Simple Summary
There is increasing interest in improving nutrient utilization in pigs and poultry and thereby reduce nutrient excretion into the environment. The present review aims to provide an overview on interactions between fermentable substrates (e.g., starch, fiber, and protein) and selected minerals on nutrient digestion and absorption to determine nutritional solutions to maximize animal performance, principally in the grower–finisher phase. Using in vitro models, the site and rate (kinetics) of nutrient digestion or fermentation of a feed ingredient or diet can be estimated. However, for minerals, no standardized methodology to assess in vitro mineral digestion exists. In vivo, the diet total tract digestibility of phosphorus might be underestimated in diets with fermentable ingredients because of increased diet-specific endogenous phosphorus losses and requires further clarification to better calculate the true total tract digestibility of phosphorus in pigs. The quantification of fiber type, composition of fiber fractions within individual raw materials, their influence on digestion kinetics, and effects on digesta pH and nutrient solubility related to fermentation should be considered. In conclusion, applications of nutrient kinetic data should be considered as part of an integrated approach to support nutrient digestion and absorption in the gastrointestinal tract of pigs, thereby helping to reduce nutrient excretion.
Abstract
Nutrient kinetic data and the timing of nutrient release along the gastrointestinal tract (GIT), are not yet widely used in current feed formulations for pigs and poultry. The present review focuses on interactions between fermentable substrates (e.g., starch, fiber, and protein) and selected minerals on nutrient digestion and absorption to determine nutritional solutions to maximize animal performance, principally in the grower–finisher phase, with the aim of minimizing environmental pollution. For phosphorus (P), myo-inositol 1,2,3,4,5,6-hexakis (dihydrogen phosphate) (InsP6), copper (Cu), and zinc (Zn), no standardized methodologies to assess in vitro mineral digestion exist. The stepwise degradation of InsP6 to lower inositol phosphate (InsP) forms in the GIT is rare, and inositol phosphate4 (InsP4) might be the limiting isomer of InsP degradation in diets with exogenous phytase. Furthermore, dietary coefficients of standardized total tract digestibility (CSTTD) of P might be underestimated in diets with fermentable ingredients because of increased diet-specific endogenous P losses (EPL), and further clarification is required to better calculate the coefficients of true total tract digestibility (CTTTD) of P. The quantification of fiber type, composition of fiber fractions, their influence on digestion kinetics, effects on digesta pH, and nutrient solubility related to fermentation should be considered for formulating diets. In conclusion, applications of nutrient kinetic data should be considered to help enhance nutrient digestion and absorption in the GIT, thereby reducing nutrient excretion.
1. Introduction
In animal nutrition, an important bio-economical challenge is the parallel development of sustainable strategies to increase feed efficiency and to decrease negative effects of animal production on the environment [1]. Improvements in precision swine nutrition and environmental sustainability can be achieved in global pork production by assessing the digestion kinetics of chemical components in feedstuffs, circadian feed behavior, gastrointestinal microbiota, and functionality of feedstuffs [2]. In pig fattening, nearly 70% of production costs are related to feed [3]. Feed formulations are based on ingredient inclusion levels and their nutrient content, digestibility data, and the assumption of additivity. However, digestibility data generally do not account for interactions among nutrients or ingredients resulting in excess nutrients in diets fed to pigs [4]. Furthermore, interest exists in strategies to increase nutrient and mineral digestibility, e.g., using exogenous enzymes and thereby reducing mineral supplementation as means to reduce the environmental impact of nitrogen, phosphorus (P), and trace elements such as copper (Cu) and zinc (Zn) [5,6,7,8]. Nitrogen and P leaching from manure may lead to the eutrophication of fresh or seawater, with ammonia leading to acidification and eutrophication, resulting in negative effects on soil, forest, and biodiversity [5]. A further global challenge is the increasing scarcity of economically viable inorganic P sources. The main source of inorganic P (rock phosphate) is non-renewable, costly, and is geographically concentrated because six countries control 90% of the world’s phosphate rock reserves [9,10]. With only 20% of the world’s mined P being consumed by humans, the development of a sustainable resource management plan with the reduced mining of phosphate is becoming particularly important [11]. In plant materials, organic P is either present as myo-inositol 1,2,3,4,5,6-hexakis (dihydrogen phosphate) (InsP6), or phytate (any salt of InsP6). In feedstuffs, 80% of zinc is bound to InsP6 [7]. In piglets, supplementation of feed with exogenous phytase increases the digestibility of Zn, whereas the quantitative relationships between phytase, InsP6, and Zn require further investigation [7]. In a comprehensive overview, the environmental impacts of Zn and Cu used in animal nutrition were found to mainly affect groundwater, from the drainage and runoff of Zn from arable land to surface water. Copper accumulation in soil seems to be a long-term environmental concern, particularly in livestock-dense regions [12]. Agriculture and aquaculture seem to be major sources of soil and water contamination with metals such as Cu and Zn, possibly leading to accumulations triggering the co-selection of antibiotic resistance [13]. The major source of Zn emission is the land application of manure, increasing Zn levels in the top 0–20 cm layer of soil by 22–68% in the next 100 years, if current Zn inputs of different origins remain the same [14]. By reducing Zn inclusion in fattening pig diets, Zn emission could be reduced by 31%, with an additional reduction of 53% with the use exogenous or intrinsic phytases, and a further reduction in maximum Zn content in complete feed from 100 to 70 mg/kg feed [7]. Zn has been limited to 150 mg Zn/kg feed in nursery pigs since June 2022 in the EU due to risks for environmental accumulation and association with development of antimicrobial resistance [15]. In contrast, Cu amounts used for feed supplementation are usually small (0.7% of total Cu used as chemical) [16], but still represent an important source in agricultural soils where the reduction in maximum Cu content of piglet feed (from 170 down to 25 mg/kg) might support a decrease in total Cu emission by 20% [8].
In this review, the focus is on potential interactions between fermentable substrates (starch, fiber, and protein), on mineral and nutrient digestion and absorption to determine nutritional solutions to maximize performance of growing-finishing pigs, with an aim to minimize environmental pollution. Data measured in growing-finishing pigs are scarce; therefore, occasionally data from other monogastric species were included in this review. Finally, an enhanced understanding of protein and fiber digestion, and the extent of fermentation among feedstuffs, may help to increase feed formulation flexibility and producer profitability.
2. In Vitro Nutrient Digestion Kinetics of Feedstuffs for Pigs
Rapid and accurate feed quality assessments of digestible nutrient contents of feedstuffs are important to help avoid reduced animal performance or increased feed cost per unit of output [17]. In vivo animal trials to determine feed quality are reliable, but also expensive and time-consuming, with inherent animal welfare considerations [18].
In vitro digestion (IVD) and fermentation models are methods that simulate the digestion and/or fermentation processes that occur in the animals’ gastrointestinal tract (GIT). The IVD model assesses feed quality by mimicking natural digestion processes by directly measuring end nutrient content to estimate digestibility. Table 1, Table 2, Table 3 and Table 4 provide an overview of the classification of feedstuffs by in vitro digestion and fermentation kinetic studies using commercially available purified enzymes for all digestion and fermentation simulation steps. Although many different IVD models exist, they can be categorized in two-step IVD models simulating the stomach (step 1) and small intestine (step 2), and sometimes include a third step (step 3) mimicking disappearance of nutrients in the large intestine [18]. In vitro simulations of hindgut fermentation require inocula from living animals such as hindgut digesta or feces [18] or the use of purified enzymes (e.g., Viscozyme) [19]. The former method, however, results in digestibility values that are closer to in vivo determined values [20]. A detailed overview of methods to predict the nutritive quality of feedstuffs in vitro has been published elsewhere [18].

Table 1.
Classification of undigested feedstuffs or diets by in vitro protein digestion kinetic studies using commercially available purified enzymes for all simulation steps.

Table 2.
Classification of undigested feedstuffs or diets by in vitro starch digestion kinetic studies using commercially available purified enzymes for all simulation steps.

Table 3.
Classification of undigested feedstuffs or diets by in vitro mineral digestion kinetic studies using commercially available purified enzymes for all simulation steps.

Table 4.
Classification of feedstuffs by in vitro fermentation kinetic studies.
In addition to quantitative information on nutrient release or metabolite production, the rates (kinetics) of nutrient digestion or fermentation are important to understand the timing of dietary nutrient release along the GIT. This information could be applied to predict effects on post-absorptive appearance of nutrients such as the net portal appearance of fermentation metabolites, and related post-absorptive metabolism such as the net portal appearance of incretin and glucagon-like peptide [61]. In vitro nutrient digestion and fermentation kinetic data can then be used to optimize feed formulations using concepts such as the nutrient synchronization of energy and protein to increase nitrogen utilization in pigs [63,64,65], or modulating specific SCFA production to promote the proliferation of beneficial microbiota in the GIT [66]. As illustrated in Table 5, studies have examined digestion kinetics of starch and protein, and fiber fermentation. Furthermore, quantitative information on differences in nutrient digestion or fermentation kinetics among different feedstuffs or purified sources were classified based on fast, slow, and resistant digestible nutrients [38,39,41,61,67,68]. Nevertheless, further research into the role of the synergistic effects of nutrient digestion is required because undigested nutrients may affect the digestion of other nutrients [18]. Finally, kinetics of a single ingredient may not reflect the kinetics of a more complex ingredient blend due to ingredient interactions.

Table 5.
Classification of feedstuffs or diets by in vitro or in vivo digestion or in vitro fermentation kinetics.
2.1. Starch Digestion and Fermentation Kinetics
2.1.1. In Vitro Starch Digestion Kinetics
Starch digestion in the small intestine yields glucose as an end product for absorption, whereas the microbial fermentation of starch throughout the intestine produces SCFA (e.g., butyrate and propionate). Starch digestion and fermentation affect feed utilization, digestive physiology, and gut health in pigs [72]. Kinetics of starch digestion are affected by several factors, such as starch chemistry (e.g., amylose:amylopectin ratio [68]), particle size, processing method, and association with other components [73]. In pigs, IVD models have been used to determine starch digestion kinetics and glucose absorption [41]. One such IVD model mimics starch digestion by a two-step IVD process using pepsin followed by enzymatic digestion with pancreatin, amyloglucosidase, and invertase, and analysis of released glucose over time at 39 °C [72]. Based on the rate and extent of in vitro enzymatic digestion [41,67], digested fractions can be classified as rapidly digestible starch (within 20 min of incubation), slowly digestible starch (between 20 and 120 min), and resistant starch (more than 120 min, not further hydrolyzed). The in vitro glucose release was linearly related (R2 = 0.95) to the cumulative portal glucose appearance in pigs after correcting for predicted gastric emptying, indicating that in vitro starch digestion kinetics adequately predict net portal glucose appearance [41].
2.1.2. In Vivo Application of In Vitro Kinetics
Gastric starch digestion is underestimated, consequently contributing to more rapid initial starch digestion in vivo, thereby challenging the prediction quality of in vitro assays [44]. In vivo starch digestion kinetics of nine diets differing in starch source (barley, corn, and high-amylose corn) and form (isolated, within cereal matrix, and extruded) were determined and compared with in vitro digestion values [67]. The in vivo starch digestion exceeded the in vitro predictions for rapidly digested starch. Within 5 min of small intestinal digestion in vivo, starch disappearance averaged 35% and resulted in the typical end products of α-amylase, whereas in vitro only 13% of starch was digested in the same time [44,45]. In particular, in the stomach and small intestine, the hydrolysis rate and digesta transport modulates the rates of nutrient absorption [18], whereas digesta transport is affected by several factors such as meal size [74], energy content [75], and nutrient-related mechanisms [76,77]. In addition, the substrate to enzyme ratio is also likely lower in vitro due to highly aqueous environment relative to GIT digesta. Notably, the rate of an enzyme-catalyzed reaction is proportional to the concentration of an enzyme–substrate complex according to the Michaels–Menten equation [78]. The solid and liquid digesta transport to the end of the small intestine was studied by feeding diets varying in starch source (barley, corn, and high-amylose corn) and form (isolated starch, ground cereal, extruded cereal) to pigs [79]. The mean retention time of digesta solids ranged between 129 and 225 min for the stomach and 86 and 124 min for the small intestine, with the greatest effect of dietary treatment on the solid digesta mean retention time in the stomach (extrusion reduced mean retention time by 29 to 75 min). The authors concluded that the mean retention time of stomach digesta is difficult to predict from dietary properties because of the complexity of chemical and physical digesta properties.
The amount of digested starch should be described as hydrolyzed starch (starch degradation by endogenous enzymes resulting in intermediate products, such as dextrins and glucose) for in vitro studies [68]. Accordingly, starch classification [67] should be updated into rapidly hydrolyzed starch (RHS; hydrolyzed within 20 min by pancreatin α-amylase, yielding mainly maltose and higher maltodextrins), slowly hydrolyzed starch (SHS; hydrolyzed within 20 to 120 min), and starch resistant to hydrolysis (RSH; not hydrolyzed within 120 min). Finally, for in vivo starch hydrolysis in pigs, starch should be classified based on the amount of starch hydrolyzed: RHS, end products of pancreatic α-amylase within 20 min after digesta enters the small intestine; RSH, not hydrolyzed at ileum site of small intestine; and SHS, difference between RHS and hydrolyzed starch at ileum [68].
Cereal and pulse grains differ in starch structure, amylose to amylopectin ratio [80], and where the protein matrix associated with the starch granules [81] contributes to variations in digestion kinetics in vitro [43]. An in vitro study assessed four diets differing in starch and protein sources, corn-, barley-, faba-bean-, and pea-based diets [46]. In a two-step IVD model and classifying the diets in fast, slow, and resistant starch [41], the pea-based diet had the greatest content of fast and resistant starch, possibly related to the crystalline structure and high-amylose content of pea starch granules [46]. In a growth performance trial [46], diets were fed to pigs and metabolic effects were determined by blood serum biochemical response criteria and glycemic and insulin post-prandial responses. In vivo glucose concentrations in blood 1 h after feeding were greater in pigs fed the corn-based diet than the barley-based diet, whereas the insulin concentration was greater for the barley-based diet 1 h and 2 h after feeding compared with the other treatments, possibly because soluble fiber (β-glucans in barley) affects hormonal release [82], but does not appear to affect the gastric emptying of starch [83,84]. Interactions among nutrients such as the amylose content and protein-starch bonds might be related to the metabolic response and might cause discrepancies in vitro [85]. Feeding a barley-based diet resulted in the greatest average daily gain (622 g/day) compared with other cereal and pulse grain treatments (corn, 495 g/day; faba bean 583 g/day; field pea, 581 g/day), but neither the feed intake nor final body weight were affected. The authors [46] suggested that the barley-based diet can be fed to pigs without reducing the growth performance compared with the corn-based diet. Feeding the faba bean-based diet resulted in lower blood glucose concentrations compared with the barley-based diet. Therefore, the effect of both types of starch and type of dietary fiber in ingredients affected nutrient digestion and absorption, including glycemic and insulinemic responses in pigs.
The broad picture emerging from these recent studies on starch kinetics is that the initial rate of starch digestion is greater in vivo than in vitro, resulting in faster initial starch digestion in vivo. Current studies strongly suggest that characteristics of the starch source affect the digesta retention time, particularly in the stomach. Considering that starch is quantitatively the main macronutrient in pig diets, it can be hypothesized that digestion kinetics of other nutrients, such as protein and minerals, are likely to be affected by starch characteristics as well.
2.2. Fiber Fermentation Kinetics
Dietary fiber comprises non-digestible carbohydrates (NSP, resistant starch, non-digestible oligosaccharides) plus lignin [86]. Fiber is poorly digested by endogenous enzymes but can be fermented by the gut microflora, affecting changes in the physicochemical properties of fiber such as the bulk, viscosity, solubility, water-holding capacity, and fermentability [72]. The rate and extent of fermentation of different dietary fiber fractions is important because the fermentation of dietary fiber mainly produces SCFA (acetate, propionate, and butyrate), lactate and gases, depending on the substrate and microbial ecology in the gut [87]. Consequently, SCFA production can be manipulated by changing substrates reaching the hindgut [88]. There is increasing interest in describing the fermentability of ingredients in the digestive tract of monogastric species to stimulate specific SCFA production through optimized diet formulations to promote beneficial microbiota with positive effects on growth performance and health [18].
The quantification of total dietary fiber (TDF) as the sum of the different fiber fractions (TDF = lignin, cellulose + insoluble hemicellulose + soluble hemicellulose + resistant starch + non-digestible oligosaccharides) is the first step to estimate the fermentability of a feed ingredient or diet [89]. In growing pigs, most soluble dietary fiber, such as soluble hemicellulose, appears to be fermented by the end of the cecum, whereas insoluble fiber is mostly fermented in the colon [90]. In addition, cellulose and lignin are fermented to a limited extent in the large intestine of growing pigs. In ruminant nutrition, the lignin concentration is inversely related to the rumen fermentation of ingredients and diets [91], and this may also be applicable for monogastric species such as pigs. Thus, lignin concentrations of ingredients and diets should be considered in determining fermentability.
In vitro models can be used to evaluate the rate of fermentation in the porcine digestive tract by measuring gas production and concentrations of SCFA [56,92]. This can help to estimate the location of fermentation within the pigs’ GIT, and to target beneficial effects of dietary fiber fermentation. However, in vitro models do not account for the ongoing production and absorption of SCFA that occurs in vivo. Furthermore, in vitro fermentation models need to consider a maximum length of 48 h for porcine studies [61] considering that mean retention times in other sections of the GIT can vary depending on the ingredient source [79]. Regarding starch, chemistry affects the post-ileal nutrient flow, nutrient digestibility, glucose, SCFA absorption, insulin, and incretin secretion in pigs [93,94,95]. Amylose contents affect starch hydrolysis, with high-amylose starch (“resistant” starch) having a greater resistance to enzymatic digestion [93]. Thus, resistant starch is largely fermented and enhances butyrate production in vitro [56,96,97] and in vivo [93], which may induce the growth of colonic epithelium, colonocyte differentiation, and immune responses [87]. With the in vitro gas production technique, fermentation characteristics in the hindgut of four purified starch sources differing in physico-chemical properties were studied: rapidly digestible (<45 g amylose/kg, rice starch); moderately rapid digestible (176 g amylose/kg, rice starch); moderately slow digestible (256 g amylose/kg, pea starch); slow digestible (569 g amylose/kg, corn starch) [61]. Rapidly digestible starch had the greatest fractional rate of degradation, indicating that rapidly digestible starch reaching the large intestine is fermented quickly. In contrast, slowly digestible starch is fermented at a slower rate than the other starch sources. Considering that rapidly digestible starch is digested rapidly [41], and completely [93], in the small intestine of pigs, except in young pigs [98], fermentability data and fermentation kinetics data, combined with coating technologies, could be used to specifically promote fermentation along the GIT [99].
In general, fermentable ingredients have a greater rate of degradation and produce more gas and SCFA than less fermentable ingredients [72]. The effect of treating undigested residues of corn and wheat distillers dried grains with solubles (DDGS) was determined with a multicarbohydrase enzyme (Trichoderma-based carbohydrase containing cellulase (20,000 U/kg hydrolyzed sample), xylanase (56,000 U//kg hydrolyzed sample), or in combination with protease (Bacillus spp. (500 U/kg hydrolyzed sample)) on in vitro fermentation characteristics using porcine fecal inoculum and the matrix structure before and after fermentation [85]. In a two-step IVD model, samples were pre-digested and their undigested residues were fermented using a mineral solution inoculated with fresh pig feces with or without enzyme supplementation. Multicarbohydrase inclusion increased fermentability (total gas and SCFA production) for corn and wheat DDGS, whereas protease in combination with multicarbohydrase inclusion reduced total gas and SCFA production and increased protein fermentation regardless of feedstuff source. The efficacy of multicarbohydrases depends on matrix porosity and ingredient source, whereas protease reduced multicarbohydrase efficacy. A later study [59] assessed the effect of supplemental xylanase (0 and 1500 U/kg diet) and mannanase (0 and 400 U/kg of diet) in an in vitro fermentation model using digested residue of corn DDGS. As a result, the addition of xylanase increased gas production after 8 h of incubation including the production of total SCFA, acetate, and propionate, indicating that supplementation with xylanase started the fermentation rapidly in the proximal part of the large intestine. The in vitro digestion and fermentation characteristics of corn wet distillers grains and corn DDGS were studied [60] without or with multi-enzyme supplementation (xylanase, glucanase, cellulase, mannanase, invertase, protease, and amylase). After a two-step IVD model, undigested residues from in vitro enzymatic digestion were evaluated using an in vitro cumulative gas-production technique. The in vitro digestibility of dry matter (DM) of wet distillers grain did not differ from DDGS, and multi-enzyme supplementation did not affect the in vitro digestibility of DM. However, the total gas production per unit weight of enzymatically unhydrolyzed residue was greater for wet distillers grain than for DDGS, indicting that wet distillers grain is more fermentable than DDGS. Furthermore, multi-enzyme supplementation increased total gas production for wet distillers grain and DDGS, improving the fermentability and degradation of both feedstuffs in the hindgut of pigs. Overall, there was no interaction of feedstuff and multiple enzymes on measured items, implying that drying wet distillers grain into DDGS did not affect the outcome of the multi-enzyme on digestibility of DDGS in pigs.
In monogastric species, fermentation in the GIT is important for animal health and the fermentability of ingredients can be used in formulating diets to stimulate beneficial microbial activity in the GIT [18]. In vitro gas and SCFA production have been used to evaluate wheat bran, soybean hulls, corn bran, oat bran, and sugar beet pulp [62]. The fermentation of wheat bran and oat bran resulted in a higher and faster gas and SCFA production compared with corn bran, sugar beet pulp, and soybean hulls, and the effects were positively correlated with TDF fractions of ingredients. However, the in vitro fermentation responses differed in microbial composition and SCFA production among ingredients. Thus, in vitro fermentation studies can not only provide information on gas and SCFA production, but also microbial composition contributing to the enhanced utilization of fibrous ingredients fed to pigs. Assessing the fermentation kinetics of feedstuffs or diets is a promising approach; however, further studies are required to incorporate kinetic information into formulation practices.
2.3. Protein Digestion Kinetics
To predict crude protein (CP) and AA digestibility, a two-step IVD model is often used. The in vitro digestibility of CP in feedstuffs is considered reliable for calculating coefficients of apparent ileal digestibility (CAID) of individual AA [31]. However, the validation of protein IVD models seems less satisfactory regarding predicted accuracy, especially for the standardized ileal digestibility of amino acids. The IVD models thus require improvement to better predict protein and AA digestibility accurately [18]. In humans [100,101], protein sources with comparable ileal protein digestibility differed in protein digestion kinetics, modulating postprandial appearance of AA and peptides in blood, and post-absorptive metabolism. In adult humans [100], postprandial AA appearance in blood was earlier for fast-digestible whey protein than slow-digestible casein. Similarly, in young men, postprandial retention was better for slowly digested casein than rapidly digested whey proteins [101].
In general, protein digestion kinetics depend on the chemical composition, protein structure, and physicochemical properties of feedstuffs [38]. The ANF may modulate the digestion and utilization of dietary protein and AA. Effects of thermomechanical and enzyme-facilitated processed SBM compared with non-processed SBM on in vitro kinetics of protein digestion and protein and AA digestibility in weaned pigs have previously been studied [39]. Processing reduced the ANF content (lectin, trypsin inhibitor activity, β-conglycinin, and glycinin) compared with non-processed SBM, and increased digested CP, tended to increase fast-digestible CP, and reduced slow and resistant CP compared with non-processed SBM. In addition, CAID and the standardized ileal digestibility of CP and of most AA were greater than in non-processed SBM indicating that processing shortened the time of digestion, increased the extent of digestion, and possibly reduced the risk of protein fermentation in the large intestine.
In broiler chickens, in vitro and in vivo protein digestibility assays can predict the rate and extent of digestion of ingredients [36,37,102]. Evidence exists that the site and rate of the digestion of protein and the absorption of AA affect broiler performance [103,104,105]. Broiler chickens were fed a protein source with either a rapid or slow protein digestion rate and two dietary fiber sources (oat hulls or sugar beet pulp) to assess the effects on growth performance [105]. Broilers fed diets containing rapidly digestible protein had greater average daily gain (ADG) and feed efficiency (gain:feed) after the starter phase. In this study, the ADG (day 28–36; day 0–36) and feed efficiency (day 28–36) of broilers fed slowly digested protein diets with oat hulls did not differ from rapidly digested protein diets supplemented with sugar beet pulp or oat hulls. However, the addition of insoluble dietary fiber, such as oat hulls, to slowly digestible protein could improve performance to the level of broilers fed costly rapidly digested protein, probably by increasing the rate of digesta passage through the distal GIT, resulting in greater feed intake. Effects of feedstuffs (corn, wheat, sorghum, soybean meal, canola meal, full-fat soybean, palm kernel meal, meat and bone meal, wheat DDGS, and wheat bran) on nitrogen and starch digestion kinetics were assessed in broiler chickens [106]. Overall, starch digestion kinetics were faster than nitrogen digestion kinetics. For nitrogen, the disappearance rate was affected by feedstuff, and interactions among feedstuffs, such as for full-fat soybean meal and soybean meal, decreased the nitrogen digestion rate by 25% compared with diets with only soybean meal or full-fat soybean meal. The authors concluded that knowledge about nutrient digestion kinetics and the additive and non-additive effects of feedstuffs in complex diets are important. The transition of dietary protein and amino acids into carcass protein in broiler chickens, and strategies to enhance this transition including nutrient digestion kinetics, were reviewed comprehensively elsewhere [107].
3. Utilization of Selected Minerals in Pigs
3.1. Digestibility of Phosphorus in Pigs
The porcine small intestine, in particular the jejunum, is the major site of P absorption [108]. In general, P homeostasis is regulated by controlling the absorption rate of inorganic phosphate in the upper small intestine and by renal phosphate excretion orchestrated mainly by parathyroid hormone and calcitriol (1,25-dihydroxycholecalciferol; 1,25-(OH)2D3) [109]. In addition to the absorption capacity of the pigs’ intestine, differences in dietary P digestibility must be considered because grains and their co-products are major ingredients in pig diets. Indeed, InsP6 is the most important source of organic P for pigs, whose dietary content ranges between 2 and 3 g/kg DM in diets for pigs and poultry depending on the ingredients, agronomy, and processing conditions [110]. In addition, InsP6 is considered an anti-nutritional factor (ANF) forming complexes with minerals and decreasing the absorption of cations and protein in pigs and poultry [111]. The hydrolysis of InsP6 is incomplete in non-ruminants because of insufficient endogenous phytase activity in the proximal GIT [112], and depends on several factors such as the intrinsic phytase activity of dietary ingredients, endogenous mucosal, and gut phytase activity [110]. The hydrolysis of InsP6 can be increased by supplementing microbial phytase to diets to increase the coefficient of apparent total tract digestibility (CATTD) of P by 26% to 65% [113]. However, studies with pigs focusing on the stepwise degradation of InsP6 to lower inositol phosphate (InsP) forms in the GIT have been rare [110], and just a few studies [113,114,115] differentiated positional InsP forms. A recent study [113] determined that pigs fed a corn–soybean-meal-based diet with up to 3000 FTU exogenous E.coli-derived 6-phytase/kg feed (Experiment 1) or a corn–soybean meal or a corn–soybean meal-rapeseed cake diet supplemented with 1500 FTU/kg feed (Experiment 2), had greater concentrations of Ins(1,2,5,6)P4 and lower concentrations of inositol phosphate5 (InsP5) isomers (Ins(1,2,3,4,5)P5; Ins(1,2,4,5,6)P5) in ileal digesta (Experiment 1) for diets supplemented with microbial phytase (1500 and 3000 FTU/kg feed). The Ins(1,2,5,6)P4 was considered the limiting isomer of InsP degradation for the used microbial phytase, in agreement with previous studies [116,117]. Thus, to increase the digestibility of dietary plant P, strategies to degrade InsP6 to lower forms of InsP are warranted because lower InsP forms can almost be completely digested by pigs [113].
Net P absorption from the hindgut of pigs is extremely limited; thus, CAID and CATTD of P do not differ widely [118]. In pigs, most InsP6 reaching the porcine hindgut is almost completely hydrolyzed (CATTD InsP6, 0.99) by endogenous phytases, probably of bacterial origin, when feeding diets based on corn and soybean meal or corn, soybean meal, and rapeseed cake. Dietary CATTD of P was the greatest at 0.64 for the corn–soybean meal diet supplemented with 1500 FYT phytase/kg diet, suggesting that the released P in the hindgut was not utilized by the pig, but excreted [113]. Endogenous secretion of P into the intestinal tract of pigs that is not absorbed is considerable [119]. The CATTD of P is not additive in pigs [120], whereas the coefficients of standardized total tract digestibility (CSTTD) or true total tract digestibility (CTTTD) of P in feed ingredients, when corrected for basal or basal and diet-specific endogenous P losses (EPLs), are favorable (i.e., additive) for diet formulation. Adequate correction of EPL is important because basal EPLs measured using a P-free diet varied greatly, ranging from 129 to 219 mg P/kg DMI [121]. The total EPLs of P estimated using regression (8 to 455 mg P/kg DMI) varied greatly [119]. The EPLs are diet-dependent and increase with increasing contents of dietary NSP, and may depend on dietary fiber properties [122,123]. In growing pigs, the effect of increasing the dietary inclusion of acacia gum, a low-viscous, fermentable fiber, on nutrient digestibility was assessed in growing pigs fed a low-P control diet (to measure basal EPL), and three additional diets including 25, 50, or 75 g/kg as-fed acacia gum at the expense of corn starch [124]. Increasing the inclusion of acacia gum tended to linearly increase the total tract EPL (basal EPL 377 mg/kg DM intake), likely due to the greater excretion of P of bacterial origin, increased epithelial cell proliferation rate, and sloughing of epithelial cells [125,126]. Moreover, increasing the inclusion of acacia gum tended to linearly decrease diet CAID and CATTD of P, and CSTTD of P calculated based on measured EPL or applying NRC’s [4] recommended value (190 mg P/kg DM intake), likely because of the increased diet-specific EPL. Specific EPLs associated with feeding ingredients high in low-viscous fermentable fiber require further clarification to better calculate CTTTD of P, thus avoid underestimating dietary P digestibility.
3.2. Mineral Digestion Kinetics
As summarized in Table 3, few studies have examined mineral digestibility In vitro, and studies on mineral kinetics are limited to Ca, InsP6, P, and Zn. For other minerals (e.g., Mg, Cu, and Fe), kinetic studies have not been conducted; consequently, IVD models could be developed to determine the rate and extent of mineral digestion to further optimize diet formulations and decrease Cu and Zn emissions. For P and InsP6, no standardized methodology to assess in vitro digestion exists, in particular including phytase supplementation [127]. In vitro assays for non-ruminants focusing on phytase and phytate have been comprehensively reviewed [127].
The digestibility of P differs between plant- and animal-based feedstuffs. In vitro P digestibility was measured in 10 plant-based (alfalfa meal, barley, canola meal, corn, grain sorghum, oats, rice bran, SBM, wheat, and wheat bran) and 4 animal-based feedstuffs (menhaden fish meal, meat and bone meal, spray-dried blood meal, and dried whey) [49]. The in vitro data of the plant-based feedstuffs correlated with in vivo P digestibility (R2 = 0.72–0.88), whereas the animal-based feedstuffs were poorly correlated (R2 = −0.26–0.70). After modification, the IVD model was validated against in vivo digestibility in growing pigs fed diets based on wheat, barley, corn, potato protein concentrate, soybean expeller, or rapeseed expeller [54]. The IVD model accurately predicted the CATTD of P in plant-based diets (R2 = 0.91) and is an inexpensive model to rapidly estimate the CATTD of P in plant feedstuffs. However, further improvements should focus on applied digestive enzymes and the time of digestion modulation using in vitro P digestion models [128], and in improving the prediction of P digestibility of animal byproducts.
3.2.1. Fiber and Mineral Digestibility
Observed effects of dietary fiber on the digestion, absorption, and utilization of minerals in pigs are not consistent [126]. In rats, the effect of Ca on inulin fermentation in the large intestine was assessed by feeding a basal fiber-free, wheat starch-casein diet, or a basal diet supplemented with 150 g/kg chicory inulin, and differing Ca content [129]. Caecal pH was lower, and mineral solubility and Ca absorption were greater in rats fed the diets supplemented with inulin compared with the fiber-free diet. The effects of feeding cellulose, corn starch, and pectin in a low-P basal diet on mineral digestibility were assessed in pigs [130]. The CAID of Ca and CATTD of P and Ca in diets were lower for the pectin diet than basal diets, leading to the conclusion that carbohydrate source affected inevitable P losses. In piglets fed barley–wheat–SBM diets supplemented with potato fiber (50 g/kg) or lignocellulose (20 g/kg), CATTD of P and Zn were greater for the potato fiber than with the lignocellulose diet, whereas CATTD of InsP6 was lower for the potato fiber than the lignocellulose diet [131]. In contrast, CAID and CATTD of P in diets supplemented with different dietary fiber sources (pectin, cellulose, straw meal, inulin) did not differ [132,133]. In this regard, differences in the content and properties of dietary fiber and InsP6 contents of ingredients must be considered to improve predictions on the effects on digesta pH and nutrient solubility affecting mineral digestibility.
Researchers have observed contradictory effects of dietary fiber on P digestibility in monogastric species; thus, the effect of type and inclusion level of dietary fiber should be considered (Table 6, Table 7, Table 8 and Table 9). In growing pigs fed corn-starch-based diets differing in ingredient compositions (SBM, corn DDGS, and CM) at three inclusion levels [134], CAID and CATTD of P were linearly increased by increasing SBM at the expense of corn starch, but there was no effect of increasing corn DDGS or CM inclusion in diets. The effects of feeding barley grain cultivars differing in amylose, β-glucan, and fiber content on mineral digestibility were compared with wheat [135]. Moderate- and low-fermentable barley, and low-fermentable wheat had greater diet CAID of P than highly fermentable barley. In addition, moderate-fermentable barley had greater diet CATTD and CSTTD of P than highly fermentable, high-β-glucan barley, concluding that cereal grains high in fermentable (slowly digestible) fiber (e.g., β-glucans) in specific hull-less barley cultivars resulted in lower dietary CAID, CATTD, and CSTTD of P. In contrast, CATTD of P did not differ between corn starch-casein diets with or without 89.5 g/kg as-fed oat β-glucan concentrate at the expense of corn starch [136]. In growing pigs, CATTD and CSTTD of Ca and CATTD of P fed in a corn-based diet were greater than in a corn-starch-based diet and added phytase and fiber (80 g/kg cellulose at the expense of corn starch) increased CATTD and CSTTD of Ca and CATTD of P in diets [137]. Tail-end dehulling of canola meal that constituted largely removal of insoluble fiber, increased CATTD and CSTTD of P in growing pigs [138]. In a two-step IVD model [139], Zn digestibility was greater in low-InsP6 whole pearl millet flour, and decorticated low-InsP6/fiber/tannin pearl millet fractions than in high-fiber/tannin bran fractions and high-InsP6 decorticated fractions. However, Zn digestibility was greater for low and high InsP6 bran fractions than in high-InsP6 decorticated fractions, possibly because of the lower InsP6:Zn molar ratio. In piglets, the effect of Zn source and dietary fiber (lignocellulose, potato fiber) on nutrient digestibility were assessed. The CATTD of P and Zn were greater, whereas CATTD of InsP6 in diets was lower for potato fiber than lignocellulose diets [131]. The CATTD of P in diets was linearly related to the ADF content of the diet, reducing CATTD of P in diets with increasing ADF content, but not to the dietary NDF content (Figure 1). Lignin concentrations might reduce fermentation in monogastric species, similar to that observed in ruminants [91]. The estimation of fermentability of feed ingredients or diets based on TDF [89] should be considered in determining the CATTD of P. Nevertheless, further research into the role of dietary fiber and minerals is still required, with special focus to be directed to individual InsP on mineral digestibility. InsP6 and the chemical structure of individual NSP affect digestion and fermentation of nutrients; therefore, future research should concentrate on ingredient and feed processing strategies that increase nutrient digestibility and animal performance, such as pre-digestion techniques using exogenous enzymes [111,140].

Table 6.
Effect of fermentable substrates (starch, fiber, and protein) on the apparent ileal digestibility of minerals and InsP6 in growing pigs (initial body weight (BW) < 30 kg).

Table 7.
Effect of fermentable substrates (starch, fiber, and protein) on apparent ileal digestibility of minerals and InsP6 in growing pigs (initial body weight (BW) > 30 kg).

Table 8.
Effect of fermentable substrates (protein and fiber) on apparent total tract digestibility of minerals and InsP6 in weaned and growing pigs (initial body weight (BW) < 30 kg).

Table 9.
Effect of fermentable substrates (protein and fiber) on apparent total tract digestibility of minerals and InsP6 in growing pigs (initial body weight (BW) > 30 kg).
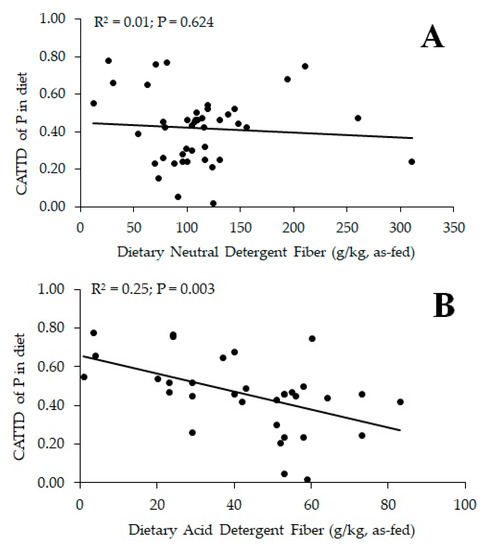
Figure 1.
Relationships between diet neutral (NDF; A) or acid detergent fiber (ADF; B) content and coefficient of apparent total tract digestibility (CATTD) of phosphorus (P) in diets fed to pigs using data in Table 8 and Table 9 [115,130,131,132,135,137,138,147]. Data were analyzed using PROC REG of SAS (version 9.4; SAS Institute) to determine linear relationships between diet NDF or ADF content and CATTD of P in diets. A value of p < 0.05 was considered significant.
3.2.2. Nutrient Kinetics and Exogenous Enzymes
In vitro, one part of InsP6 in cereals and oilseeds is readily degraded, whereas the residual part requires a longer digestion time [148]. The less degradable part of InsP6 may consist of complexes formed with minerals and/or proteins, or InsP6 may be encapsulated within the cell wall matrix, reducing its accessibility to exogenous enzymes such as phytase [50,53,149]. Using an IVD model simulating the fish stomach, effects of E. coli phytase (2500 FYT/kg DM) supplementation on P and protein digestibility in eight plant ingredients (SBM, field pea, broad bean meal, chickpea protein isolate, lupin meal, canola meal, wheat middlings, and wheat flour) was assessed [150]. Degradation of InsP6 by exogenous phytase increased protein solubility in all ingredients, except wheat flour (pH-dependent: broad bean meal, field peas, acidic and neutral pH; SBM, chickpea protein isolate, acidic pH; lupin meal, pH 4.0, 5.0; wheat middlings, pH 2.0, 3.0, 4.0, 5.0; canola meal, pH 2.0, 3.0). Exogenous phytase might increase protein solubility in leguminous seeds, but to a lesser extent in cereals and canola meal. Such conclusions are in agreement with a broiler study feeding seven plant ingredients (corn, SBM, wheat, wheat middlings, barley, defatted rice bran, and canola) as the sole source of P with and without added phytase (600 FYT phytase/kg diet [151]). Total tract degradation of InsP6 was greatest for the SBM and barley diet and lowest for the wheat and defatted rice diet. In broilers, addition of exogenous Buttiauxella phytase (500 FYT/kg feed) to a corn-based diet increased proximal jejunal, distal ileal starch, proximal, distal jejunal and ileal protein apparent digestibility, and reduced starch:protein disappearance rate ratios [152]. Exogenous phytase inclusion increased the apparent AA digestibility in four intestine segments, particularly the proximal jejunum. In addition, the apparent digestibility of Na and P was greater for phytase-supplemented diets in the proximal and distal small intestine, whereas Na CAID was correlated with starch and protein CAID. Increasing protein disappearance rates in the proximal ileum would be advantageous, whereas increasing starch disappearance rates would be disadvantageous in terms of weight gain over 40 days, emphasizing that starch and protein digestion kinetics and the post-enteral availability of glucose and AA at sites of protein synthesis are important for broiler growth. The authors concluded that supplementation with exogenous phytase and the effect on Na apparent digestibility in the small intestine might be relevant for glucose and AA absorption.
Phytate sources (phytate content, location, and ingredient matrix) might determine the extent of InsP6 degradation [127]. Moreover, the degradation of InsP is reduced by increasing the dietary Ca content in monogastric species [153]. Increasing pH [154] and small calcium carbonate particle size (28 µm) with greater solubility (>70%) increased Ca–phytate complex formation or inhibited phytase efficacy [127,155].
In rats, Zn forms insoluble complexes with phytate in the GIT, reducing Zn digestibility [156,157]. A two-step IVD model was applied to estimate the Ca, Mg, Fe, Cu, and Zn digestibility of eight different breads (white, brown, whole meal wheat, rye, brown bread with sunflower seed, white bread with hazelnut, sourdough fermented brown, and sourdough fermented brown bread with sunflower seed) varying in InsP6 content [158]. During pancreatic digestion, the in vitro digestibility, measured as dialysability, was decreased for Ca, Mg, Fe, and Cu with increasing pH (from 6.6 to 7.1), whereas the digestibility of Zn was not affected, suggesting a strong effect of pH on mineral digestibility. In addition, the InsP6 content of the breads might have reduced the digestibility of Ca, Fe, and Zn, possibly by forming insoluble Ca–Zn–phytate complexes with increasing pH [159]. Effects of dietary Zn source included in corn–SBM-based diets differing in Zn, phytate, and exogenous phytase (500 FYT Aspergillus niger phytase/kg feed) content were assessed in piglets [160]. Phytase supplementation increased soluble Zn in the stomach and tended to increase soluble Zn content in the intestine, possibly by lowering gastric pH and resulting in increased mineral solubility [145,161].
In grower pigs, the effects of wheat millrun inclusion (200 or 400 g/kg), xylanase (0 or 4375 U/kg feed), and phytase (0 or 500 U/kg feed) level on nutrient digestibility and growth performance were assessed [142]. The CAID and CATTD of P and Ca were reduced linearly with the increasing inclusion of wheat millrun. The supplementation of xylanase increased CAID of P in diets. There was a synergistic effect of xylanase and phytase increasing CATTD of P, resulting in a similar CATTD of P for the 200 g/kg wheat millrun than in the wheat control diet. The authors concluded that NSP and phytate limit nutrient digestibility in wheat co-products, and exogenous phytase and xylanase supplementation increased P digestibility. The effect of the dietary fiber content of rapeseed meal with and without added microbial phytase (Aspergillus niger 500 FYT/kg feed) on mineral digestibility in growing pigs was studied [147]. Feeding exogenous phytase increased CATTD of P, and gastric and cecal inorganic P solubility was greater in pigs fed diets with exogenous phytase. The dietary inclusion of 45 or 90 g/kg rapeseed hulls quadratically increased inorganic P solubility in the caecum. The decrease in digesta pH from the distal ileum into the cecum increased inorganic P solubility and might increase P absorption. In the caecum, dietary fiber might affect nutrient solubility by increasing the effect of microbial phytase on P digestibility.
The digestibility of P for other co-products, such as DDGS, is enhanced by the fermentation process lowering the phytate content, resulting in a greater CATTD of P of wheat–pea-based diets with corn-, wheat-, or corn–wheat DDGS than in wheat–pea diet [144] without DDGS. Similarly, the CATTD of P was 60% lower for the wheat diet than wheat–DDGS diet and was not affected by supplementation with 4000 U/kg feed xylanase [143]. Feed processing, such as the fermentation of wheat bran, resulted in greater dietary CATTD of P than feeding untreated or extruded wheat bran, probably because of a lower phytate content in fermented wheat bran diets [146]. Further research into the effects of feed processing technologies on nutrient and mineral digestion kinetics is warranted.
4. Conclusions
Current feed formulations for pigs and poultry are largely based on ingredient inclusion levels, nutrient level constraints, and nutrient digestibility data to increase feed efficiency and reduce nutrient excretion. However, the rate and extent of nutrient digestion or fermentation, and information about the timing of dietary nutrient release along the GIT, are missing. Nutrient kinetic data could be applied to help predict effects on post-absorptive nutrient appearance, and to further improve nutrient utilization and diet formulation practices. Novel approaches such as the combination of in vitro and in silico methods for feedstuff evaluation to predict in silico digestive processes such as digesta transit, nutrient hydrolysis, and absorption kinetics of feedstuffs can be used to evaluate the extent of nutrient digestion of feedstuffs in the GIT [162].
Although results on the interaction between minerals and fermentable substrates are inconsistent, several studies have shown that CSTTD of P of diets might be underestimated in diets with fermentable ingredients because of increased diet-specific EPL; thus, the CTTTD of P should be used. The quantification of TDF (and/or other fiber fractions) to estimate fermentability should be considered when formulating diets to influence digesta pH and nutrient solubility, and to predict mineral and protein digestibility. Due to differences in P digestibility, and the formation of individual InsP because of variations in InsP6 content, the intrinsic and exogenous phytase effects of feed ingredients should be considered. Nevertheless, further research regarding standardized methodologies to assess in vitro digestion is still required, in particular to improve the use and application of digestive enzymes and time of digestion, but also on the effects of undigested nutrients on digestive processes.
Author Contributions
Conceptualization, C.M.E.H., N.W.J., G.I.P. and R.T.Z.; writing—original draft preparation, C.M.E.H.; writing—review and editing, C.M.E.H., N.W.J., G.I.P. and R.T.Z.; funding acquisition, C.M.E.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Mitacs Elevate Postdoctoral Fellowship Program cofunded by Trouw Nutrition Canada, grant number IT18163 awarded to Charlotte M. E. Heyer.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors certify that they have no conflict of interest to declare. The authors, Neil W. Jaworski and Greg I. Page, are employees of Trouw Nutrition, This research was funded by Mitacs Elevate Postdoctoral Fellowship Program cofunded by Trouw Nutrition Canada, grant number IT18163 awarded to Charlotte M. E. Heyer. The origin of author salaries does not alter authors’ adherence to journal policies and materials.
References
- Carus, M.; Dammer, L. The circular bioeconomy–concepts, opportunities, and limitations. Ind. Biotechnol. 2018, 14, 83–91. [Google Scholar] [CrossRef]
- Shurson, G.C.; Hung, Y.-T.; Jang, J.C.; Urriola, P.E. Measures matter—Determining the true nutri-physiological value of feed ingredients for swine. Animals 2021, 11, 1259. [Google Scholar] [CrossRef] [PubMed]
- Pomar, C.; Hauschild, L.; Zhang, G.H.; Pomar, J.; Lovatto, P.A. Applying precision feeding techniques in growing-finishing pig operations. Rev. Bras. Zootecn. 2009, 38, 226–237. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Swine, 11th ed.; The National Academies Press: Washington, DC, USA, 2012. [Google Scholar]
- Dourmad, J.-Y.; Jondreville, C. Impact of nutrition on nitrogen, phosphorus, Cu and Zn in pig manure, and on emissions of ammonia and odours. Livest. Sci. 2007, 112, 192–198. [Google Scholar] [CrossRef]
- Rodehutscord, M. Approaches for saving limited phosphate resources. Arch. Tierz. 2008, 51, 39–48. [Google Scholar]
- European Food Safety Authority (EFSA) FEEDAP (Additives and Products or Substances Used in Animal Feed) Panel. Scientific opinion on the potential reduction of the currently authorised maximum zinc content in complete feed. EFSA J. 2014, 12, 3668–3745. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA) FEEDAP (Additives and Products or Substances Used in Animal Feed) Panel. Revision of the currently authorised maximum copper content in complete feed. EFSA J. 2016, 14, 4563–4663. [Google Scholar] [CrossRef]
- Bennett, E.M.; Carpenter, S.R.; Caraco, N.F. Human impact on erodable phosphorus and eutrophication: A global perspective: Increasing accumulation of phosphorus in soil threatens rivers, lakes, and coastal oceans with eutrophication. BioScience 2001, 51, 227–234. [Google Scholar] [CrossRef]
- Cordell, D.; Neset, T.S.S. Phosphorus vulnerability: A qualitative framework for assessing the vulnerability of national and regional food systems to the multidimensional stressors of phosphorus scarcity. Glob. Environ. Chang. Policy Dimens. 2014, 24, 108–122. [Google Scholar] [CrossRef]
- Cordell, D.; Drangert, J.-O.; White, S. The story of phosphorus: Global food security and food for thought. Glob. Environ. Chang. 2009, 19, 292–305. [Google Scholar] [CrossRef]
- Monteiro, S.C.; Lofts, S.; Boxall, A.B.A. Pre-assessment of environmental impact of zinc and copper used in animal nutrition. EFSA Supporting Publ. 2010, 7, 74E. [Google Scholar] [CrossRef]
- Seiler, C.; Berendonk, T.U. Heavy metal driven co-selection of antibiotic resistance in soil and water bodies impacted by agriculture and aquaculture. Front. Microbiol. 2012, 3, 101–110. [Google Scholar] [CrossRef]
- Imseng, M.; Wiggenhauser, M.; Müller, M.; Keller, A.; Frossard, E.; Wilcke, W.; Bigalke, M. The fate of Zn in agricultural soils: A stable isotope approach to anthropogenic impact, soil formation, and soil-plant cycling. Environ. Sci. Technol. 2019, 53, 4140–4149. [Google Scholar] [CrossRef]
- Commission Implementing Decision. Available online: https://ec.europa.eu/health/documents/community-register/2017/20170626136754/dec_136754_en.pdf (accessed on 18 September 2020).
- Voluntary Risk Assessment Reports—Copper and Copper Compounds. Available online: https://echa.europa.eu/copper-voluntary-risk-assessment-reports (accessed on 22 September 2020).
- De Lange, C.F.M.; Birkett, S.H. Characterization of useful energy content in swine and poultry feed ingredients. Can. J. Anim. Sci. 2005, 85, 269–280. [Google Scholar] [CrossRef]
- Wang, L.F.; Zijlstra, R.T. Prediction of bioavailable nutrients and energy. In Feed Evaluation Science; Moughan, P.J., Hendriks, W.H., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2018; pp. 337–386. [Google Scholar]
- Boisen, S.; Fernández, J.A. Prediction of the total tract digestibility of energy in feedstuffs and pig diets by in vitro analyses. Anim. Feed Sci. Technol. 1997, 68, 277–286. [Google Scholar] [CrossRef]
- Urriola, P.E.; Stein, H.H. Evaluation of in vitro procedures to measure digestibility of fiber in distillers dried grains with solubles. J. Anim. Sci. 2010, 88 (E-Suppl. S2), 368–369. [Google Scholar]
- Decuypere, J.A.; Knockaert, P.; Henderickx, H.K. In Vitro and In Vivo protein digestion in pigs fed diets containing soybean protein isolates with different physical properties. J. Anim. Sci. 1981, 53, 1297–1308. [Google Scholar] [CrossRef]
- Wiesemüller, W.; Poppe, S. Protein digestion in pigs measured in vitro. Arch. Anim. Nutr. 1990, 40, 689–693. [Google Scholar] [CrossRef]
- Babinszky, L.; Van der Meer, J.M.; Boer, H.; Den Hartog, L.A. An in-vitro method for prediction of the digestible crude protein content in pig feeds. J. Sci. Food Agri. 1990, 50, 173–178. [Google Scholar] [CrossRef]
- Cone, J.W.; Van der Poel, A.F.B. Prediction of apparent ileal protein digestibility in pigs with a 2-step in-vitro method. J. Sci. Food Agri. 1993, 62, 393–400. [Google Scholar] [CrossRef]
- Boisen, S.; Fernández, J.A. Prediction of the apparent ileal digestibility of protein and amino-acids in feedstuffs and feed mixtures for pigs by in-vitro analyses. Anim. Feed Sci. Technol. 1995, 51, 29–43. [Google Scholar] [CrossRef]
- Beames, R.M.; Helm, J.H.; Eggum, B.O.; Boisen, S.; Bach Knudsen, K.E.; Swift, M.L. A comparison of methods for measuring the nutritive value for pigs of a range of hulled and hulless barley cultivars. Anim. Feed Sci. Technol. 1996, 62, 189–201. [Google Scholar] [CrossRef]
- Huang, G.; Sauer, W.C.; He, J.; Hwangbo, J.; Wang, X. The nutritive value of hulled and hulless barley for growing pigs. 1. Determination of energy and protein digestibility with the in vivo and in vitro method. J. Anim. Feed Sci. 2003, 12, 759–769. [Google Scholar] [CrossRef]
- Qiao, Y.; Lin, X.; Odle, J.; Whittaker, A.; Van Kempen, T.A.T.G. Refining in vitro digestibility assays: Fractionation of digestible and indigestible peptides. J. Anim. Sci. 2004, 82, 1669–1677. [Google Scholar] [CrossRef]
- Kies, A.K.; De Jonge, L.H.; Kemme, P.A.; Jongbloed, A.W. Interaction between protein, phytate, and microbial phytase. In vitro studies. J. Agric. Food Chem. 2006, 54, 1753–1758. [Google Scholar] [CrossRef]
- Pujol, S.; Torrallardona, D. Evaluation of in vitro methods to estimate the in vivo nutrient digestibility of barley in pigs. Livest. Sci. 2007, 109, 186–188. [Google Scholar] [CrossRef]
- Boisen, S. In vitro analyses for predicting standardised ileal digestibility of protein and amino acids in actual batches of feedstuffs and diets for pigs. Livest. Sci. 2007, 109, 182–185. [Google Scholar] [CrossRef]
- Chiang, C.; Croom, J.; Chuang, S.; Chiou, P.W.S.; Yu, B. A dynamic system simulating pigs’ stomach to evaluate the protein digestibility of pig diet. J. Chin. Soc. Anim. Sci. 2008, 37, 131–143. [Google Scholar]
- Wilfart, A.; Jaguelin-Peyraud, Y.; Simmins, H.; Noblet, J.; van Milgen, J.; Montagne, L. Kinetics of enzymatic digestion of feeds as estimated by a stepwise in vitro method. Anim. Feed Sci. Technol. 2008, 141, 171–183. [Google Scholar] [CrossRef]
- Meunier, J.P.; Manzanilla, E.G.; Anguita, M.; Denis, S.; Pérez, J.F.; Gasa, J.; Cardot, J.M.; Garcia, F.; Moll, X.; Alric, M. Evaluation of a dynamic in vitro model to simulate the porcine ileal digestion of diets differing in carbohydrate composition. J. Anim. Sci. 2008, 86, 1156–1163. [Google Scholar] [CrossRef]
- Cho, J.H.; Kim, I.H. Evaluation of the apparent ileal digestibility (AID) of protein and amino acids in nursery diets by in vitro and in vivo methods. Asian-Aust. J. Anim. Sci. 2011, 24, 1007–1010. [Google Scholar] [CrossRef]
- Bryan, D.D.S.L.; Abbott, D.A.; Classen, H.L. Development of an in vitro protein digestibility assay mimicking the chicken digestive tract. Anim Nutr. 2018, 4, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Bryan, D.D.S.L.; Abbott, D.A.; Classen, H.L. Digestion kinetics of protein sources determined using an in vitro chicken model. Anim. Feed Sci. Technol. 2019, 248, 106–113. [Google Scholar] [CrossRef]
- Chen, H.; Wierenga, P.A.; Hendriks, W.H.; Jansman, A.J.M. In Vitro protein digestion kinetics of protein sources for pigs. Animal 2019, 13, 1154–1164. [Google Scholar] [CrossRef]
- Ton Nu, M.A.; Lupatsch, I.; Zannatta, J.S.; Schulze, H.; Zijlstra, R.T. Thermomechanical and enzyme-facilitated processing of soybean meal enhanced in vitro kinetics of protein digestion and protein and amino acid digestibility in weaned pigs. J. Anim. Sci. 2020, 98, skaa224. [Google Scholar] [CrossRef]
- Sun, T.; Lærke, H.N.; Jørgensen, H.; Bach Knudsen, K.E. The effect of extrusion cooking of different starch sources on the in vitro and in vivo digestibility in growing pigs. Anim. Feed Sci. Technol. 2006, 131, 67–86. [Google Scholar] [CrossRef]
- Van Kempen, T.A.T.G.; Regmi, P.R.; Matte, J.J.; Zijlstra, R.T. In vitro starch digestion kinetics, corrected for estimated gastric emptying, predict portal glucose appearance in pigs. J. Nutr. 2010, 140, 1227–1233. [Google Scholar] [CrossRef]
- Giuberti, G.; Gallo, A.; Masoero, F. Plasma glucose response and glycemic indices in pigs fed diets differing in in vitro hydrolysis indices. Animal 2012, 6, 1068–1076. [Google Scholar] [CrossRef]
- Martens, B.M.J.; Gerrits, W.J.J.; Bruininx, M.A.M.; Schols, H.A. Amylopectin structure and crystallinity explains variation in digestion kinetics of starches across botanic sources in an in vitro pig model. J. Anim. Sci. Biotechnol. 2018, 9, 91. [Google Scholar] [CrossRef]
- Martens, B.M.J.; Flécher, T.; de Vries, S.; Schols, H.A.; Bruininx, E.M.A.M.; Gerrits, W.J.J. Starch digestion kinetics and mechanisms of hydrolysing enzymes in growing pigs fed processed and native cereal-based diets. Br. J. Nutr. 2019, 121, 1124–1136. [Google Scholar] [CrossRef]
- Martens, B.M.J.; Bruininx, E.M.A.M.; Gerrits, W.J.J.; Schola, H.A. The importance of amylase action in the porcine stomach to starch digestion kinetics. Anim. Feed Sci. Technol. 2020, 267, 114546. [Google Scholar] [CrossRef]
- Lombardi, P.; Musco, N.; Calabrò, S.; Tudisco, R.; Mastellone, V.; Vastolo, A.; Infascelli, F.; Cutrignelli, M.I. Different carbohydrate sources affect swine performance and post-prandial glycaemic response. Ital. J. Anim. Nutr. 2020, 19, 421–430. [Google Scholar] [CrossRef]
- Sandberg, A.-S.; Svanberg, U. Phytate hydrolysis by phytase in cereals; Effects on in vitro estimation of iron availability. J. Food Sci. 1991, 56, 1330–1333. [Google Scholar] [CrossRef]
- Liu, J.; Ledoux, R.; Veum, T.L. In Vitro procedure for predicting the enzymatic dephosphorylation of phytate in corn−soybean meal diets for growing swine. J. Agric. Food Chem. 1997, 45, 2612–2617. [Google Scholar] [CrossRef]
- Liu, J.; Ledoux, D.R.; Veum, T.L. In vitro prediction of phosphorus availability in feed ingredients for swine. J. Agric. Food Chem. 1998, 46, 2678–2681. [Google Scholar] [CrossRef]
- Newkirk, R.W.; Classen, H.L. In vitro hydrolysis of phytate in canola meal with purified and crude sources of phytase. Anim. Feed Sci. Technol. 1998, 72, 315–327. [Google Scholar] [CrossRef]
- Näsi, M.; Piironen, J.; Partanen, K. Efficacy of Trichoderma reesei phytase and acid phosphatase activity ratios in phytate phosphorus degradation in vitro and in pigs fed maize-soybean meal or barley-soybean meal diets. Anim. Feed Sci. Technol. 1999, 77, 125–137. [Google Scholar] [CrossRef]
- Rodriguez, E.; Porres, J.M.; Han, Y.; Lei, X.G. Different sensitivity of recombinant Aspergillus niger phytase (r-PhyA) and Escherichia coli pH 2.5 acid phosphatase (r-AppA) to trypsin and pepsin in vitro. Arch. Biochem. Biophys. 1999, 365, 262–267. [Google Scholar] [CrossRef]
- Bohn, L.; Josefsen, L.; Meyer, A.S.; Rasmussen, S.K. Quantitative analysis of phytate globoids isolated from wheat bran and characterization of their sequential dephosphorylation by wheat phytase. J. Agric. Food Chem. 2007, 55, 7547–7552. [Google Scholar] [CrossRef]
- Schlegel, P.; Ampuero Kragten, S.; Gutzwiller, A. Validation of an in vitro method for the estimation of apparent total tract digestibility of phosphorus in plant feed ingredients for pigs. Anim. Feed Sci. Technol. 2014, 198, 341–346. [Google Scholar] [CrossRef]
- Awati, A.; Williams, B.A.; Bosch, M.W.; Li, Y.C.; Verstegen, M.W.A. Use of the in vitro cumulative gas production technique for pigs: An examination of alterations in fermentation products and substrate losses at various time points. J. Anim. Sci. 2006, 84, 1110–1118. [Google Scholar] [CrossRef]
- Jha, R.; Bindelle, J.; Rossnagel, B.; Van Kessel, A.G.; Leterme, P. In vitro evaluation of the fermentation characteristics of the carbohydrate fractions of hulless barley and other cereals in the gastrointestinal tract of pigs. Anim. Feed Sci. Technol. 2011, 163, 185–193. [Google Scholar] [CrossRef]
- Jonathan, M.C.; van den Borne, J.J.G.C.; van Wiechen, P.; Souza da Silva, C.; Schols, H.A.; Gruppen, H. In vitro fermentation of 12 dietary fibres by faecal inoculum from pigs and humans. Food Chem. 2012, 133, 889–897. [Google Scholar] [CrossRef]
- Jha, R.; Woyengo, T.A.; Li, J.; Bedford, M.R.; Vasanthan, T.; Zijlstra, R.T. Enzymes enhance degradation of the fiber–starch–protein matrix of distillers dried grains with solubles as revealed by a porcine in vitro fermentation model and microscopy. J. Anim. Sci. 2015, 93, 1039–1051. [Google Scholar] [CrossRef]
- Tiwari, U.P.; Chen, H.; Kim, S.W.; Jha, R. Supplemental effect of xylanase and mannanase on nutrient digestibility and gut health of nursery pigs studied using both in vivo and in vitro models. Anim. Feed Sci. Technol. 2018, 245, 77–90. [Google Scholar] [CrossRef]
- Zangaro, C.A.; Patterson, R.; Woyengo, T.A. Porcine in vitro digestion and fermentation characteristics of corn wet distillers’ grains and dried distillers grains with solubles without or with multi-enzyme. Anim. Feed Sci. Technol. 2019, 245, 114205. [Google Scholar] [CrossRef]
- Jha, R.; Zijlstra, R.T. Physico-chemical properties of purified starch affect their in vitro fermentation characteristics and are linked to in vivo fermentation characteristics in pigs. Anim. Feed Sci. Technol. 2019, 253, 74–80. [Google Scholar] [CrossRef]
- Bai, Y.; Zhao, J.; Tao, S.; Zhao, X.; Pi, Y.; Gerrits, W.J.J.; Johnston, L.J.; Zhang, S.; Yang, H.; Ling, L.; et al. Effect of dietary fiber fermentation on short-chain fatty acid production and microbial composition in vitro. J. Agric. Food Sci. 2020, 100, 4282–4291. [Google Scholar] [CrossRef]
- Van den Borne, J.J.G.C.; Schrama, J.W.; Heetkamp, M.J.W.; Verstegen, M.W.A.; Gerrits, W.J.J. Synchronising the availability of amino acids and glucose increases protein retention in pigs. Animal 2007, 1, 666–674. [Google Scholar] [CrossRef]
- Drew, M.D.; Schafer, T.C.; Zijlstra, R.T. Glycemic index of starch affects nitrogen retention in grower pigs. J. Anim. Sci. 2012, 90, 1233–1241. [Google Scholar] [CrossRef]
- Nguyen, N.; Fledderus, J.; Busink, R.; Smits, C.; Ramaekers, P.J.L.; Jaworski, N.W. Interaction between protein sources (wheat gluten and protein concentrate from soy and potato) and starch sources (pre-gelatinized and native pea starch) on weanling pig growth performance and diarrhea incidence. J. Anim. Sci. 2018, 96 (Suppl. S2), 141. [Google Scholar] [CrossRef]
- Tiwari, U.P.; Singh, A.K.; Jha, R. Fermentation characteristics of resistant starch, arabinoxylan, and β-glucan and their effects on the gut microbial ecology of pigs: A review. Anim. Nutr. 2019, 5, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Englyst, H.N.; Kingman, S.M.; Cummings, J.H. Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 1992, 46, 33–50. [Google Scholar]
- Martens, B.M.J. Starch Digestion Kinetics in Pigs: The Impact of Starch Structure, Feed Processing, and Digesta Passage Behaviour. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 5 July 2019. [Google Scholar] [CrossRef]
- Van Kempen, T.; Pujol, S.; Tibble, S.; Balfagon, A. In vitro characterization of starch digestion and its implications for pigs. In Paradigms in Pig Science; Wiseman, J., Varley, M.A., McOrist, S., Kemp, B., Eds.; Nottingham University Press: Nottingham, UK, 2007; pp. 515–526. [Google Scholar]
- Ørskov, E.R.; McDonald, I. The estimation of protein degradability in the rumen from incubation measurements weighted according to rate of passage. J. Agric. Sci. 1979, 92, 499–503. [Google Scholar] [CrossRef]
- France, J.; Dhanoa, M.S.; Theodorou, M.K.; Lister, S.J.; Davies, D.R.; Isac, D. A model to interpret gas accumulation profiles associated with in vitro degradation of ruminant feeds. J. Theor. Biol. 1993, 163, 99–111. [Google Scholar] [CrossRef]
- Zijlstra, R.T.; Jha, R.; Woodward, A.D.; Fouhse, J.; van Kempen, T.A.T.G. Starch and fiber properties affect their kinetics of digestion and thereby digestive physiology in pigs. J. Anim. Sci. 2012, 90, 49–58. [Google Scholar] [CrossRef]
- Giuberti, G.; Gallo, A.; Masoero, F.; Moschini, M. New insight into the role of resistant starch in pig nutrition. Anim. Feed Sci. Technol. 2015, 201, 1–13. [Google Scholar] [CrossRef]
- Gregory, P.C.; McFadyen, M.; Rayner, D.V. Pattern of gastric emptying in the pig: Relation to feeding. Br. J. Nutr. 1990, 64, 45–58. [Google Scholar] [CrossRef]
- Collins, P.J.; Horowitz, M.; Cook, D.J.; Harding, P.E.; Shearman, D.J. Gastric emptying in normal subjects—A reproducible technique using a single scintillation camera and computer system. Gut 1983, 24, 1117–1125. [Google Scholar] [CrossRef]
- Van Citters, G.W.; Lin, H.C. Ileal brake: Neuropeptidergic control of intestinal transit. Curr. Gastroenterol. Rep. 2006, 8, 367–373. [Google Scholar] [CrossRef]
- Maljaars, P.W.J.; Peters, H.P.F.; Mela, D.J.; Masclee, A.A.M. Ileal brake: A sensible food target for appetite control. A review. Physiol. Behav. 2008, 95, 271–281. [Google Scholar] [CrossRef]
- Johnson, K.A.; Goody, R.S. The Original Michaelis Constant: Translation of the 1913 Michaelis-Menten Paper. Biochemistry 2011, 50, 8264–8269. [Google Scholar] [CrossRef]
- Martens, B.M.J.; Noorloos, M.; de Vries, S.; Schols, H.A.; Bruininx, E.M.A.M.; Gerrirts, W.J.J. Whole digesta properties as influenced by feed processing explain variation in gastrointestinal transit times in pigs. Br. J. Nutr. 2019, 122, 1242–1254. [Google Scholar] [CrossRef]
- Singh, N. Functional and physicochemical properties of pulse starch. In Pulse Foods, 2nd ed.; Tiwari, B.K., McKenna, B., Gowen, A., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 87–112. [Google Scholar] [CrossRef]
- Baldwin, P.M. Starch granule-associated proteins and polypeptides: A review. Starch 2001, 53, 475–503. [Google Scholar] [CrossRef]
- Glore, S.R.; Van Treeck, D.; Knehans, A.W.; Guild, M. Soluble fiber and serum lipids (a literature review). J. Am. Diet Assoc. 1994, 94, 425–436. [Google Scholar] [CrossRef]
- Rainbird, A.L.; Low, A.G. Effect of various types of dietary fibre on gastric emptying in growing pigs. Br. J. Nutr. 1986, 55, 111–121. [Google Scholar] [CrossRef]
- Johansen, H.N.; Knudsen, K.E.; Sandström, B.; Skjøth, F. Effects of varying content of soluble dietary fibre from wheat flour and oat milling fractions on gastric emptying in pigs. Br. J. Nutr. 1996, 75, 339–351. [Google Scholar] [CrossRef]
- Regmi, P.R.; Matte, J.J.; van Kempen, T.; Zijlstra, R.T. Starch chemistry affects kinetics of glucose absorption and insulin response in swine. Livest. Sci. 2010, 134, 44–46. [Google Scholar] [CrossRef]
- Phillips, G.O.; Cui, S.W. An introduction: Evolution and finalisation of the regulatory definition of dietary fibre. Food Hydrocoll. 2011, 25, 139–143. [Google Scholar] [CrossRef]
- Jha, R.; Berrocoso, J.D. Review: Dietary fiber utilization and its effects on physiological functions and gut health of swine. Animal 2015, 9, 1441–1452. [Google Scholar] [CrossRef]
- Bach Knudsen, K.E. Microbial degradation of whole-grain complex carbohydrates and impact on short-chain fatty acids and health. Adv. Nutr. Int. Rev. J. 2015, 6, 206–213. [Google Scholar] [CrossRef]
- Navarro, D.M.D.L.; Bruininx, E.M.A.M.; de Jong, L.; Stein, H.H. Effects of inclusion rate of high fiber dietary ingredients on apparent ileal, hindgut, and total tract digestibility of dry matter and nutrients in ingredients fed to growing pigs. Anim. Feed Sci. Technol. 2019, 248, 1–9. [Google Scholar] [CrossRef]
- Jaworski, N.W.; Stein, H.H. Disappearance of nutrients and energy in the stomach and small intestine, cecum, and colon of pigs fed corn-soybean meal diets containing distillers dried grains with solubles, wheat middlings, or soybean hulls. J. Anim. Sci. 2017, 95, 727–739. [Google Scholar] [CrossRef]
- Grabber, J.H.; Mertens, D.R.; Kim, H.; Funk, C.; Lu, F.; Ralph, J. Cell wall fermentation kinetics are impacted more by lignin content and ferulate cross-linking than by lignin composition. J. Sci. Food Agric. 2009, 89, 122–129. [Google Scholar] [CrossRef]
- Williams, B.A.; Bosch, M.W.; Boer, H.; Verstegen, M.W.A.; Tamminga, S. An in vitro batch culture method to assess potential fermentability of feed ingredients for monogastric diets. Anim. Feed Sci. Technol. 2005, 123, 445–462. [Google Scholar] [CrossRef]
- Regmi, P.R.; Metzler-Zebeli, B.U.; Gänzle, M.G.; van Kempen, T.A.T.G.; Zijlstra, R.T. Starch with high amylose content and low in vitro digestibility increases intestinal nutrient flow and microbial fermentation and selectively promotes Bifidobacteria in pigs. J. Nutr. 2011, 141, 1273–1280. [Google Scholar] [CrossRef]
- Regmi, P.R.; van Kempen, T.A.T.G.; Matte, J.J.; Zijlstra, R.T. Starch with high amylose and low in vitro digestibility increases short-chain fatty acid absorption, reduces peak insulin secretion, and modulates incretin secretion in pigs. J. Nutr. 2011, 141, 398–405. [Google Scholar] [CrossRef]
- Fouhse, J.M.; Gänzle, M.G.; Regmi, P.R.; van Kempen, T.A.T.G.; Zijlstra, R.T. High amylose starch with low in vitro digestibility stimulates hindgut fermentation and has a bifidogenic effect in weaned pigs. J. Nutr. 2015, 145, 2464–2470. [Google Scholar] [CrossRef]
- Jha, R.; Owusu-Asiedu, A.; Simmins, P.H.; Pharazyn, A.; Zijlstra, R.T. Degradation and fermentation characteristics of wheat co-products from flour milling in the pig intestine, studied in vitro. J. Anim. Sci. 2012, 90 (E-Suppl. S4), 173–175. [Google Scholar] [CrossRef]
- Giuberti, G.; Gallo, A.; Moschini, M.; Masoero, F. In vitro production of short-chain fatty acids from resistant starch by pig faecal inoculum. Animal 2013, 7, 1446–1453. [Google Scholar] [CrossRef] [PubMed]
- Gdala, J.; Johansen, H.N.; Bach Knudsen, K.E.; Knap, I.H.; Wagner, P.; Jørgensen, O.B. The digestibility of carbohydrates, protein and fat in the small and large intestine of piglets fed non-supplemented and enzyme supplemented diets. Anim. Feed Sci. Technol. 1997, 65, 15–33. [Google Scholar] [CrossRef]
- Williams, B.A.; Verstegen, M.W.A.; Tamminga, S. Fermentation in the large intestine of single-stomached animals and its relationship to animal health. Nutr. Res. Rev. 2001, 14, 207–227. [Google Scholar] [CrossRef] [PubMed]
- Boirie, Y.; Dangin, M.; Gachon, P.; Vasson, M.-P.; Maubois, J.L.; Beaufrère, B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc. Natl. Acad. Sci. USA 1997, 94, 14930–14935. [Google Scholar] [CrossRef]
- Dangin, M.; Boirie, Y.; Garcia-Rodenas, C.; Gachon, P.; Fauquant, J.; Callier, P.; Ballèvre, O.; Beaufrère, B. The digestion rate of protein is an independent regulating factor of postprandial protein retention. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E340–E348. [Google Scholar] [CrossRef]
- Bryan, D.D.S.L.; Van Kessel, A.G.; Classen, H.L. In vivo digestion characteristics of protein sources fed to broilers. Poult. Sci. 2019, 98, 3313–3325. [Google Scholar] [CrossRef]
- Liu, S.Y.; Selle, P.H.; Raubenheimer, D.; Cadogan, D.J.; Simpson, S.J.; Cowieson, A.J. An assessment of the influence of macronutrients on growth performance and nutrient utilisation in broiler chickens by nutritional geometry. Br. J. Nutr. 2017, 116, 2129–2138. [Google Scholar] [CrossRef]
- Truong, H.H.; Chrystal, P.V.; Moss, A.F.; Selle, P.H.; Liu, S.Y. Rapid protein disappearance rates along the small intestine advantage poultry performance and influence the post-enteral availability of amino acids. Br. J. Nutr. 2017, 118, 1031–1042. [Google Scholar] [CrossRef]
- Berrocoso, J.D.; García-Ruiz, A.; Page, G.; Jaworski, N.W. The effect of added oat hulls or sugar beet pulp to diets containing rapidly or slowly digestible protein sources on broiler growth performance from 0 to 36 days of age. Poult. Sci. 2020, 99, 6859–6866. [Google Scholar] [CrossRef]
- Pedersen, N.B.; Hanigan, M.; Zaefarian, F.; Cowieson, A.J.; Nielsen, M.O.; Storm, A.C. The influence of feed ingredients on CP and starch disappearance rate in complex diets for broiler chickens. Poult. Sci. 2021, 100, 101068. [Google Scholar] [CrossRef]
- Macelline, S.P.; Chrystal, P.V.; Liu, S.Y.; Selle, P.H. The dynamic conversion of dietary protein and amino acids into chicken-meat protein. Animals 2021, 11, 2288. [Google Scholar] [CrossRef]
- Breves, G.; Schröder, B. Comparative aspects of gastrointestinal phosphorus metabolism. Nutr. Res. Rev. 1991, 4, 125–140. [Google Scholar] [CrossRef]
- Schröder, B.; Breves, G.; Rodehutscord, M. Mechanisms of intestinal phosphorus absorption and availability of dietary phosphorus in pigs. Dtsch. Tierärztl. Wochenschr. 1996, 103, 209–214. [Google Scholar]
- Rodehutscord, M.; Rosenfelder, P. Update on phytate degradation pattern in the gastrointestinal tract of pigs and broiler chickens. In Phytate Destruction—Consequences for Precision Animal Nutrition; Walk, C.L., Kühn, I., Stein, H.H., Kidd, M.T., Rodehutscord, M., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2016; pp. 15–32. [Google Scholar]
- Woyengo, T.A.; Nyachoti, C.M. Review: Anti-nutritional effects of phytic acid in diets for pigs and poultry—Current knowledge and directions for future research. Can. J. Anim. Sci. 2013, 93, 9–21. [Google Scholar] [CrossRef]
- Selle, P.H.; Ravindran, V. Phytate-degrading enzymes in pig nutrition. Livest. Sci. 2008, 113, 99–122. [Google Scholar] [CrossRef]
- Rosenfelder-Kuon, P.; Klein, N.; Zegowitz, B.; Schollenberger, M.; Kühn, I.; Thuringer, L.; Seifert, J.; Rodehutscord, M. Phytate degradation cascade in pigs as affected by phytase supplementation and rapeseed cake inclusion in corn–soybean meal-based diets. J. Anim. Sci. 2020, 98, skaa053. [Google Scholar] [CrossRef]
- Kemme, P.A.; Schlemmer, U.; Mroz, Z.; Jongbloed, A.W. Monitoring the stepwise phytate degradation in the upper gastrointestinal tract of pigs. J. Sci. Food Agric. 2006, 86, 612–622. [Google Scholar] [CrossRef]
- Heyer, C.M.E.; Schmucker, S.; Burbach, K.; Weiss, E.; Eklund, M.; Aumiller, T.; Capezzone, F.; Steuber, J.; Rodehutscord, M.; Hoelzle, L.E.; et al. Phytate degradation, intestinal microbiota, microbial metabolites and immune values are changed in growing pigs fed diets with varying calcium–phosphorus concentration and fermentable substrates. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1185–1197. [Google Scholar] [CrossRef] [PubMed]
- Kühn, I.; Schollenberger, M.; Männer, K. Effect of dietary phytase level on intestinal phytate degradation and bone mineralization in growing pigs. J. Anim. Sci. 2016, 94, 264–267. [Google Scholar] [CrossRef]
- Mesina, V.G.R.; Lagos, L.V.; Sulabo, R.C.; Walk, C.L.; Stein, H.H. Effects of microbial phytase on mucin synthesis, gastric protein hydrolysis, and degradation of phytate along the gastrointestinal tract of growing pigs. J. Anim. Sci. 2019, 97, 756–767. [Google Scholar] [CrossRef] [PubMed]
- Stein, H.H. Procedures for determining digestibility of amino acids, lipids, starch, fibre, phosphorus, and calcium in feed ingredients fed to pigs. Anim. Prod. Sci. 2017, 57, 2317–2324. [Google Scholar] [CrossRef]
- Zhang, F.; Adeola, O. Techniques for evaluating digestibility of energy, amino acids, phosphorus, and calcium in feed ingredients for pigs. Anim. Nutr. 2017, 3, 344–352. [Google Scholar] [CrossRef]
- Fan, M.Z.; Sauer, W.C. Additivity of apparent ileal and fecal phosphorus digestibility values measured in single feed ingredients for growing-finishing pigs. Can. J. Anim. Sci. 2002, 82, 183–191. [Google Scholar] [CrossRef]
- Almeida, F.N.; Stein, H.H. Standardized total tract digestibility of phosphorus in blood products fed to weanling pigs. Rev. Colomb. Cienc. Pec. 2011, 24, 617–622. [Google Scholar]
- Son, A.R.; Kim, B.G. Effects of dietary cellulose on the basal endogenous loss of phosphorus in growing pigs. Asian-Aust. J. Anim. Sci. 2015, 28, 369–373. [Google Scholar] [CrossRef]
- Bikker, P.; van der Peet-Schwering, C.M.C.; Gerrits, W.J.J.; Sips, V.; Walvoort, C.; van Laar, H. Endogenous phosphorus losses in growing-finishing pigs and gestating sows. J. Anim. Sci. 2017, 95, 1637–1643. [Google Scholar] [CrossRef]
- Heyer, C.M.E.; Wang, L.F.; Zijlstra, R.T. Increasing inclusion of fermentable fiber decreases nutrient digestibility in grower pigs. J. Anim. Sci. 2020, 98 (Suppl. S3), 85. [Google Scholar] [CrossRef]
- Mosenthin, R.; Sauer, W.C.; Ahrens, F. Dietary pectin’s effect on ileal and fecal amino acid digestibility and exocrine pancreatic secretions in growing pigs. J. Nutr. 1994, 124, 1222–1229. [Google Scholar] [CrossRef]
- Metzler, B.U.; Mosenthin, R. A review of interactions between dietary fiber and the gastrointestinal microbiota and their consequences on intestinal phosphorus metabolism in growing pigs. Asian-Aust. J. Anim. Sci. 2008, 21, 603–615. [Google Scholar] [CrossRef]
- Sommerfeld, V.; Santos, R.R. In vitro assays for evaluating phytate degradation in non-ruminants: Chances and limitations. J. Sci. Food Agric. 2021, 101, 3117–3122. [Google Scholar] [CrossRef]
- Li, D.; Lu, Q.; Zhang, H.; Zhuang, X.; Chen, L. Effects of digestive enzymes and digestive time on evaluating in vitro digestibility of phosphorus in common plant-origin feeds by bionic digestion system for growing pigs. Chin. J. Anim. Nutr. 2013, 25, 2051–2058. [Google Scholar]
- Rémésy, C.; Levrat, M.-A.; Gamet, L.C.; Demigné, C. Cecal fermentations in rats fed oligosaccharides (inulin) are modulated by dietary calcium level. Am. J. Physiol. 1993, 264, G855–G862. [Google Scholar] [CrossRef]
- Baumgärtel, T.; Metzler, B.U.; Mosenthin, R.; Greiner, R.; Rodehutscord, M. Precaecal and postileal metabolism of P, Ca and N in pigs as affected by different carbohydrate sources fed at low level of P intake. Arch. Anim. Nutr. 2008, 62, 169–181. [Google Scholar] [CrossRef]
- Barszcz, M.; Taciak, M.; Tuśnio, A.; Čobanová, K.; Grešáková, L. The effect of organic and inorganic zinc source, used in combination with potato fiber, on growth, nutrient digestibility and biochemical blood profile in growing pigs. Livest. Sci. 2019, 227, 37–43. [Google Scholar] [CrossRef]
- Den Hartog, L.A.; Huisman, J.; Thielen, W.J.G.; Van Schayk, G.H.A.; Boer, H.; Van Weerden, E.J. The effect of including various structural polysaccharides in pig diets on ileal and faecal digestibility of amino acids and minerals. Livest. Prod. Sci. 1988, 18, 157–170. [Google Scholar] [CrossRef]
- Vanhoof, K.; De Schrijver, R. Availability of minerals in rats and pigs fed non-purified diets containing inulin. Nutr. Res. 1996, 16, 1017–1022. [Google Scholar] [CrossRef]
- Liu, J.B.; Shen, X.Y.; Zhai, H.X.; Chen, L.; Zhang, H.F. Dietary sources of phosphorus affect postileal phosphorus digestion in growing pigs. J. Anim. Sci. 2017, 95, 4490–4498. [Google Scholar] [CrossRef]
- Heyer, C.M.E.; Fouhse, J.M.; Vasanthan, T.; Zijlstra, R.T. Cereal grain fiber composition modifies phosphorus digestibility in grower pigs. J. Anim. Sci. 2022, 100, skac181. [Google Scholar] [CrossRef]
- Metzler-Zebeli, B.U.; Gänzle, M.G.; Mosenthin, R.; Zijlstra, R.T. Oat β-glucan and dietary calcium and phosphorus differentially modify intestinal expression of proinflammatory cytokines and monocarboxylate transporter 1 and cecal morphology in weaned pigs. J. Nutr. 2012, 142, 668–674. [Google Scholar] [CrossRef] [PubMed]
- González-Vega, J.C.; Walk, C.L.; Stein, H.H. Effect of phytate, microbial phytase, fiber, and soybean oil on calculated values for apparent and standardized total tract digestibility of calcium and apparent total tract digestibility of phosphorus in fish meal fed to growing pig. J. Anim. Sci. 2015, 93, 4808–4818. [Google Scholar] [CrossRef]
- Mejicanos, G.A.; Kim, J.W.; Nyachoti, C.M. Tail-end dehulling of canola meal improves apparent and standardized total tract digestibility of phosphorus when fed to growing pigs. J. Anim. Sci. 2018, 96, 1430–1440. [Google Scholar] [CrossRef]
- Lestienne, I.; Besançon, P.; Caporiccio, B.; Lullien-Péllerin, V.; Tréche, S. Iron and zinc availability in pearl millet flours (Pennisetum glaucum) with varying phytate, tannin, and fiber contents. J. Agric. Food Chem. 2005, 53, 3240–3247. [Google Scholar] [CrossRef] [PubMed]
- Metzler-Zebeli, B.U.; Hooda, S.; Mosenthin, R.; Gänzle, M.G.; Zijlstra, R.T. Bacterial fermentation affects net mineral flux in the large intestine of pigs fed diets with viscous and fermentable nonstarch polysaccharides. J. Anim. Sci. 2010, 88, 3351–3362. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, M.; Gómez, R.; Fierro, S.; Barrera, M.A.; Morales, A.; Araiza, B.A.; Zijlstra, R.T.; Sánchez, J.E.; Sauer, W.C. Ileal digestibility of amino acids, phosphorus, phytate and energy in pigs fed sorghum-based diets supplemented with phytase and pancreatin. J. Anim. Physiol. Anim. Nutr. 2011, 95, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Nortey, T.N.; Patience, J.F.; Simmins, P.H.; Trottier, N.L.; Zijlstra, R.T. Effects of individual or combined xylanase and phytase supplementation on energy, amino acid, and phosphorus digestibility and growth performance of grower pigs fed wheat-based diets containing wheat millrun. J. Anim. Sci. 2007, 85, 1432–1443. [Google Scholar] [CrossRef] [PubMed]
- Widyaratne, G.P.; Patience, J.F.; Zijlstra, R.T. Effect of xylanase supplementation of diets containing wheat distiller’s dried grains with solubles on energy, amino acid and phosphorus digestibility and growth performance of grower-finisher pigs. Can. J. Anim. Sci. 2009, 89, 91–95. [Google Scholar] [CrossRef]
- Widyaratne, G.P.; Zijlstra, R.T. Nutritional value of wheat and corn distiller’s dried grain with solubles: Digestibility and digestible contents of energy, amino acids and phosphorus, nutrient excretion and growth performance of grower-finisher pigs. Can. J. Anim. Sci. 2007, 87, 103–114. [Google Scholar] [CrossRef]
- Blank, R.; Naatjes, M.; Baum, C.; Köhling, K.; Ader, P.; Roser, U.; Susenbeth, A. Effects of formic acid and phytase supplementation on digestibility and use of phosphorus and zinc in growing pigs. J. Anim. Sci. 2012, 90, 212–214. [Google Scholar] [CrossRef]
- Kraler, M.; Schedle, K.; Domig, K.J.; Heine, D.; Michlmayr, H.; Kneifel, W. Effects of fermented and extruded wheat bran on total tract apparent digestibility of nutrients, minerals and energy in growing pigs. Anim. Feed Sci. Technol. 2014, 197, 121–129. [Google Scholar] [CrossRef]
- Bournazel, M.; Lessire, M.; Duclos, M.J.; Magnin, M.; Même, N.; Peyronnet, C.; Recoules, E.; Quinsac, A.; Labussière, E.; Narcy, A. Effects of rapeseed meal fiber content on phosphorus and calcium digestibility in growing pigs fed diets without or with microbial phytase. Animal 2018, 12, 34–42. [Google Scholar] [CrossRef]
- Blaabjerg, K.; Carlsson, N.-G.; Hansen-Møller, J.; Poulsen, H.D. Effect of heat-treatment, phytase, xylanase and soaking time on inositol phosphate degradation in vitro in wheat, soybean meal and rapeseed cake. Anim. Feed Sci. Technol. 2010, 162, 123–134. [Google Scholar] [CrossRef]
- Blaabjerg, K.; Jørgensen, H.; Tauson, A.-H.; Poulsen, H.D. The presence of inositol phosphates in gastric pig digesta is affected by time after feeding a nonfermented or fermented liquid wheat- and barley-based diet. J. Anim. Sci. 2011, 89, 3153–3162. [Google Scholar] [CrossRef]
- Morales, G.A.; Saenz de Rodrigañez, M.; Márquez, L.; Díaz, M.; Moyano, F.J. Solubilisation of protein fractions induced by Escherichia coli phytase and its effects on in vitro fish digestion of plant proteins. Anim. Feed Sci. Technol. 2013, 181, 54–64. [Google Scholar] [CrossRef]
- Leske, K.L.; Coon, C.N. A bioassay to determine the effect of phytase on phytate phosphorus hydrolysis and total phosphorus retention of feed ingredients as determined with broilers and laying hens. Poult. Sci. 1999, 78, 1151–1157. [Google Scholar] [CrossRef]
- Truong, H.H.; Bold, R.M.; Liu, S.Y.; Selle, P.H. Standard phytase inclusion in maize-based broiler diets enhances digestibility coefficients of starch, amino acids and sodium in four small intestinal segments and digestive dynamics of starch and protein. Anim. Feed Sci. Technol. 2015, 209, 240–248. [Google Scholar] [CrossRef]
- Selle, P.H.; Cowieson, A.J.; Ravindran, V. Consequences of calcium interactions with phytate and phytase for poultry and pigs. Livest. Sci. 2009, 124, 126–141. [Google Scholar] [CrossRef]
- Siener, R.; Heynck, H.; Hesse, A. Calcium-binding capacities of different brans under simulated gastrointestinal pH conditions. In vitro study with 45Ca. J. Agric. Food Chem. 2001, 49, 4397–4401. [Google Scholar] [CrossRef]
- McCuaig, L.W.; Davies, M.I.; Motzok, I. Intestinal alkaline phosphatase and phytase of chicks: Effect of dietary magnesium, calcium, phosphorus and thyroactive casein. Poult. Sci. 1972, 51, 526–530. [Google Scholar] [CrossRef]
- Davies, N.T.; Nightingale, R. The effects of phytate on intestinal absorption and secretion of zinc, and whole-body retention of Zn, copper, iron and manganese in rats. Br. J. Nutr. 1975, 34, 243–258. [Google Scholar] [CrossRef]
- Windisch, W.; Kirchgessner, M. Zinc absorption and excretion in adult rats at zinc deficiency induced by dietary phytate additions: I. Quantitative zinc metabolism of 65Zn-labelled adult rats at zinc deficiency. J. Anim. Physiol. Anim. Nutr. 1999, 82, 106–115. [Google Scholar] [CrossRef]
- Wolters, M.G.E.; Schreuder, H.A.W.; van den Heuvel, G.; van Lonkhuijsen, H.A.W.; Hermus, R.J.J.; Voragen, A.G.J. A continuous in vitro method for estimation of the bioavailability of minerals and trace elements in foods: Application to breads varying in phytic acid content. Br. J. Nutr. 1993, 69, 849–861. [Google Scholar] [CrossRef]
- Champagne, E.T.; Phillippy, B.Q. Effects of pH on calcium, zinc, and phytate solubilities and complexes following in vitro digestions of soy protein isolate. J. Food Sci. 1989, 54, 587–592. [Google Scholar] [CrossRef]
- Schlegel, P.; Nys, Y.; Jondreville, C. Zinc availability and digestive zinc solubility in piglets and broilers fed diets varying in their phytate contents, phytase activity and supplemented zinc source. Animal 2010, 4, 200–209. [Google Scholar] [CrossRef]
- Jongbloed, A.W.; Mroz, Z.; van der Weij-Jongbloed, R.; Kemme, P.A. The effects of microbial phytase, organic acids and their interaction in diets for growing pigs. Livest. Prod. Sci. 2000, 67, 113–122. [Google Scholar] [CrossRef]
- Gerrits, W.J.J.; Schop, M.T.A.; de Vries, S.; Dijkstra, J. ASAS-NANP symposium: Digestion kinetics in pigs: The next step in feed evaluation and a ready-to-use modeling exercise. J. Anim. Sci. 2021, 99, skab020. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).