Immunohistochemical Expression of p62 in Feline Mammary Carcinoma and Non-Neoplastic Mammary Tissue
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cases Included in the Study and Feline Mammary Carcinoma Grading System
2.2. Immunohistochemical Analysis and Immunoreactivity Scoring System for p62
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bjorkoy, G.; Lamark, T.; Pankiv, S.; Overvatn, A.; Brech, A.; Johansen, T. Monitoring autophagic degradation of p62/SQSTM1. Methods Enzymol. 2009, 452, 181–197. [Google Scholar] [CrossRef]
- Ciani, B.; Layfield, R.; Cavey, J.R.; Sheppard, P.W.; Searle, M.S. Structure of the ubiquitin-associated domain of p62 (SQSTM1) and implications for mutations that cause Paget’s disease of bone. J. Biol. Chem. 2003, 278, 37409–37412. [Google Scholar] [CrossRef] [PubMed]
- Pankiv, S.; Clausen, T.H.; Lamark, T.; Brech, A.; Bruun, J.-A.; Outzen, H.; Øvervatn, A.; Bjørkøy, G.; Johansen, T. p62/SQSTM1 Binds Directly to Atg8/LC3 to Facilitate Degradation of Ubiquitinated Protein Aggregates by Autophagy. J. Biol. Chem. 2007, 282, 24131–24145. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Inflammaging: Disturbed interplay between autophagy and inflammasomes. Aging 2012, 4, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Horne, S.D.N.; Pollick, S.A.; Heng, H.H. Evolutionary mechanism unifies the hallmarks of cancer. Int. J. Cancer 2015, 136, 2012–2021. [Google Scholar] [CrossRef] [PubMed]
- Moscat, J.; Diaz-Meco, M.T. p62 at the crossroads of autophagy, apoptosis, and cancer. Cell 2009, 137, 1001–1004. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.Y.; Ellis, R.A.; Lovat, P.E. Prognostic Impact of Autophagy Biomarkers for Cutaneous Melanoma. Front. Oncol. 2016, 6, 236. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, K.J.; Ademi, C.; Bertram, S.; Schmid, K.W.; Baba, H.A. Prognostic relevance of autophagy-related markers LC3, p62/sequestosome 1, Beclin-1 and ULK1 in colorectal cancer patients with respect to KRAS mutational status. World J. Surg. Oncol. 2016, 14, 189. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Lum, J.J.; Hatzivassiliou, G.; Thompson, C.B. The biology of cancer: Metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008, 7, 11–20. [Google Scholar] [CrossRef]
- Moscat, J.; Diaz-Meco, M.T. p62: A versatile multitasker takes on cancer. Trends Biochem. Sci. 2012, 37, 230–236. [Google Scholar] [CrossRef]
- Moscat, J.; Karin, M.; Diaz-Meco, M.T. p62 in Cancer: Signaling Adaptor Beyond Autophagy. Cell 2016, 167, 606–609. [Google Scholar] [CrossRef]
- Bao, L.; Chandra, P.K.; Moroz, K.; Zhang, X.; Thung, S.N.; Wu, T.; Dash, S. Impaired autophagy response in human hepatocellular carcinoma. Exp. Mol. Pathol. 2014, 96, 149–154. [Google Scholar] [CrossRef]
- Kuo, W.L.; Sharifi, M.N.; Lingen, M.W.; Ahmed, O.; Liu, J.; Nagilla, M.; Macleod, K.F.; Cohen, E.E. p62/SQSTM1 accumulation in squamous cell carcinoma of head and neck predicts sensitivity to phosphatidylinositol 3-kinase pathway inhibitors. PLoS ONE 2014, 9, e90171. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, H.; Torigoe, T.; Asanuma, H.; Hisasue, S.I.; Suzuki, K.; Tsukamoto, T.; Satoh, M.; Sato, N. Cytosolic overexpression of p62 sequestosome 1 in neoplastic prostate tissue. Histopathology 2006, 48, 157–161. [Google Scholar] [CrossRef]
- Shi, F.D.; Zhang, J.Y.; Liu, D.; Rearden, A.; Elliot, M.; Nachtsheim, D.; Daniels, T.; Casiano, C.A.; Heeb, M.J.; Chan, E.K.; et al. Preferential humoral immune response in prostate cancer to cellular proteins p90 and p62 in a panel of tumor-associated antigens. Prostate 2005, 63, 252–258. [Google Scholar] [CrossRef]
- Su, Y.; Qian, H.; Zhang, J.; Wang, S.; Shi, P.; Peng, X. The diversity expression of p62 in digestive system cancers. Clin. Immunol. 2005, 116, 118–123. [Google Scholar] [CrossRef]
- Kim, H.M.; Kim, E.S.; Koo, J.S. Expression of Autophagy-Related Proteins in Different Types of Thyroid Cancer. Int. J. Mol. Sci. 2017, 18, 540. [Google Scholar] [CrossRef]
- Adams, O.; Dislich, B.; Berezowska, S.; Schlafli, A.M.; Seiler, C.A.; Kroll, D.; Tschan, M.P.; Langer, R. Prognostic relevance of autophagy markers LC3B and p62 in esophageal adenocarcinomas. Oncotarget 2016, 7, 39241–39255. [Google Scholar] [CrossRef] [PubMed]
- Thompson, H.G.; Harris, J.W.; Wold, B.J.; Lin, F.; Brody, J.P. p62 overexpression in breast tumors and regulation by prostate-derived Ets factor in breast cancer cells. Oncogene 2003, 22, 2322–2333. [Google Scholar] [CrossRef]
- Iwadate, R.; Inoue, J.; Tsuda, H.; Takano, M.; Furuya, K.; Hirasawa, A.; Aoki, D.; Inazawa, J. High Expression of SQSTM1/p62 Protein Is Associated with Poor Prognosis in Epithelial Ovarian Cancer. Acta Histochem. Cytochem. 2014, 47, 295–301. [Google Scholar] [CrossRef]
- Goldschmidt, M.H.; Peña, L.; Zappulli, V. Tumors of the Mammary Gland. In Tumors in Domestic Animals, 5th ed.; Meuten, D.J., Ed.; John Wiley & Sons, Inc.: Ames, IA, USA, 2017; pp. 723–765. [Google Scholar]
- Mills, S.W.; Musil, K.M.; Davies, J.L.; Hendrick, S.; Duncan, C.; Jackson, M.L.; Kidney, B.; Philibert, H.; Wobeser, B.K.; Simko, E. Prognostic value of histologic grading for feline mammary carcinoma: A retrospective survival analysis. Vet. Pathol. 2015, 52, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Di Cerbo, A.; Giusti, S.; Mariotti, F.; Spaterna, A.; Rossi, G.; Magi, G.E. Increased Expression of Toll-Like Receptor 4 in Skin of Dogs with Discoid Lupus Erythematous (DLE). Animals 2021, 11, 1044. [Google Scholar] [CrossRef] [PubMed]
- Mariotti, F.; Magi, G.E.; Gavazza, A.; Vincenzetti, S.; Komissarov, A.; Shneider, A.; Venanzi, F.M. p62/SQSTM1 expression in canine mammary tumours: Evolutionary notes. Vet. Comp. Oncol. 2019, 17, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Zappulli, V.; Rasotto, R.; Caliari, D.; Mainenti, M.; Pena, L.; Goldschmidt, M.H.; Kiupel, M. Prognostic evaluation of feline mammary carcinomas: A review of the literature. Vet. Pathol. 2015, 52, 46–60. [Google Scholar] [CrossRef] [PubMed]
- Rolland, P.; Madjd, Z.; Durrant, L.; Ellis, I.O.; Layfield, R.; Spendlove, I. The ubiquitin-binding protein p62 is expressed in breast cancers showing features of aggressive disease. Endocr. Relat. Cancer 2007, 14, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Rich, T.; Dean, R.T.; Lamm, C.G.; Ramiro-Ibanez, F.; Stevenson, M.L.; Patterson-Kane, J.C. p62/Sequestosome-1: Mapping Sites of Protein-Handling Stress in Canine Cutaneous Mast Cell Tumors. Vet. Pathol. 2015, 52, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Ponomarenko, D.M.; Klimova, I.D.; Chapygina, Y.A.; Dvornichenko, V.V.; Zhukova, N.V.; Orlova, R.V.; Manikhas, G.M.; Zyryanov, A.V.; Burkhanova, L.A.; Badrtdinova, I.I.; et al. Safety and efficacy of p62 DNA vaccine ELENAGEN in a first-in-human trial in patients with advanced solid tumors. Oncotarget 2017, 8, 53730–53739. [Google Scholar] [CrossRef]
- Cai-McRae, X.; Karantza, V. p62: A hub of multiple signaling pathways in HER2-induced mammary tumorigenesis. Mol. Cell Oncol. 2015, 2, e975035. [Google Scholar] [CrossRef][Green Version]
- Duran, A.; Linares, J.F.; Galvez, A.S.; Wikenheiser, K.; Flores, J.M.; Diaz-Meco, M.T.; Moscat, J. The signaling adaptor p62 is an important NF-kappaB mediator in tumorigenesis. Cancer Cell 2008, 13, 343–354. [Google Scholar] [CrossRef]
- Nagel, R.; Semenova, E.A.; Berns, A. Drugging the addict: Non-oncogene addiction as a target for cancer therapy. EMBO Rep. 2016, 17, 1516–1531. [Google Scholar] [CrossRef]
- Nozaki, F.; Hirotani, Y.; Nakanishi, Y.; Yamaguchi, H.; Nishimaki, H.; Noda, H.; Tang, X.; Yamamoto, H.; Suzuki, A.; Seki, T.; et al. p62 Regulates the Proliferation of Molecular Apocrine Breast Cancer Cells. Acta Histochem. Cytochem. 2016, 49, 125–130. [Google Scholar] [CrossRef]
- Xu, L.Z.; Li, S.S.; Zhou, W.; Kang, Z.J.; Zhang, Q.X.; Kamran, M.; Xu, J.; Liang, D.P.; Wang, C.L.; Hou, Z.J.; et al. p62/SQSTM1 enhances breast cancer stem-like properties by stabilizing MYC mRNA. Oncogene 2017, 36, 304–317. [Google Scholar] [CrossRef]
- Rodriguez, A.; Duran, A.; Selloum, M.; Champy, M.F.; Diez-Guerra, F.J.; Flores, J.M.; Serrano, M.; Auwerx, J.; Diaz-Meco, M.T.; Moscat, J. Mature-onset obesity and insulin resistance in mice deficient in the signaling adapter p62. Cell Metab. 2006, 3, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Outschoorn, U.E.; Sotgia, F.; Lisanti, M.P. Metabolic asymmetry in cancer: A “balancing act” that promotes tumor growth. Cancer Cell 2014, 26, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Valencia, T.; Kim, J.Y.; Abu-Baker, S.; Moscat-Pardos, J.; Ahn, C.S.; Reina-Campos, M.; Duran, A.; Castilla, E.A.; Metallo, C.M.; Diaz-Meco, M.T.; et al. Metabolic reprogramming of stromal fibroblasts through p62-mTORC1 signaling promotes inflammation and tumorigenesis. Cancer Cell 2014, 26, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Di Cerbo, A.; Palmieri, B.; De Vico, G.; Iannitti, T. Onco-epidemiology of domestic animals and targeted therapeutic attempts: Perspectives on human oncology. J. Cancer Res. Clin. Oncol. 2014, 140, 1807–1814. [Google Scholar] [CrossRef] [PubMed]

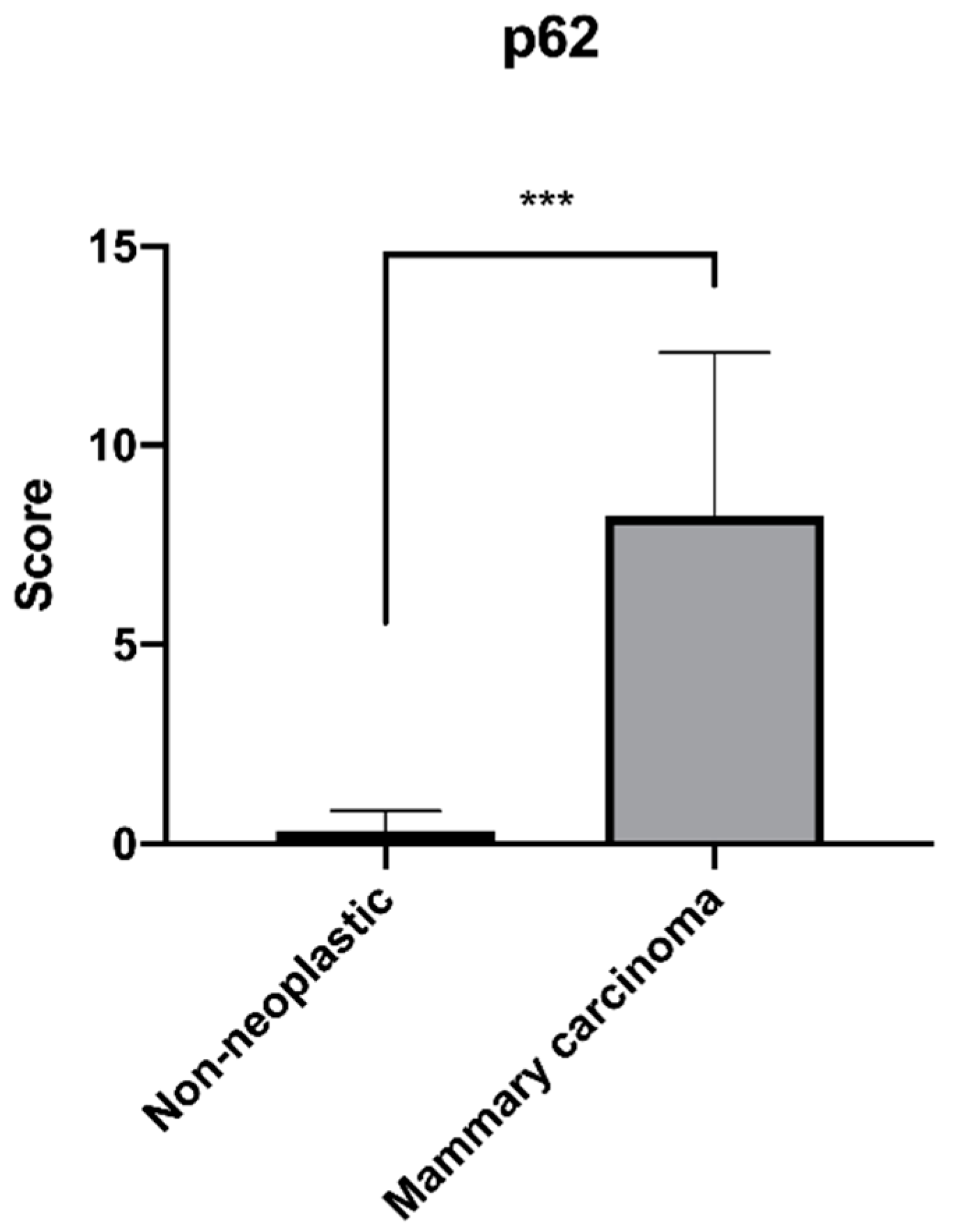
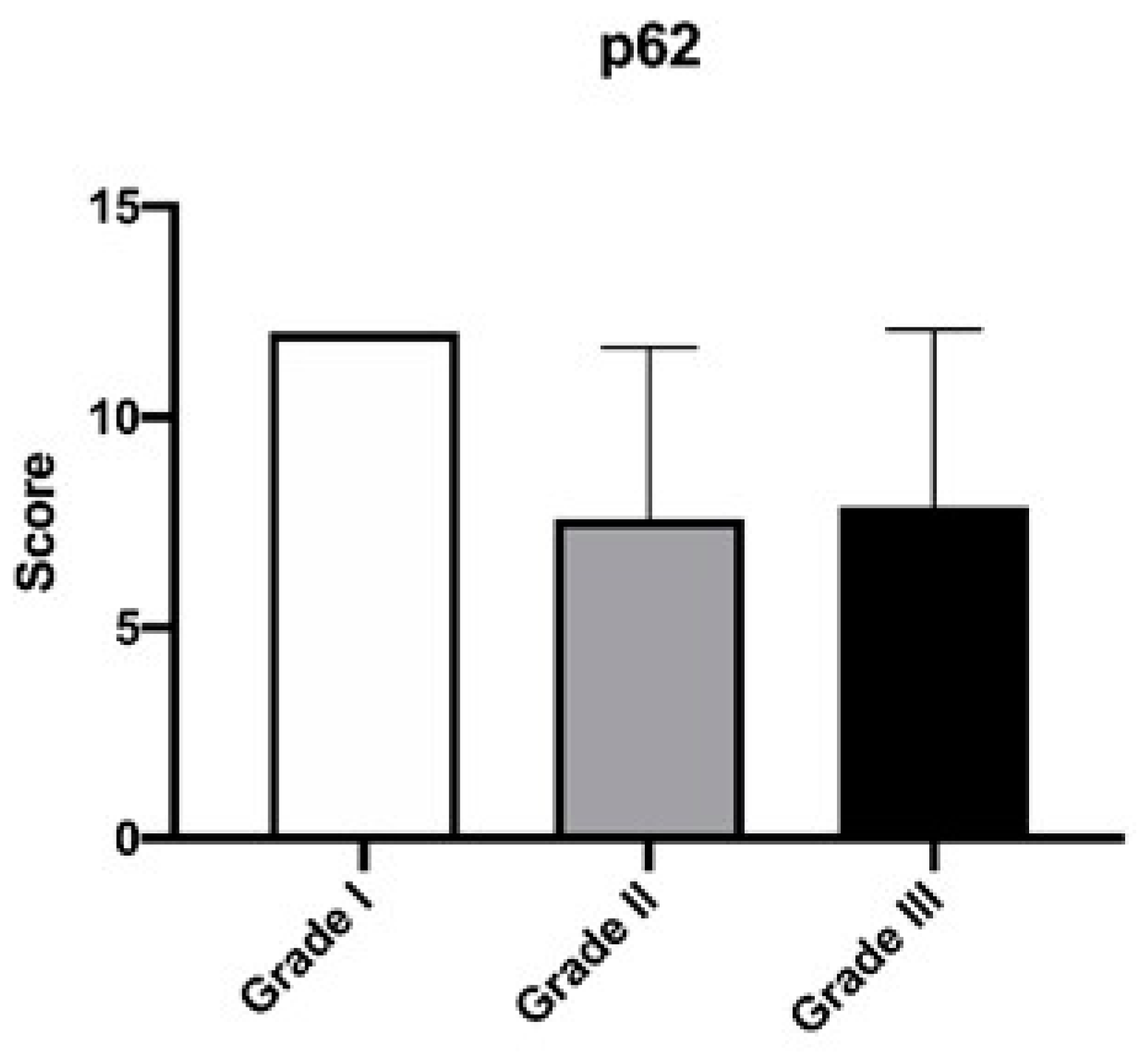

| p62 Positivity Score | ||||
|---|---|---|---|---|
| Low (1–2) | Intermediate (3–4) | High (≥5) | p-Value | |
| Grade I Carcinoma (n = 5) | - | - | 100% (n = 5) | 0.0919 |
| Grade II Carcinoma (n = 10) | 20% (n = 2) | 10% (n = 1) | 70% (n = 7) | 0.0666 |
| Grade III Carcinoma (n = 23) | 21.7% (n = 5) | 8.7% (n = 2) | 69.6% (n = 16) | >0.9999 |
| Cases with Lymphovascular Invasion and p62 Immunoreactivity | ||||
|---|---|---|---|---|
| Low p62 (1–2) | Intermediate p62 (3–4) | High p62 (≥ 5) | p-Value | |
| Carcinoma (n = 10) | 20% (n = 2) | 10% (n = 1) | 70% (n = 7) | |
| Grade II Carcinoma (n = 2) | - | - | 100% (n = 2) | 0.5556 |
| Grade III Carcinoma (n = 8) | 25% (n = 2) | 12.5% (n = 1) | 62.5% (n = 5) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magi, G.E.; Mariotti, F.; Pallotta, L.; Di Cerbo, A.; Venanzi, F.M. Immunohistochemical Expression of p62 in Feline Mammary Carcinoma and Non-Neoplastic Mammary Tissue. Animals 2022, 12, 1964. https://doi.org/10.3390/ani12151964
Magi GE, Mariotti F, Pallotta L, Di Cerbo A, Venanzi FM. Immunohistochemical Expression of p62 in Feline Mammary Carcinoma and Non-Neoplastic Mammary Tissue. Animals. 2022; 12(15):1964. https://doi.org/10.3390/ani12151964
Chicago/Turabian StyleMagi, Gian Enrico, Francesca Mariotti, Lorenzo Pallotta, Alessandro Di Cerbo, and Franco Maria Venanzi. 2022. "Immunohistochemical Expression of p62 in Feline Mammary Carcinoma and Non-Neoplastic Mammary Tissue" Animals 12, no. 15: 1964. https://doi.org/10.3390/ani12151964
APA StyleMagi, G. E., Mariotti, F., Pallotta, L., Di Cerbo, A., & Venanzi, F. M. (2022). Immunohistochemical Expression of p62 in Feline Mammary Carcinoma and Non-Neoplastic Mammary Tissue. Animals, 12(15), 1964. https://doi.org/10.3390/ani12151964







