef] [PubMed]
- Misztal, I.; Tsuruta, S.; Strabel, T.; Auvray, B.; Druet, T.; Lee, D. BLUPF90 and related programs (BGF90). In Proceedings of the 7th World Congress on Genetics Applied to Livestock Production, Montpellier, France, 19–23 August 2002. [Google Scholar]
- Misztal, I.; Legarra, A.; Aguilar, I. Using recursion to compute the inverse of the genomic relationship matrix. J. Dairy Sci. 2014, 97, 3943–3952. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, I.; Misztal, I.; Johnson, D.; Legarra, A.; Tsuruta, S.; Lawlor, T. Hot topic: A unified approach to utilize phenotypic, full pedigree, and genomic information for genetic evaluation of Holstein final score. J. Dairy Sci. 2010, 93, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.A.; Gere, T.; Kishk, W.H. Additive genetic variance and covariance in some reproduetive disorders in Hungarian Holstein Friesian using multi-trait animal model. Arch. Anim. Breed. 2000, 43, 573–582. [Google Scholar] [CrossRef]
- Carthy, T.; Ryan, D.P.; Fitzgerald, A.; Evans, R.; Berry, D. Genetic parameters of ovarian and uterine reproductive traits in dairy cows. J. Dairy Sci. 2015, 98, 4095–4106. [Google Scholar] [CrossRef]
- Oliver, K.F.; Wahl, A.M.; Dick, M.; Toenges, J.A.; Kiser, J.N.; Galliou, J.M.; Moraes, J.G.; Burns, G.W.; Dalton, J.; Spencer, T.E. Genomic analysis of spontaneous abortion in Holstein Heifers and Primiparous cows. Genes 2019, 10, 954. [Google Scholar] [CrossRef] [PubMed]
- Bamber, R.; Shook, G.; Wiltbank, M.; Santos, J.; Fricke, P. Genetic parameters for anovulation and pregnancy loss in dairy cattle. J. Dairy Sci. 2009, 92, 5739–5753. [Google Scholar] [CrossRef] [PubMed]
- Keshavarzi, H.; Sadeghi-Sefidmazgi, A.; Kristensen, A.R.; Stygar, A.H. Abortion studies in Iranian dairy herds: I. Risk factors for abortion. Livest. Sci. 2017, 195, 45–52. [Google Scholar] [CrossRef]
- Jousan, F.; Drost, M.; Hansen, P. Factors associated with early and mid-to-late fetal loss in lactating and nonlactating Holstein cattle in a hot climate. J. Anim. Sci. 2005, 83, 1017–1022. [Google Scholar] [CrossRef]
- Stangaferro, M.; Wijma, R.; Masello, M.; Thomas, M.J.; Giordano, J. Economic performance of lactating dairy cows submitted for first service timed artificial insemination after a voluntary waiting period of 60 or 88 days. J. Dairy Sci. 2018, 101, 7500–7516. [Google Scholar] [CrossRef]
- Erb, R.; Morrison, R. Effects of twinning on reproductive efficiency in a Holstein-Friesian herd. J. Dairy Sci. 1959, 42, 512–519. [Google Scholar] [CrossRef]
- Day, J.D.; Weaver, L.D.; Franti, C.E. Twin pregnancy diagnosis in Holstein cows: Discriminatory powers and accuracy of diagnosis by transrectal palpation and outcome of twin pregnancies. Can. Vet. J. 1995, 36, 93. [Google Scholar]
- Hossein-Zadeh, N.G.; Nejati-Javaremi, A.; Miraei-Ashtiani, S.; Kohram, H. An observational analysis of twin births, calf stillbirth, calf sex ratio, and abortion in Iranian Holsteins. J. Dairy Sci. 2008, 91, 4198–4205. [Google Scholar] [CrossRef] [PubMed]
- Dahl, M.O.; Maunsell, F.P.; de Vries, A.; Galvao, K.N.; Risco, C.A.; Hernandez, J.A. Evidence that mastitis can cause pregnancy loss in dairy cows: A systematic review of observational studies. J. Dairy Sci. 2017, 100, 8322–8329. [Google Scholar] [CrossRef] [PubMed]
- Sangsritavong, S.; Combs, D.; Sartori, R.; Armentano, L.; Wiltbank, M. High feed intake increases liver blood flow and metabolism of progesterone and estradiol-17β in dairy cattle. J. Dairy Sci. 2002, 85, 2831–2842. [Google Scholar] [CrossRef]
- De Vries, A. Symposium review: Why revisit dairy cattle productive lifespan? J. Dairy Sci. 2020, 103, 3838–3845. [Google Scholar] [CrossRef]

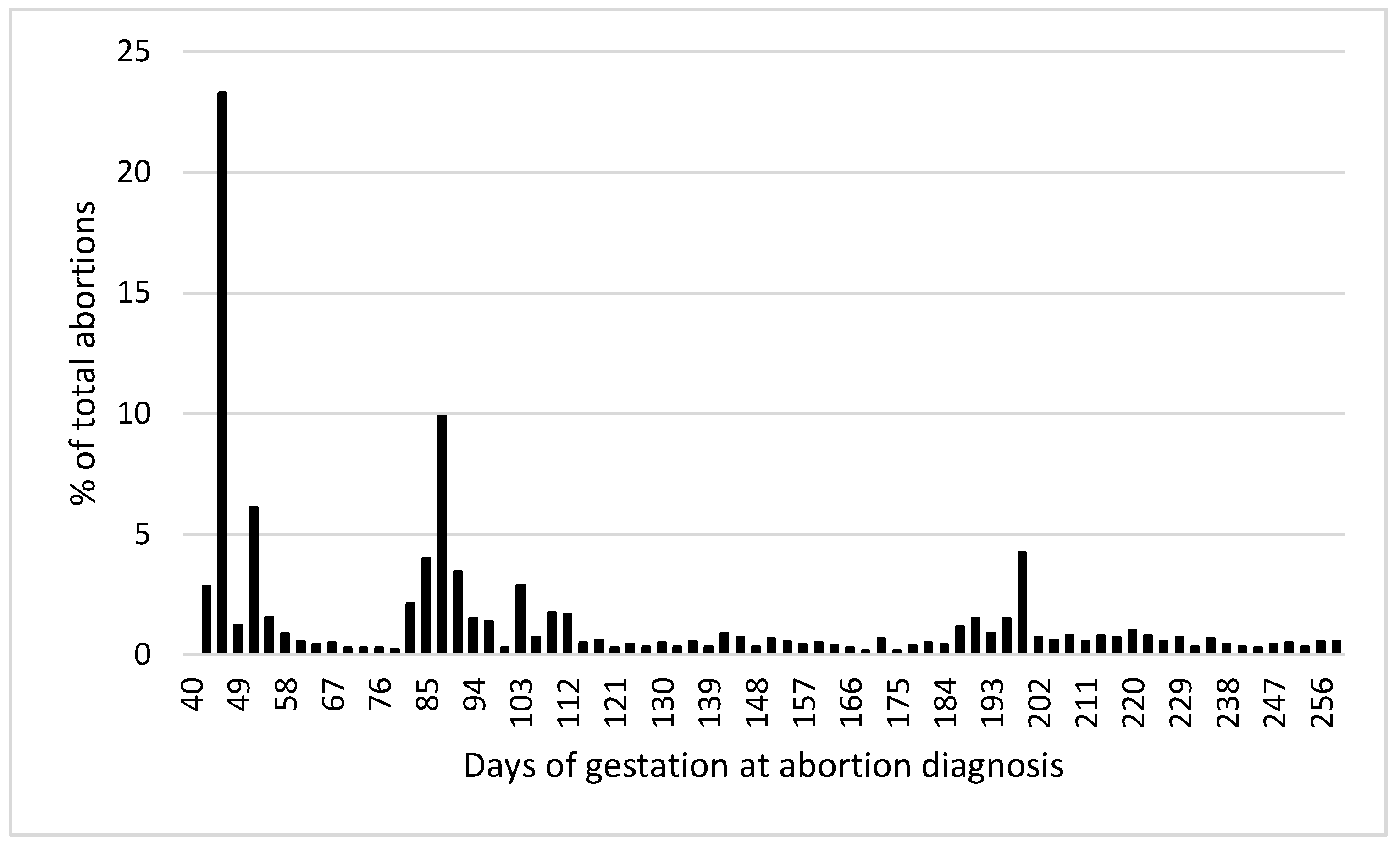

| Item | Count |
|---|---|
| Total animals in the evaluation | 4,955,087 |
| Phenotypic records total | 3,838,805 |
| Animals with phenotypes | 2,038,425 |
| Animals with genotypes | 1,662,251 |
| Animals with genotypes and phenotypes | 109,267 |
| Incidence of abortion | 11.67% |
| Trait | σ2g | σ2pe | σ2hys | σ2ss | σ2e | h2 | r2 |
|---|---|---|---|---|---|---|---|
| Z_ABORT | 0.1235 | 0.0941 | 0.3748 | 0.0049 | 1.0 | 0.0773 | 0.1362 |
| All Animals with Genotypes | |||||
|---|---|---|---|---|---|
| Variables * | N | Mean | Std Dev | Minimum | Maximum |
| Z_Abort_PTA | 1,662,251 | −0.01 | 2.1 | −8.8 | 12.4 |
| Z_Abort _STA | 1,662,251 | 99.0 | 4.8 | 71.0 | 119.0 |
| Z_Abort _REL | 1,662,251 | 42.4 | 6.0 | 0.0 | 99.6 |
| Animals with genotype (no phenotype, no progeny) | |||||
| Z_Abort_PTA | 1,232,143 | 0.1 | 2.1 | −8.8 | 11.8 |
| Z_Abort _STA | 1,232,143 | 98.8 | 4.7 | 73.0 | 119.0 |
| Z_Abort _REL | 1,232,143 | 41.1 | 5.3 | 0.0 | 62.4 |
| Animals with both genotype and phenotype (no progeny) | |||||
| Z_Abort_PTA | 39,036 | −0.5 | 2.3 | −8.1 | 12.4 |
| Z_Abort _STA | 39,036 | 100.1 | 5.1 | 71.0 | 117.0 |
| Z_Abort _REL | 39,036 | 49.5 | 4.5 | 10.1 | 62.1 |
| Zoetis Wellness Traits * | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DWP$ | Z_CALF DIAR | Z_CALF RESP | Z_CALF LIV | Z_RETP | Z_METR | Z_MAST | Z_LAME | Z_KETO | Z_DA | Z_MFV | Z_TWIN | Z_RESP | Z_CYST | |
| Z_Abort | 0.017 | 0.013 | 0.078 | 0.190 | 0.285 | 0.189 | 0.087 | 0.068 | 0.037 | 0.039 | 0.069 | 0.284 | 0.145 | 0.013 |
| CDCB Traits ** | ||||||||||||||
| NM$ | Milk | Fat | Prot | PL | LIV | SCS | DPR | HCR | CCR | |||||
| Z_Abort | −0.180 | −0.224 | −0.254 | −0.264 | −0.016 | 0.184 | −0.005 | 0.289 | 0.051 | 0.236 | ||||
| Z_Abort STA Genetic Group | Marginal Means (Incidence) | SEM | Abortion Cost per Cow ($) |
|---|---|---|---|
| Worst 25% | 0.166 a | 0.013 | 92 |
| 25–50% | 0.136 b | 0.011 | 76 |
| 50–75% | 0.115 c | 0.010 | 64 |
| Best 25% | 0.110 c | 0.010 | 61 |
| Lactation Group | Marginal Means (Incidence) | SEM |
|---|---|---|
| Lact 1 | 0.112 a | 0.009 |
| Lact 2 | 0.120 a | 0.010 |
| Lact 3 | 0.140 b | 0.012 |
| Lact 4 | 0.154 b | 0.016 |
| DPR gPTA Genetic Group | Marginal Means (Incidence) | SEM | Abortion Cost per Cow ($) |
|---|---|---|---|
| Worst 25% | 0.151 a | 0.013 | USD 84 |
| 25–50% | 0.127 bc | 0.011 | USD 71 |
| 50–75% | 0.133 b | 0.011 | USD 74 |
| Best 25% | 0.116 c | 0.010 | USD 64 |
| HH0 | HH1 | HH2 | HH3 | HH4 | HH5 | |
|---|---|---|---|---|---|---|
| AI Bulls | 2.6% | 0.4% | 1.6% | 2.4% | 0.2% | 4.8% |
| Abort | 4.4% | 2.5% | 4.7% | 6.4% | 1.6% | 2.8% |
| No Abort | 4.7% | 2.6% | 3.7% | 6.9% | 1.1% | 3.2% |
| Carrier × carrier matings (n) | 1 | 1 | 1 | 0 | 0 | 1 |
| Z_Abort STA Genetic Group | HH0 | HH1 | HH2 | HH3 | HH4 | HH5 |
|---|---|---|---|---|---|---|
| Worst 25% | 4.5 | 2.7 | 5.0 a | 6.1 | 0.1 | 2.5 |
| 25–50% | 3.9 | 2.6 | 4.2 a | 5.9 | 0.1 | 3.4 |
| 50–75% | 5.2 | 3.9 | 4.1 a | 8.7 | 0.1 | 3.3 |
| Best 25% | 4.2 | 2.5 | 2.1 b | 6.7 | 0.04 | 4.7 |
| p-Value | 0.19 | 0.83 | <0.001 | 0.07 | 0.05 | 0.24 |
| DPR gPTA Genetic Group | |||||
|---|---|---|---|---|---|
| Worst 25% | 25–50% | 50–75% | Best 25% | ||
| Z_Abort STA Genetic Group | Worst 25% | 8.2 | 6.8 | 5.3 | 3.9 |
| 25–50% | 6.8 | 6.8 | 6.3 | 5.7 | |
| 50–75% | 5.4 | 6.1 | 7.3 | 7.2 | |
| Best 25% | 4.2 | 5.6 | 6.4 | 8.1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wijma, R.; Weigel, D.J.; Vukasinovic, N.; Gonzalez-Peña, D.; McGovern, S.P.; Fessenden, B.C.; McNeel, A.K.; Di Croce, F.A. Genomic Prediction for Abortion in Lactating Holstein Dairy Cows. Animals 2022, 12, 2079. https://doi.org/10.3390/ani12162079
Wijma R, Weigel DJ, Vukasinovic N, Gonzalez-Peña D, McGovern SP, Fessenden BC, McNeel AK, Di Croce FA. Genomic Prediction for Abortion in Lactating Holstein Dairy Cows. Animals. 2022; 12(16):2079. https://doi.org/10.3390/ani12162079
Chicago/Turabian StyleWijma, Robert, Daniel J. Weigel, Natascha Vukasinovic, Dianelys Gonzalez-Peña, Shaileen P. McGovern, Brenda C. Fessenden, Anthony K. McNeel, and Fernando A. Di Croce. 2022. "Genomic Prediction for Abortion in Lactating Holstein Dairy Cows" Animals 12, no. 16: 2079. https://doi.org/10.3390/ani12162079
APA StyleWijma, R., Weigel, D. J., Vukasinovic, N., Gonzalez-Peña, D., McGovern, S. P., Fessenden, B. C., McNeel, A. K., & Di Croce, F. A. (2022). Genomic Prediction for Abortion in Lactating Holstein Dairy Cows. Animals, 12(16), 2079. https://doi.org/10.3390/ani12162079







