Locally Injected Autologous Platelet-Rich Plasma Improves Cutaneous Wound Healing in Cats
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Anesthesia
2.3. Preparation of Autologous PRP
2.4. Skin Wound Creation and PRP Application
2.5. Postoperative Care
2.6. Evaluation of Wound Healing
2.7. Macroscopic Clinical Evaluation
2.8. Laser Doppler Flowmetry
2.9. Photoplanimetry
n/original wound area day 0 × 100)
n/original wound area day 0
2.10. Histologic Evaluation
2.11. Metalloproteinases-2 and -9, and TIMP-1 mRNA Expression
2.12. Statistical Analysis
3. Results
3.1. PRP Analysis
3.2. Clinical Evaluation
3.3. Photoplanimetry
3.3.1. Epithelialization
3.3.2. Contraction
3.3.3. Total Wound Healing
3.4. Laser Doppler flowmetry
3.5. Histologic Evaluation
3.6. Metalloproteinases
3.6.1. MMP-2
3.6.2. MMP-9
3.6.3. TIMP-1
4. Discussion
5. Conclusions
Author Contributions
Funding

Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mitsui, A.; Mathews, K.G.; Linder, K.E.; Kruse, M.A.; Roe, S.C. Effects of fascial abrasion, fasciotomy, and fascial excision on cutaneous wound healing in cats. Am. J. Vet. Res. 2009, 70, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Bohling, M.W.; Henderson, R.A. Differences in cutaneous wound healing between dogs and cats. Vet. Clin. North Am. Small Anim. Pract. 2006, 36, 687–692. [Google Scholar] [CrossRef]
- Bohling, M.W.; Henderson, R.A.; Swaim, S.F.; Kincaid, S.A.; Wright, J.C. Comparison of the role of the subcutaneous tissues in cutaneous wound healing in the dog and cat. Vet. Surg. 2006, 35, 3–14. [Google Scholar] [CrossRef]
- Affolter, V.K.; Moore, P.F. Histologie features of normal canine and feline skin. Clin. Dermatol. 1994, 12, 491–497. [Google Scholar] [CrossRef]
- Taylor, G.I.; Minabe, T. The angiosomes of the mammals and other vertebrates. Plast. Reconstr. Surg. 1992, 89, 181–215. [Google Scholar] [CrossRef] [PubMed]
- Caley, M.P.; Martins, V.; O’Toole, E. Metalloproteinases and Wound Healing. Adv. Wound Care 2015, 4, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Krejner, A.; Litwiniuk, M.; Grzela, T. Matrix metalloproteinases in the wound microenvironment: Therapeutic perspectives. Chronic Wound Care Manag. Res. 2016, 3, 29–39. [Google Scholar]
- Nguyen, T.T.; Mobashery, S.; Chang, M. Roles of Matrix Metalloproteinases in Cutaneous Wound Healing. In Wound Healing-New Insights into Ancient Challenges; Alexandrescu, V.A., Ed.; IntechOpen: London, UK, 2016. [Google Scholar]
- Michopoulou, A.; Rousselle, P. How do epidermal matrix metalloproteinases support re-epithelialization during skin healing? Eur. J. Dermatol. 2015, 25 (Suppl. S1), 33–42. [Google Scholar] [CrossRef]
- Ladwig, G.P.; Robson, M.C.; Liu, R.; Kuhn, M.A.; Muir, D.F.; Schultz, G.S. Ratios of activated matrix metalloproteinase-9 to tissue inhibitor of matrix metalloproteinase-1 in wound fluids are inversely correlated with healing of pressure ulcers. Wound Repair Regen. 2002, 10, 26–37. [Google Scholar] [CrossRef]
- Lana, S.E.; Ogilvie, G.K.; Hansen, R.A.; Powers, B.E.; Dernell, W.S.; Withrow, S.J. Identification of matrix metalloproteinases in canine neoplastic tissue. Am. J. Vet. Res. 2000, 61, 111–114. [Google Scholar] [CrossRef]
- Loukopoulos, P.; Mungall, B.A.; Straw, R.C.; Thornton, J.R.; Robinson, W.F. Matrix metalloproteinase-2 and -9 involvement in canine tumors. Vet. Pathol. 2003, 40, 382–394. [Google Scholar] [CrossRef] [PubMed]
- Krupakaran, P. Matrix Metalloproteinase-9 (Mmp-9) activity in urine samples of dogs affected with mammary tumor. CIBTech J. Bio-Protoc. 2013, 2, 22–28. [Google Scholar]
- Saxena, S.; Shrivastava, S.; Arora, R.; Hussain, S.; Jena, S.C.; Kumar, M.; Vasu, R.K.; Srivastava, S.; Sharma, P.; Kumar, N.; et al. Development of Real-Time PCR Assays for Detecting Matrix Metalloproteinases-2 & 9 Over-expression in Canine Mammary Tumours. Adv. Anim. Vet. Sci. 2016, 4, 342–345. [Google Scholar]
- Farghali, H.A.; AbdelKader, N.A.; Khattab, M.S.; AbuBakr, H.O. Evaluation of subcutaneous infiltration of autologous platelet-rich plasma on skin-wound healing in dogs. Biosci. Rep. 2017, 37, BSR20160503. [Google Scholar] [CrossRef] [PubMed]
- Nurden, A.T.; Nurden, P.; Sanchez, M.; Andia, I.; Anitua, E. Platelets and wound healing. Front. Biosci. 2008, 13, 3532–3548. [Google Scholar] [CrossRef]
- Roukis, T.S.; Zgonis, T.; Tiernan, B. Autologous platelet-rich plasma for wound and osseous healing: A review of the literature and commercially available products. Adv. Ther. 2006, 23, 218–237. [Google Scholar] [CrossRef]
- Tambella, A.M.; Attili, A.R.; Dini, F.; Piccionello, A.P.; Vullo, C.; Serri, E.; Scrollavezza, P.; Dupré, G. Autologous platelet gel to treat chronic decubital ulcers: A randomized, blind controlled clinical trial in dogs. Vet. Surg. 2014, 43, 726–733. [Google Scholar] [CrossRef]
- Eppley, B.L.; Pietrzak, W.S.; Blanton, M. Platelet-rich plasma: A review of biology and applications in plastic surgery. Plast. Reconstr. Surg. 2006, 118, 147e–159e. [Google Scholar] [CrossRef]
- Saunders, W.B.; Bearden, R.N.; Franklin, S.P. Platelet-Rich Plasma and Autologous Conditioned Sera. In Veterinary Surgery Small Animal, 2nd ed.; Johnston, S.A., Tobias, K.M., Eds.; Elsevier: St Louis, MO, USA, 2018; Volume 1, pp. 40–48. [Google Scholar]
- Ferrari, J.T.; Schwartz, P. Prospective Evaluation of Feline Sourced Platelet-Rich Plasma Using Centrifuge-Based Systems. Front. Vet. Sci. 2020, 7, 1–6. [Google Scholar] [CrossRef]
- Alves, R.; Grimalt, R. A Review of Platelet-Rich Plasma: History, Biology, Mechanism of Action, and Classification. Ski. Appendage Disord 2018, 4, 18–24. [Google Scholar] [CrossRef]
- Marx, R.E. Platelet-rich plasma (PRP): What is PRP and what is not PRP? Implant Dent. 2001, 10, 225–228. [Google Scholar] [CrossRef]
- Gonshor, A. Technique for producing platelet-rich plasma and platelet concentrate: Background and process. Int. J. Periodontics Restor. Dent. 2002, 22, 547–557. [Google Scholar]
- Cortese, L.; Christopherson, P.W.; Pelagalli, A. Platelet Function and Therapeutic Applications in Dogs: Current Status and Future Prospects. Animals 2020, 10, 201. [Google Scholar] [CrossRef] [PubMed]
- McLellan, J.; Plevin, S. Does it matter which platelet-rich plasma we use? Equine Vet. Educ. 2011, 23, 101–104. [Google Scholar] [CrossRef]
- Franklin, S.P.; Garner, B.C.; Cook, J.L. Characteristics of canine platelet-rich plasma prepared with five commercially available systems. Am. J. Vet. Res. 2015, 76, 822–827. [Google Scholar] [CrossRef] [PubMed]
- Foster, T.E.; Puskas, B.L.; Mandelbaum, B.R.; Gerhardt, M.B.; Rodeo, S.A. Platelet-rich plasma: From basic science to clinical applications. Am. J. Sports Med. 2009, 37, 2259–2272. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Woodell-May, J.; Ponticiello, M.; Yang, Z.; Nimni, M. The effect of thrombin activation of platelet-rich plasma on demineralized bone matrix osteoconductivity. J. Bone Joint Surg. Am. 2009, 91, 1459–1470. [Google Scholar] [CrossRef] [PubMed]
- Arguelles, D.; Carmona, J.U.; Pastor, J.; Iborra, A.; Viñals, L.; Martínez, P.; Bach, E.; Prades, M. Evaluation of single and double centrifugation tube methods for concentrating equine platelets. Res. Vet. Sci. 2006, 81, 237–245. [Google Scholar] [CrossRef]
- Bhanot, S.; Alex, J.C. Current Applications of Platelet Gels in Facial Plastic Surgery. Facial Plast. Surg. 2002, 18, 27–34. [Google Scholar] [CrossRef]
- Froum, S.J.; Wallace, S.S.; Tarnow, D.P.; Cho, S.C. Effect of platelet-rich plasma on bone growth and osseointegration in human maxillary sinus grafts: Three bilateral case reports. Int. J. Periodontics Restor. Dent. 2002, 22, 45. [Google Scholar]
- Alio, J.L.; Arnalich-Montiel, F.; Rodriguez, A.E. The role of “eye platelet rich plasma” (E-PRP) for wound healing in ophthalmology. Curr. Pharm. Biotechnol. 2012, 13, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Knop, E.; De Paula, L.E.; Fuller, R. Platelet-rich plasma for osteoarthritis treatment. Rev. Bras. Reumatol. Engl. Ed. 2016, 56, 152–164. [Google Scholar] [CrossRef] [PubMed]
- Mlynarek, R.A.; Kuhn, A.W.; Bedi, A. Platelet-Rich Plasma (PRP) in Orthopedic Sports Medicine. Am. J. Orthop. 2016, 45, 290–326. [Google Scholar] [PubMed]
- Emer, J. Platelet-Rich Plasma (PRP): Current Applications in Dermatology. Skin Therapy Lett. 2019, 24, 1–6. [Google Scholar]
- Peng, G.L. Platelet-Rich Plasma for Skin Rejuvenation: Facts, Fiction, and Pearls for Practice. Facial Plast. Surg. Clin. North. Am. 2019, 27, 405–411. [Google Scholar]
- Li, H.; Zou, X.; Xue, Q.; Egund, N.; Lind, M.; Bünger, C. Anterior lumbar interbody fusion with carbon fiber cage loaded with bioceramics and platelet-rich plasma. An experimental study on pigs. Eur. Spine J. 2004, 13, 354–358. [Google Scholar] [CrossRef][Green Version]
- Al-Bayati, A.H.; Al-Asadi, R.N.; Mahdi, A.K.; Al-Falahi, N.H. Effects of Autologous Platelets Rich Plasma on Full-thickness Cutaneous Wounds Healing in Goats. Int. J. Anim. Vet. Adv. 2013, 5, 233–239. [Google Scholar] [CrossRef]
- Bauer, J.A.; Correa, L.; Lima, F.L.M.; Lima, L.A.P.A.; Pustiglioni, F.E. Efeitos do plasma rico em plaquetas no processo de reparacËão de feridas deÂrmicas padronizadas em ratos. R. Periodontia. 2009, 19, 98–108. [Google Scholar]
- Blanton, M.W.; Hadad, I.; Johnstone, B.H.; Mund, J.A.; Rogers, P.I.; Eppley, B.L.; March, K.L. Adipose stromal cells and platelet-rich plasma therapies synergistically increase revascularization during wound healing. Plast. Reconstr. Surg. 2009, 123 (Suppl. S2), 56S–64S. [Google Scholar] [CrossRef]
- Hadad, I.; Johnstone, B.H.; Brabham, J.G.; Blanton, M.W.; Rogers, P.I.; Fellers, C.; Solomon, J.L.; Merfeld-Clauss, S.; DesRosiers, C.M.; Dynlacht, J.R.; et al. Development of a porcine delayed wound-healing model and its use in testing a novel cell-based therapy. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 888–896. [Google Scholar] [CrossRef]
- Yang, H.S.; Shin, J.; Bhang, S.H.; Shin, J.Y.; Park, J.; Im, G.I.; Kim, C.S.; Kim, B.S. Enhanced skin wound healing by a sustained release of growth factors contained in platelet-rich plasma. Exp. Mol. Med. 2011, 43, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Dionyssiou, D.; Demiri, E.; Foroglou, P.; Cheva, A.; Saratzis, N.; Aivazidis, C.; Karkavelas, C. The effectiveness of intralesional injection of platelet-rich plasma in accelerating the healing of chronic ulcers: An experimental and clinical study. Int. Wound J. 2013, 10, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.F.; Carmona, J.U.; Rezende, C.M.F. Intra-articular injections of autologous platelet concentrates in dogs with surgical reparation of cranial cruciate ligament rupture: A pilot study. Vet. Comp. Orthop. Traumatol. 2013, 26, 285–290. [Google Scholar] [PubMed]
- Abegão, K.G.B.; Bracale, B.N.; Delfim, I.G.; Santos, E.S.; Laposy, C.B.; Nai, G.A.; Giuffrida, R.; Nogueira, R.M.B. Effects of heterologous platelet-rich plasma gel on standardized dermal wound healing in rabbits. Acta. Cir. Bras. 2015, 30, 209–215. [Google Scholar] [CrossRef] [PubMed]
- De Souza, M.V.; Silva, M.B.; de Oliveira Pinto, J.; de Souza Lima, M.B.; Crepaldi, J.; Lopes, G.F.M.; dos Santos, H.B.; Ribeiro, R.I.M.A.; Thome, R.G. Immunohistochemical Expression of Collagens in the Skin of Horses Treated with Leukocyte-Poor Platelet-Rich Plasma. BioMed. Res. Int. 2015, 2015, 1–12. [Google Scholar] [CrossRef]
- Karayannopoulou, M.; Psalla, D.; Kazakos, G.; Loukopoulos, P.; Giannakas, N.; Savvas, I.; Kritsepi-Konstantinou, M.; Chantes, A.; Papazoglou, L.G. Effect of locally injected autologous platelet-rich plasma on second intention wound healing of acute full-thickness skin defects in dogs. Vet. Comp. Orthop. Traumatol. 2015, 28, 172–178. [Google Scholar]
- Gemignani, F.; Perazzi, A.; Iacopetti, I. Use of canine sourced platelet-rich plasma in a feline contaminated cutaneous wound. Can. Vet. J. 2017, 58, 141–144. [Google Scholar]
- Gokulakrishnan, M.; Βabu, M.S.S.; Nagarajan, L.; Shafiuzama, M.; D’Souza, N.J. Management of Large Chronic Non-healing Wounds by Autogenous Platelet Rich Plasma and Reconstructive Surgery in Three Cats. Iran. J. Vet. Surg. 2016, 11, 61–66. [Google Scholar]
- Penning, L.C.; Vrieling, H.E.; Brinkhof, B.; Riemers, F.M.; Rothuizen, J.; Rutteman, G.R.; Hazewinkel, H.A.W. A validation of 10 feline reference genes for gene expression measurements in snap-frozen tissues. Vet. Immunol. Immunopathol. 2007, 120, 212–222. [Google Scholar] [CrossRef]
- Kessler, Y.; Helfer-Hungerbuehler, A.K.; Cattori, V.; Meli, M.L.; Zellweger, B.; Ossent, P.; Riond, B.; Reusch, C.E.; Lutz, H.; Hofmann-Lehmann, R. Quantitative TaqMan®real-time PCR assays for gene expression normalisation in feline tissues. BMC Mol. Biol. 2009, 10, 106. [Google Scholar] [CrossRef]
- Jursza, E.; Skarzynski, D.J.; Siemieniuch, M.J. Validation of reference genes in the feline endometrium. Reprod. Biol. 2014, 14, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Konstantinidis, A.O.; Adamama-Moraitou, K.K.; Pardali, D.; Dovas, C.I.; Brellou, G.D.; Papadopoulos, T.; Jergens, A.E.; Allenspach, K.; Rallis, T.S. Colonic mucosal and cytobrush sample cytokine mRNA expression in canine inflammatory bowel disease and their correlation with disease activity, endoscopic and histopathologic score. PLoS ONE 2021, 16, e0245713. [Google Scholar]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Tsioli, V.; Gouletsou, P.G.; Galatos, A.D.; Psalla, D.; Lymperis, A.; Papazoglou, L.G.; Karayannopoulou, M. Effects of two occlusive, hydrocolloid dressings on healing of full-thickness skin wounds in cats. Vet. Comp. Orthop. Traumatol. 2016, 29, 298–305. [Google Scholar]
- De Rossi, R.; Coelho, A.C.A.; de Mello, G.S.; Frazilio, F.O.; Leal, C.R.B.; Facco, G.G.; Brum, K.B. Effects of platelet-rich plasma gel on skin healing in surgical wound in horses. Acta Cir. Bras. 2009, 24, 276–281. [Google Scholar] [CrossRef]
- Chicharro, D.; Carrillo, J.M.; Rubio, M.; Cugat, R.; Cuervo, B.; Guil, S.; Forteza, J.; Moreno, V.; Vilar, J.M.; Sopena, J. Combined plasma rich in growth factors and adipose-derived mesenchymal stem cells promotes the cutaneous wound healing in rabbits. BMC Vet. Res. 2018, 14, 288. [Google Scholar] [CrossRef]
- Iacopetti, I.; Patruno, M.; Melotti, L.; Martinello, T.; Bedin, S.; Badon, T.; Righetto, E.M.; Perazzi, A. Autologous Platelet-Rich Plasma Enhances the Healing of Large Cutaneous Wounds in Dogs. Front. Vet. Sci. 2020, 7, 575449. [Google Scholar]
- Carr, B.J.; Canapp, S.O.; Mason, D.R.; Cox, C.; Hess, T. Canine Platelet-Rich Plasma Systems: A Prospective Analysis. Front. Vet. Sci. 2015, 2, 73. [Google Scholar] [CrossRef]
- Hee, H.T.; Majd, M.E.; Holt, R.T.; Myers, L. Do autologous growth factors enhance transforaminal lumbar interbody fusion? Eur. Spine J. 2003, 12, 400–407. [Google Scholar] [CrossRef]
- Sanchez, M.; Anitua, E.; Azofra, J.; Andia, I.; Padilla, S.; Mujika, I. Comparison of surgically repaired Achilles tendon tears using platelet-rich fibrin matrices. Am. J. Sports Med. 2007, 35, 245–251. [Google Scholar] [CrossRef]
- Sanchez, M.; Anitua, E.; Andia, I. Poor standardization in platelet-rich therapies hampers advancement. Arthroscopy 2010, 26, 725–726. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Li, Z.; Li, J.; Liao, W.; Qin, Y.; Zhang, N.; Huo, X.; Mao, N.; Zhu, H. Optimization of the Platelet-Rich Plasma Concentration for Mesenchymal Stem Cell Applications. Tissue Eng. Part A 2019, 25, 333–351. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, R.; Terashima, H.; Yoneyama, S.; Tadano, S.; Ohkohchi, N. Effects of platelet-rich plasma on intestinal anastomotic healing in rats: PrP concentration is a key factor. J. Surg. Res. 2012, 173, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Giusti, I.; D’Ascenzo, S.; Mancò, A.; Di Stefano, G.; Di Francesco, M.; Rughetti, A.; Dal Mas, A.; Properzi, G.; Calvisi, V.; Dolo, V. Platelet Concentration in Platelet-Rich Plasma Affects Tenocyte Behavior In Vitro. Biomed Res. Int. 2014, 2014, 1–12. [Google Scholar]
- Weibrich, G.; Kleis, W.K.G.; Hafner, G.; Hitzler, W.E. Growth factor levels in platelet-rich plasma and correlations with donor age, sex, and platelet count. J. Craniomaxillofac. Surg. 2002, 30, 97–102. [Google Scholar] [CrossRef]
- Pietrzak, W.; Eppley, B.L. Platelet rich plasma: Biology and new technology. J. Craniofac. Surg. 2005, 16, 1043–1054. [Google Scholar] [CrossRef]
- Fadadu, P.P.; Mazzola, A.J.; Hunter, C.W.; Davis, T.T. Review of concentration yields in commercially available platelet-rich plasma (PRP) systems: A call for PRP standardization. Reg. Anesth. Pain Med. 2019, 16, 652–659. [Google Scholar] [CrossRef]
- Chun, N.; Canapp, S.; Carr, B.J.; Wong, V.; Curry, J. Validation and Characterization of Platelet-Rich Plasma in the Feline: A Prospective Analysis. Front. Vet. Sci. 2020, 7, 512. [Google Scholar] [CrossRef]
- Jee, C.H.; Eom, N.Y.; Jang, H.M.; Jung, H.W.; Choi, E.S.; Won, J.H.; Hong, I.H.; Kang, B.T.; Jeong, D.W.; Jung, D.I. Effect of autologous platelet-rich plasma application on cutaneous wound healing in dogs. J. Vet. Sci. 2016, 17, 79–87. [Google Scholar] [CrossRef]
- Stief, M.; Gottschalk, J.; Ionita, J.C.; Einspanier, A.; Oechtering, G.; Böttcher, P. Concentration of platelets and growth factors in canine autologous conditioned plasma. Vet. Comp. Orthop. Traumatol. 2011, 24, 122–125. [Google Scholar] [CrossRef]
- Lacci, K.M.; Dardik, A. Platelet-rich plasma: Support for its use in wound healing. Yale J. Biol. Med. 2010, 83, 1–9. [Google Scholar]
- Pelletier, M.H.; Malhotra, A.; Brighton, T.; Walsh, W.R.; Lindeman, R. Platelet function and constituents of platelet rich plasma. Int. J. Sports. Med. 2013, 34, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Ehrenfest, D.M.D.; Rasmusson, L.; Albrektsson, T. Classification of platelet concentrates: From pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends Biotechnol. 2009, 27, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Aguirre, J.J.; Algorta, J.; Ayerdi, E.; Cabezas, A.I.; Orive, G.; Andia, I. Effectiveness of autologous preparation rich in growth factors for the treatment of chronic cutaneous ulcers. J. Biomed. Mater. Res. B. Appl. Biomater. 2008, 84, 415–421. [Google Scholar] [CrossRef]
- Schnabel, L.V.; Mohammed, H.O.; Miller, B.J.; McDermott, W.G.; Jacobson, M.S.; Santangelo, K.S.; Fortier, L.A. Platelet rich plasma (PRP) enhances anabolic gene expression patterns in flexor digitorum superficialis tendons. J. Orthop. Res. 2007, 25, 230–240. [Google Scholar] [CrossRef] [PubMed]
- McCarrel, T.; Fortier, L. Temporal growth factor release from platelet rich plasma, trehalose lyophilized platelets and bone marrow aspirate and their effect on tendon and ligament gene expression. J. Orthop. Res. 2009, 27, 1033–1042. [Google Scholar] [CrossRef]
- Parrish, W.R.; Roides, B. Physiology of Blood Components in Wound Healing: An Appreciation of Cellular Co-Operativity in Platelet Rich Plasma Action. J. Exerc. Sports Orthop. 2017, 4, 1–14. [Google Scholar] [CrossRef]
- Lana, J.F.; Macedo, A.; Ingrao, I.L.G.; Huber, S.C.; Santos, G.S.; Santana, M.H.A. Leukocyte-rich PRP for knee osteoarthritis: Current concepts. J. Clin. Orthop. Trauma 2019, 10 (Suppl. S1), S179–S182. [Google Scholar] [CrossRef]
- Perego, R.; Spada, E.; Moneta, E.; Baggiani, L.; Proverbio, D. Use of Autologous Leucocyte- and Platelet-Rich Plasma (L-PRP) in the Treatment of Aural Hematoma in Dogs. Vet. Sci. 2021, 8, 172. [Google Scholar] [CrossRef]
- Le, A.D.K.; Enweze, L.; DeBaun, M.R.; Dragoo, J.L. Current Clinical Recommendations for Use of Platelet-Rich Plasma. Curr. Rev. Musculoskelet. Med. 2018, 11, 624–634. [Google Scholar] [CrossRef]
- Zimmermann, R.; Jakubietz, R.; Jakubietz, M.; Strasser, E.; Schlegel, A.; Wiltfang, J.; Eckstein, R. Different preparation methods to obtain platelet components as a source of growth factors for local application. Transfusion 2001, 41, 1217–1224. [Google Scholar] [CrossRef] [PubMed]
- Collins, T.; Alexander, D.; Barkatali, B. Platelet-rich plasma: A narrative review. EFORT Open Rev. 2021, 6, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Bohling, M.W.; Henderson, R.A.; Swaim, S.F.; Kincaid, S.A.; Wright, J.C. Cutaneous Wound Healing in the Cat: A Macroscopic Description and Comparison with Cutaneous Wound Healing in the Dog. Vet. Surg. 2004, 33, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Eppley, B.L.; Woodell, J.E.; Higgins, J. Platelet quantification and growth factor analysis from platelet-rich plasma: Implications for wound healing. Plast. Reconstr. Surg. 2004, 114, 1502–1508. [Google Scholar] [CrossRef] [PubMed]
- Greenhalgh, D.G.; Sprugel, K.H.; Murray, M.J.; Rosst, R. PDGF and FGF Stimulate Wound Healing in the Genetically Diabetic Mouse. Am. J. Pathol. 1990, 136, 1235–1246. [Google Scholar] [PubMed]
- Kim, N.; Choi, K.U.; Lee, E.; Lee, S.; Oh, J.; Kim, W.K.; Woo, S.H.; Kim, D.Y.; Kim, W.H.; Kweon, O.K. Therapeutic effects of platelet derived growth factor overexpressed-mesenchymal stromal cells and sheets in canine skin wound healing model. Histol. Histopathol. 2020, 35, 751–767. [Google Scholar]
- Jeffcoate, W.J.; Musgrove, A.J.; Lincoln, N.B. Using image J to document healing in ulcers of the foot in diabetes. Int. Wound J. 2017, 14, 1137–1139. [Google Scholar] [CrossRef]
- Pavletic, M.M. Atlas of Small Animal Wound Management and Reconstructive Surgery, 4th ed.; John Wiley & Sons: Pondicherry, India, 2018; pp. 17–31. [Google Scholar]
- Li, J.; Chen, J.; Kirsner, R. Pathophysiology of acute wound healing. Clin. Dermatol. 2007, 25, 9–18. [Google Scholar] [CrossRef]
- Tambella, A.M.; Attili, A.R.; Dupré, G.; Cantalamessa, A.; Martin, S.; Cuteri, V.; Marcazzan, S.; Del Fabbro, M. Platelet-rich plasma to treat experimentally-induced skin wounds in animals: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0191093. [Google Scholar] [CrossRef]
- Manning, T.O.; Monteiro-Riviere, N.A.; Bristol, D.G.; Riviere, J.E. Cutaneous laser-Doppler velocimetry in nine animal species. Am. J. Vet. Res. 1991, 52, 1960–1964. [Google Scholar]
- Bircher, A.; Boer, E.M.; Agner, T.; Wahlberg, J.E.; Serup, J. Guidelines for measurement of cutaneous blood flow by laser Doppler flowmetry. A report from the Standardization Group of the European Society of Contact Dermatitis. Contact. Dermatitis. 1994, 30, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Gargiulo, S.; Gramanzini, M.; Liuzzi, R.; Greco, A.; Brunetti, A.; Vesce, G. Effects of some anesthetic agents on skin microcirculation evaluated by laser Doppler perfusion imaging in mice. BMC Vet. Res. 2013, 9, 255. [Google Scholar] [CrossRef] [PubMed]
- Fossum, T.W. Small Animal Surgery, 5th ed.; Elsevier: Philadelphia, PA, USA, 2019; pp. 179–265. [Google Scholar]
- Beldon, P. Basic science of wound healing. Surg 2010, 28, 409–412. [Google Scholar] [CrossRef]
- Balsa, I.M.; Culp, W.T.N. Wound Care. Vet. Clin. North Am. Small Anim. Pract. 2015, 45, 1049–1065. [Google Scholar] [CrossRef]
- Pourkarim, R.; Farahpour, M.R.; Rezaei, S.A. Comparison effects of platelet-rich plasma on healing of infected and non-infected excision wounds by the modulation of the expression of inflammatory mediators: Experimental research. Eur. J. Trauma Emerg. Surg. 2022, 1–9. [Google Scholar] [CrossRef]
- Farghali, H.A.; AbdElKader, N.A.; AbuBakr, H.O.; Aljuaydi, S.H.; Khattab, M.S.; Elhelw, R.; Elhariri, M. Antimicrobial action of autologous platelet-rich plasma on MRSA-infected skin wounds in dogs. Sci. Rep. 2019, 9, 12722. [Google Scholar] [CrossRef]
- Xu, P.; Wu, Y.; Zhou, L.; Yang, Z.; Zhang, X.; Hu, X.; Yang, J.; Wang, M.; Wang, B.; Luo, G.; et al. Platelet-rich plasma accelerates skin wound healing by promoting re-epithelialization. Burns Trauma. 2020, 8, tkaa028. [Google Scholar] [CrossRef]
- Wang, B.; Geng, Q.; Hu, J.; Shao, J.; Ruan, J.; Zheng, J. Platelet-rich plasma reduces skin flap inflammatory cells infiltration and improves survival rates through induction of angiogenesis: An experiment in rabbits. J. Plast. Surg. Hand Surg. 2016, 50, 239–245. [Google Scholar] [CrossRef]
- Fujihara, M.; Yamamizu, K.; Wildt, D.E.; Songsasen, N. Expression pattern of matrix metalloproteinases changes during folliculogenesis in the cat ovary. Reprod. Domest. Anim. 2016, 51, 717–725. [Google Scholar] [CrossRef]
- Martins, V.L.; Caley, M.; O’Toole, E.O. Matrix metalloproteinases and epidermal wound repair. Cell Tissue Res. 2013, 351, 255–268. [Google Scholar] [CrossRef]
- Hattori, N.; Mochizuki, S.; Kishi, K.; Nakajima, T.; Takaishi, H.; D’Armiento, J.; Okada, Y. MMP-13 Plays a Role in Keratinocyte Migration, Angiogenesis, and Contraction in Mouse Skin Wound Healing. Am. J. Pathol. 2009, 175, 533–546. [Google Scholar] [CrossRef] [PubMed]
- Utz, E.R.; Elster, E.A.; Tadaki, D.K.; Gage, F.; Perdue, P.W.; Forsberg, J.A.; Stojadinovic, A.; Hawksworth, J.S.; Brown, T.S. Metalloproteinase Expression is Associated with Traumatic Wound Failure. J. Surg. Res. 2010, 159, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Lazaro, J.L.; Izzo, V.; Meaume, S.; Davies, A.H.; Lobmann, R.; Uccioli, L. Elevated levels of matrix metalloproteinases and chronic wound healing: An updated review of clinical evidence. J. Wound Care 2016, 25, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Franz, M.G.; Robson, M.C.; Steed, D.L.; Barbul, A.; Brem, H.; Cooper, D.M.; Leaper, D.; Milner, S.M.; Payne, W.G.; Wachtel, T.L.; et al. Guidelines to aid healing of acute wounds by decreasing impediments of healing. Wound Repair Regen. 2008, 16, 723–748. [Google Scholar] [CrossRef]
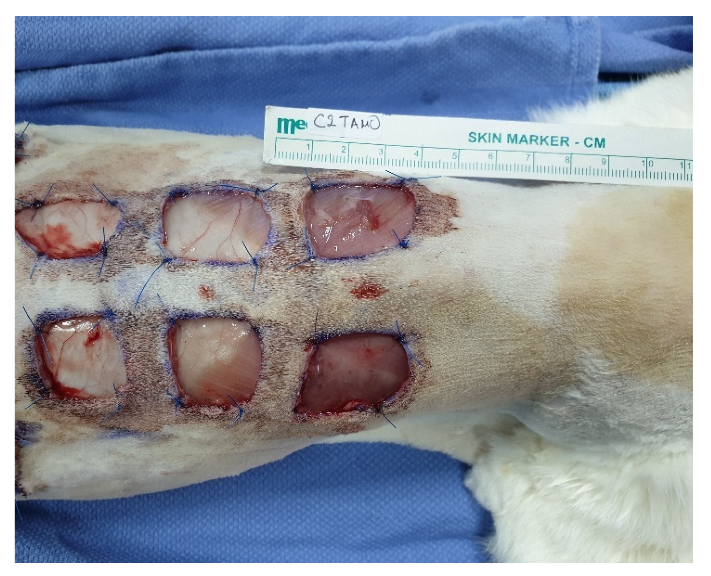
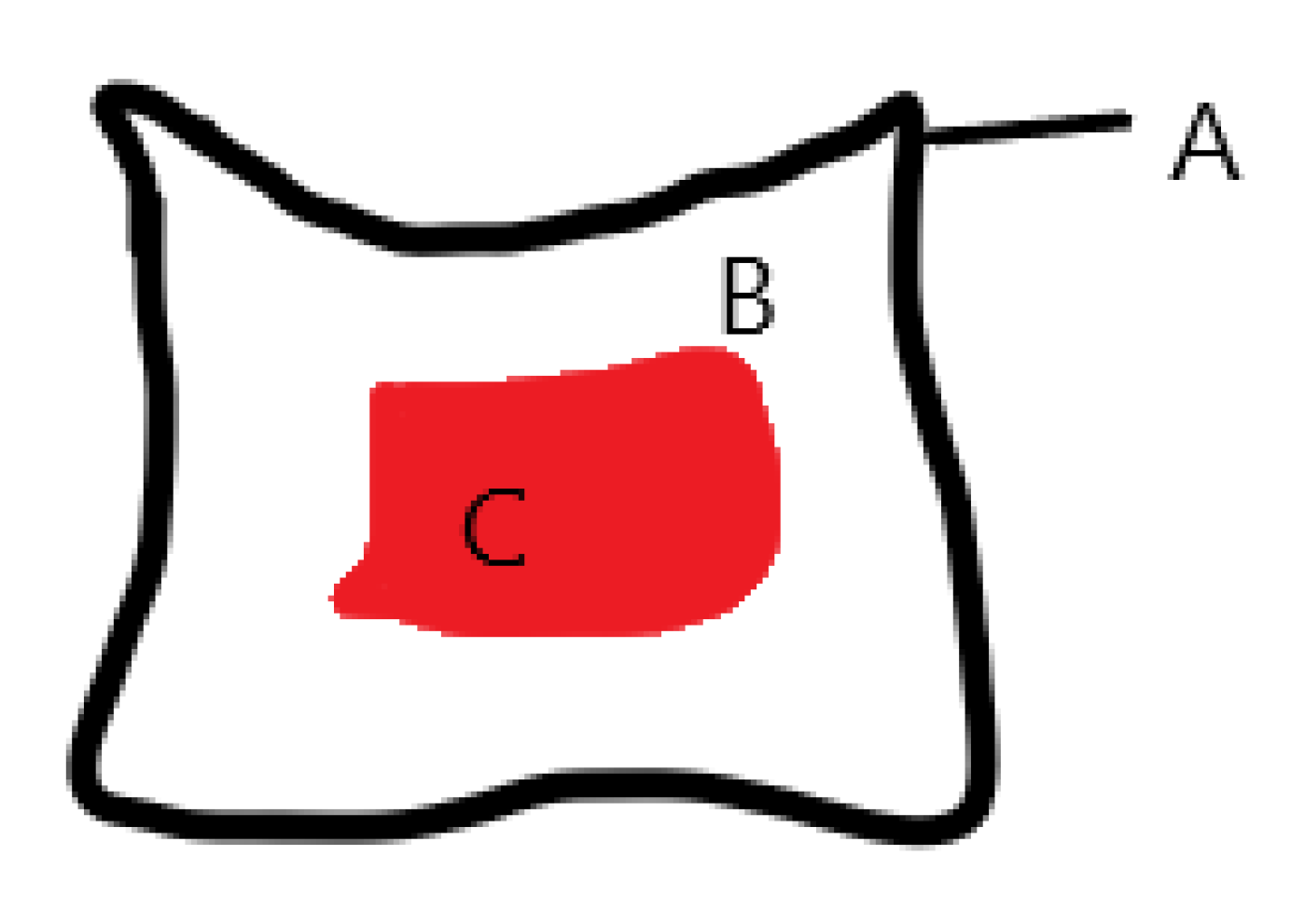
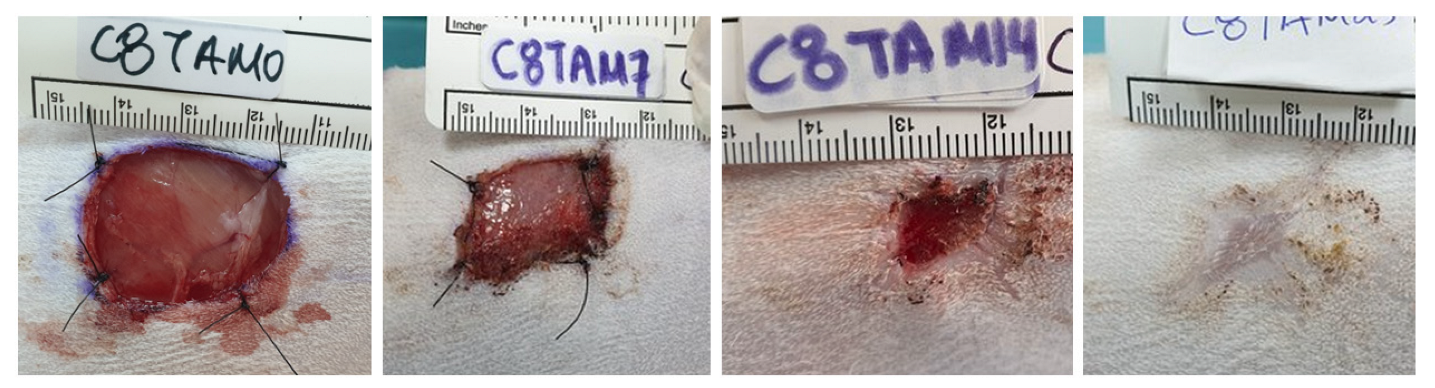

| Cat Number | Dorsal Side | PRP Treatment |
|---|---|---|
| 1 | Right (R) | No |
| Left (L) | Yes | |
| 2 | R | No |
| L | Yes | |
| 3 | R | No |
| L | Yes | |
| 4 | R | No |
| L | Yes | |
| 5 | R | Yes |
| L | No | |
| 6 | R | No |
| L | Yes | |
| 7 | R | Yes |
| L | No | |
| 8 | R | Yes |
| L | No |
| Cat | PLTs before Centrifugation (K/μL) | PLTs in PRP (K/μL) | Percentage (%) | RBCs (M/μL)/WBCs (K/μL) before Centrifugation | RBCs (M/μL)/WBCs (K/μL) after Centrifugation |
|---|---|---|---|---|---|
| 1 | 305,000 | 2,503,000 | 8.2 | 6.38/6 | 0.0/0.5 |
| 2 | 397,000 | 2,107,000 | 5.3 | 7.2/13.1 | 0.0/0.4 |
| 3 | 250,000 | 497,000 | 2 | 6.9/7.2 | 0.0/1.2 |
| 4 | 320,000 | 730,000 | 2.3 | 6.61/6.4 | 0.0/1.4 |
| 5 | 239,000 | 1,373,000 | 5.8 | 6.82/5.5 | 0.0/0.8 |
| 6 | 257,000 | 943,000 | 3.7 | 7.14/6.2 | 0.0/1 |
| 7 | 210,000 | 550,000 | 2.6 | 6.3/8 | 0.0/0.4 |
| 8 | 260,000 | 751,000 | 2.9 | 6.4/5.4 | 0.0/0.2 |
| Mean | 279,750 | 1,181,750 | 4.1 | 6.71/7.22 | 0.0/0.73 |
| SD | 55.140 | 702.938 | 2.01 | 0.32/2.36 | 0.0/0.4 |
| Group | Day | Measurements in Full Thickness Wounds (Mean ± Standard Deviation) | ||
|---|---|---|---|---|
| Epithelialization (%) | Contraction (%) | Total Wound Healing (%) | ||
| Control | 0 | |||
| 7 | 10.613 (±7.719) * | 5.775(±5.844) * | 16.462 (±11.612) *,$ | |
| 14 | 48.395 (±4.88) * | 42.73 (±16.028) * | 70.212 (±8.686) * | |
| 25 | 84.395 (±14.14) * | 73.728 (±19.48) * | 96.9 (±2.343) * | |
| PRP | 0 | |||
| 7 | 10.475 (±7927) * | 21.512 (±10.03) *,$ | 30.887 (±9.614) *,$,# | |
| 14 | 46.597 (±12.16) * | 59.251 (±14.834) *,$ | 77.741 (±8.098) *,$ | |
| 25 | 83.817 (±20.683) * | 75.287 (±15.588) *,$ | 96.887 (±4.114) *,$ | |
| Group | Tissue Flowmetry in Full Thickness Wounds (Mean ± Standard Deviation) | |||
|---|---|---|---|---|
| Day 0 | Day 7 | Day 14 | Day 25 | |
| Control | 1.866 (±0.373) + | 2.125 (±0.766) + | 2.551 (±0.983) *,+ | 1.871 (±0.58) $,+ |
| PRP | 1.842 (±0.41) + | 3.151 (±1.1) + | 3.096 (±0.855) *,+ | 2.106 (±0.88) $,+ |
| Histologic Parameters | Group | Day 0 | Day 7 | Day 14 | Day 25 |
|---|---|---|---|---|---|
| Inflammatory cell infiltration score | Control | 1 * | 3 (±1.069) * | 3.38 * (±0.744) | 2.88 * (±0.835) |
| PRP | 1 | 2.75 (±1.282) | 3 (±1.069) | 2.5 (±0.756) | |
| Edema score | Control | 1.13 (±0.354) & | 2.88 & (±0.991) | 2 (±1.069) | 1(±0) & |
| PRP | 2.5 $ (±0.926) | 1.88 (±0.835) | 1 (±0) $ | ||
| Collagen production score | Control | 3 (±0) # | 2.13 # (±0.835) | 3.13 (±1.126) | 3.88 # (±0.354) |
| PRP | 3 (±0) | 2.13 ® (±0.641) | 3.5 ® (±0.535) | 3.75 ® (±0.463) | |
| Angiogenesis | Control | 1 (±0) © | 1.38 © (±0.744) | 2.75 © (±0.886) | 2.5 © (±0.535) |
| PRP | 1 (±0) | 2.38 (±0.916) | 3.25 (±0.463) | 3 (±0.535) | |
| Epidermis thickness | Control | 3.5 (±0.535) | |||
| PRP | 3.75 (0.463) |
| Metalloproteinases | Group | Day 0 | Day 14 | Day 25 |
|---|---|---|---|---|
| MMP-2 | Control | 1 * | 6.73 (±3.08) *,& | 0.73 (±0.25) & |
| PRP | 1 * | 8.31 (±3.87) *,& | 0.86 (±0.29) & | |
| MMP-9 | Control | 1 | 13.56 (±6.8) | 14.52 (±8.57) |
| PRP | 1 | 37.87 (±25.02) | 9.17 (±3.98) | |
| TIMP-1 | Control | 1 $ | 17.2 (±7.89) $ | 1.16 (±0.29) $ |
| PRP | 1 $ | 15.12 (±7.09) $ | 1.4 (±0.27) $ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angelou, V.; Psalla, D.; Dovas, C.I.; Kazakos, G.M.; Marouda, C.; Chatzimisios, K.; Kyrana, Z.; Moutou, E.; Karayannopoulou, M.; Papazoglou, L.G. Locally Injected Autologous Platelet-Rich Plasma Improves Cutaneous Wound Healing in Cats. Animals 2022, 12, 1993. https://doi.org/10.3390/ani12151993
Angelou V, Psalla D, Dovas CI, Kazakos GM, Marouda C, Chatzimisios K, Kyrana Z, Moutou E, Karayannopoulou M, Papazoglou LG. Locally Injected Autologous Platelet-Rich Plasma Improves Cutaneous Wound Healing in Cats. Animals. 2022; 12(15):1993. https://doi.org/10.3390/ani12151993
Chicago/Turabian StyleAngelou, Vasileia, Dimitra Psalla, Chrysostomos I. Dovas, George M. Kazakos, Christina Marouda, Kyriakos Chatzimisios, Zacharenia Kyrana, Evangelia Moutou, Maria Karayannopoulou, and Lysimachos G. Papazoglou. 2022. "Locally Injected Autologous Platelet-Rich Plasma Improves Cutaneous Wound Healing in Cats" Animals 12, no. 15: 1993. https://doi.org/10.3390/ani12151993
APA StyleAngelou, V., Psalla, D., Dovas, C. I., Kazakos, G. M., Marouda, C., Chatzimisios, K., Kyrana, Z., Moutou, E., Karayannopoulou, M., & Papazoglou, L. G. (2022). Locally Injected Autologous Platelet-Rich Plasma Improves Cutaneous Wound Healing in Cats. Animals, 12(15), 1993. https://doi.org/10.3390/ani12151993







