Variability among Animals and Incubation Protocols for Ruminant In Situ Degradation Studies with Tropical Feeds
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Samples Characterization
2.2. Ruminal Incubations Procedures
2.3. Evaluation of Variability among Animals Regarding Degradation Rates
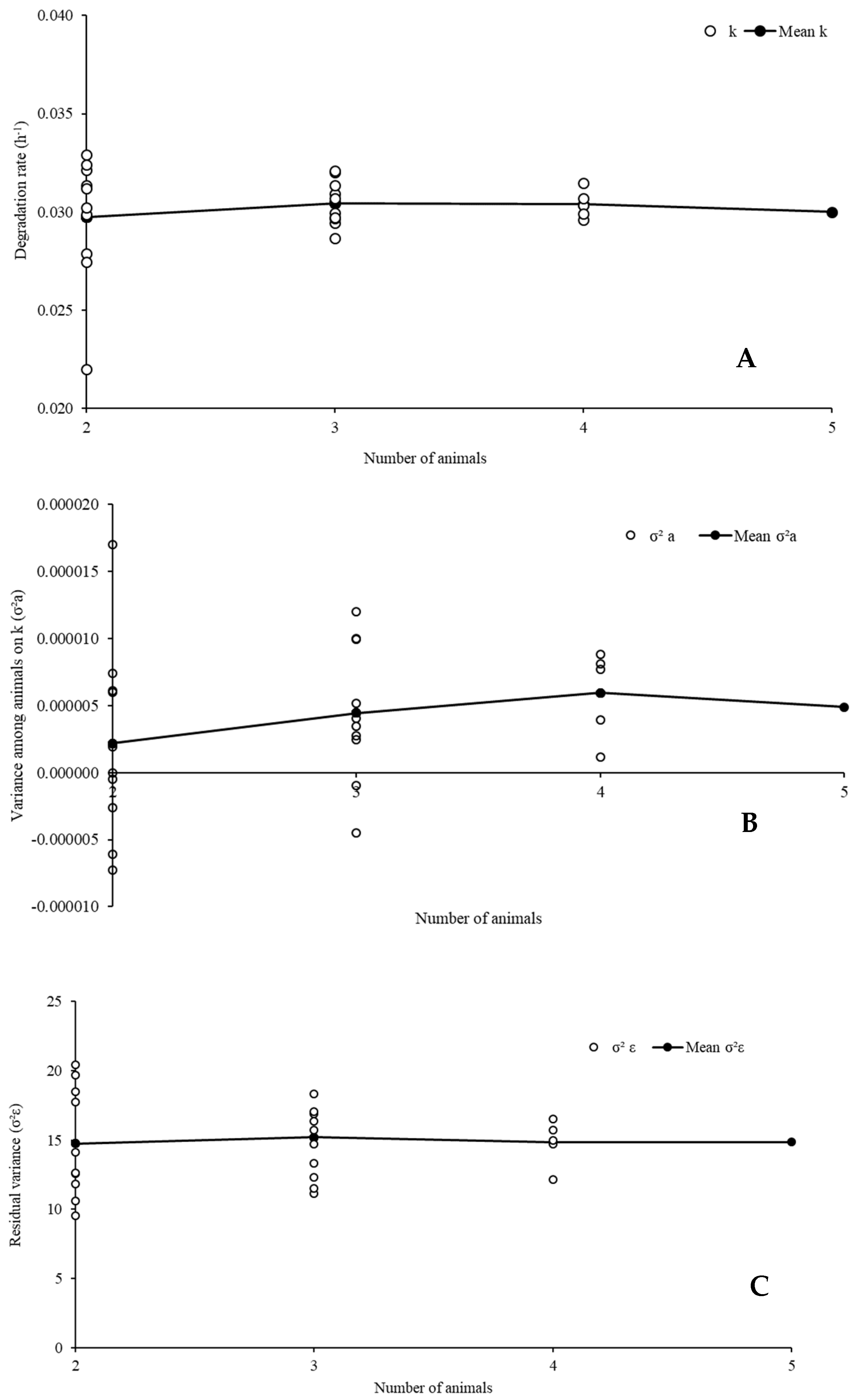
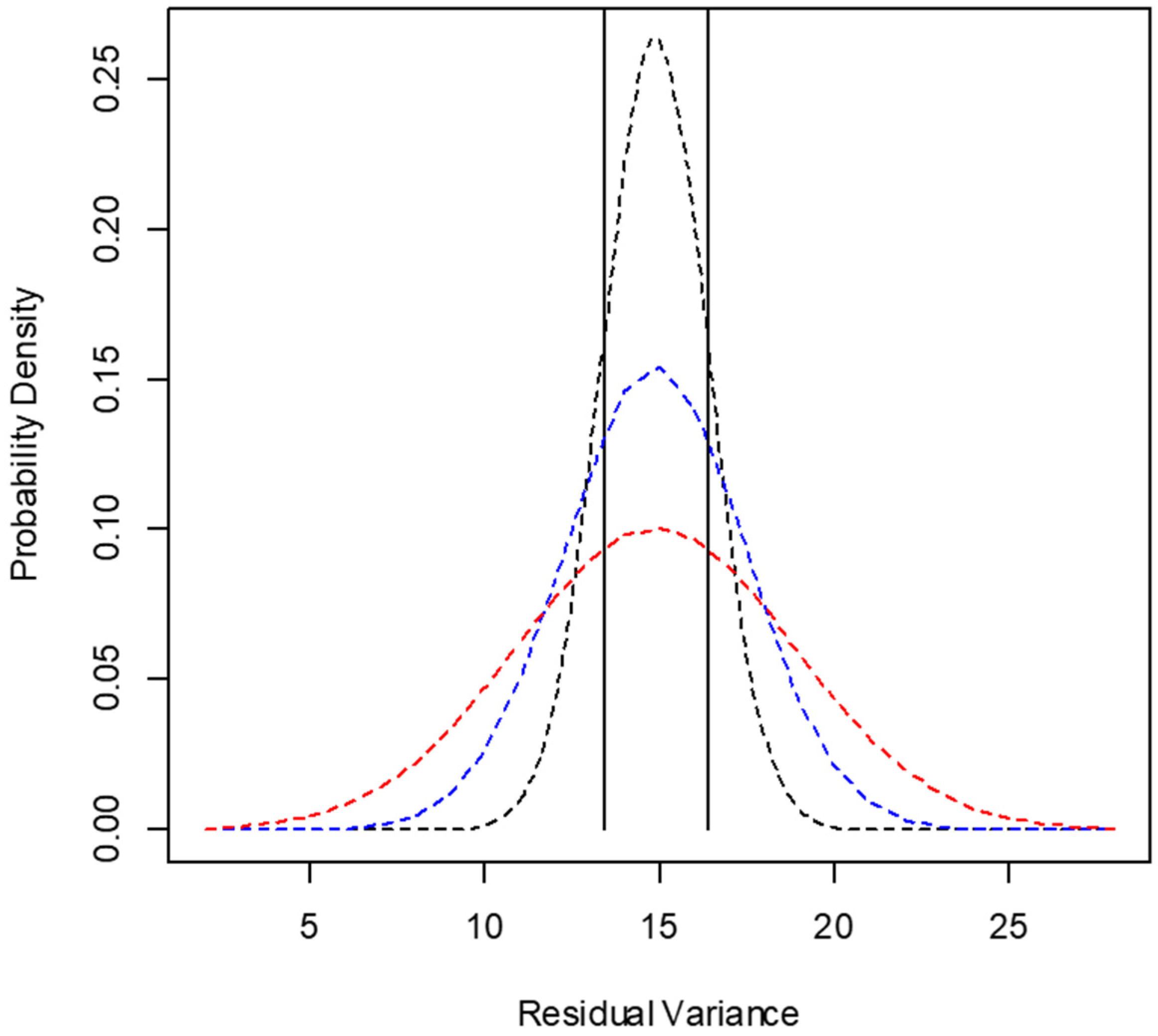
2.4. Evaluation of the Minimum Number of Animals for In Situ Degradation Essay
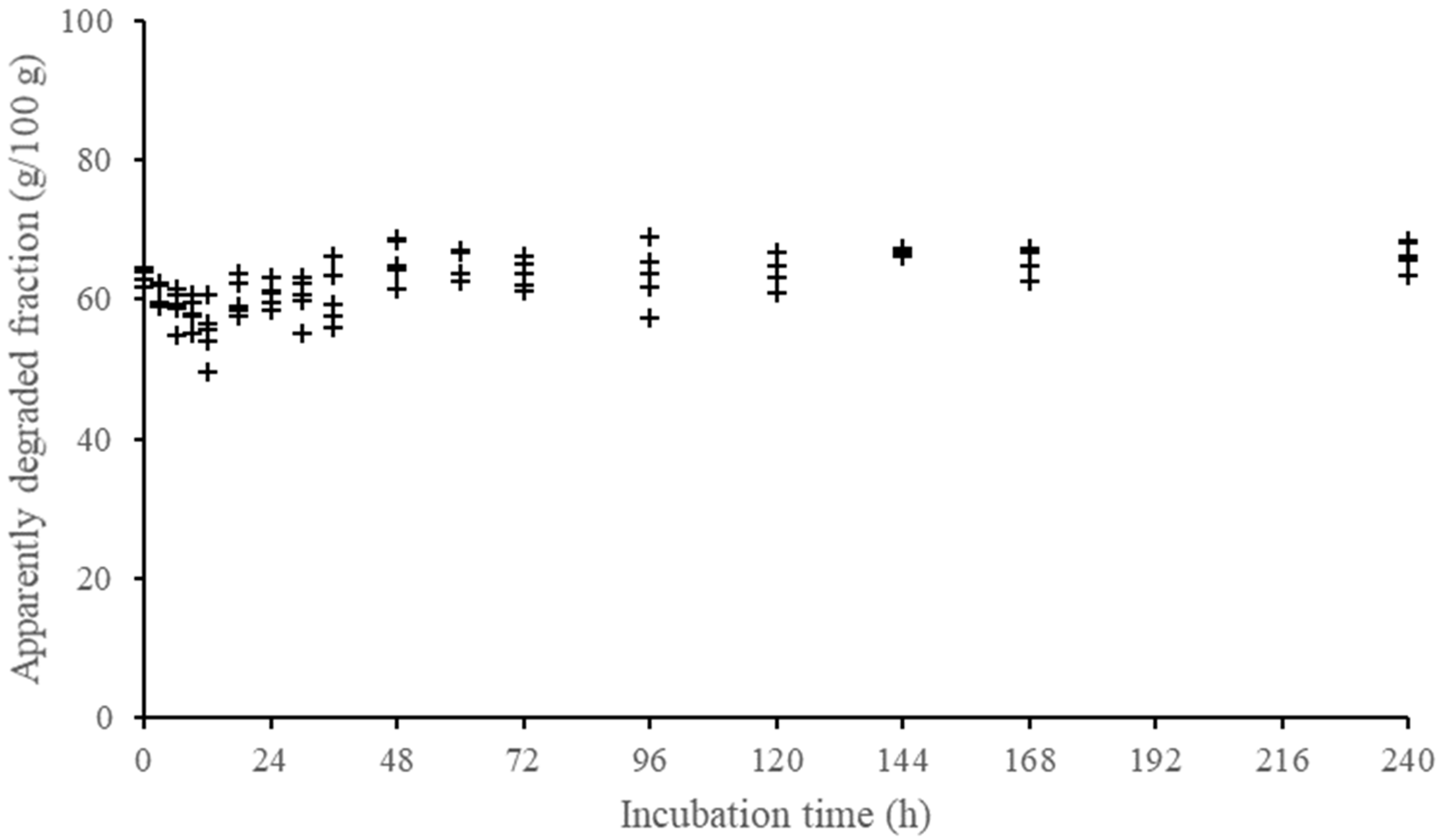
2.5. Establishment of a Minimum Design of Sampling Times for Incubations
3. Results and Discussion
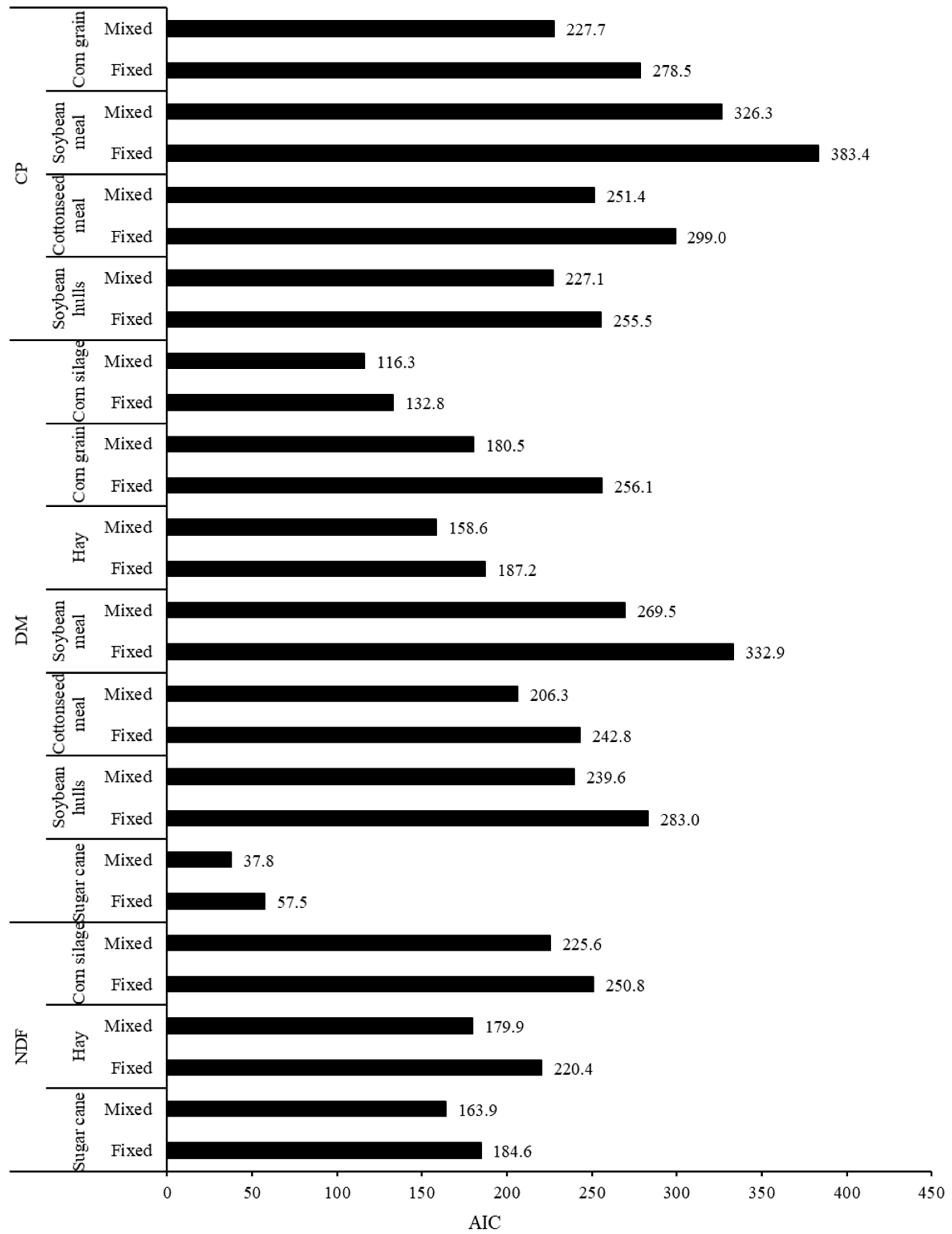
3.1. Comparison of Adjustments Using Fixed or Mixed Models Approach
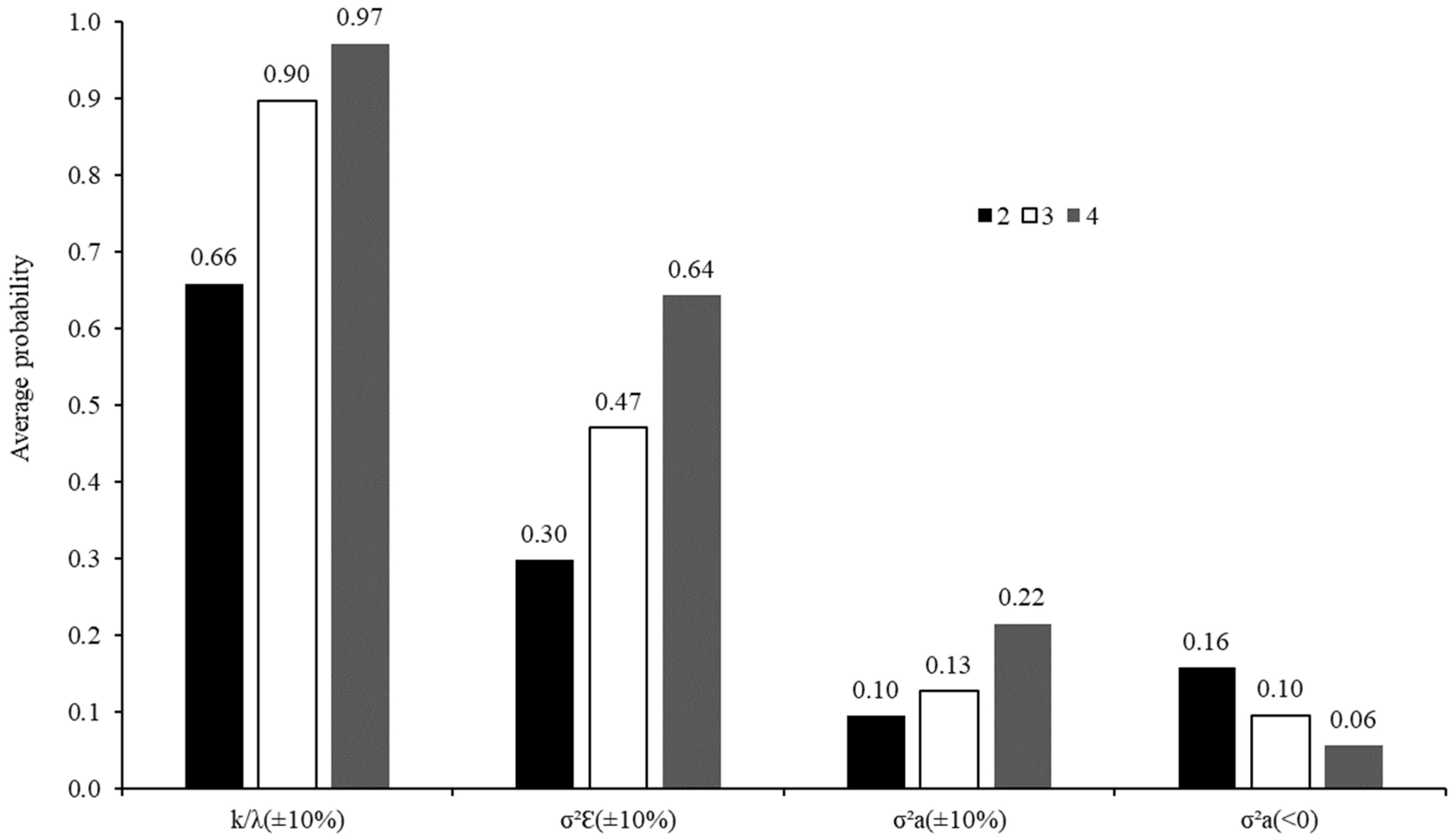
3.2. Evaluation of the Number of Animals Used in the Incubation Procedures
3.3. Definition of Minimum Scheme of Incubation Times
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huhtanen, P.; Nousiainen, J.; Rinne, M. Recent developments in forage evaluation with special reference to practical applications. Agric. Food Sci. 2006, 15, 293–323. [Google Scholar] [CrossRef] [Green Version]
- Harmon, D.L.; Richards, C.J. Considerations for gastrointestinal cannulations in ruminants. J. Anim. Sci. 1997, 75, 2248–2255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broderick, G.A.; Cochran, R.C. In vitro and in situ methods for estimating digestibility with references to protein degradability. In Feeding Systems and Feed Evaluation Models; Theodorou, M.K., France, J., Eds.; CAB International: Wallingford, UK, 2000; pp. 53–85. [Google Scholar]
- Warner, R.G.; Flatt, W.P.; Loosli, J.K. Ruminant nutrition, dietary factors influencing the development of the ruminant stomach. J. Agric. Food Chem. 1956, 9, 788–792. [Google Scholar] [CrossRef]
- Huhtanen, P.; Kaustell, K.; Jaakkola, S. The use of internal markers to predict total digestibility and duodenal flow of nutrients in cattle given six different diets. Anim. Feed. Sci. Technol. 1994, 48, 211–227. [Google Scholar] [CrossRef]
- Mertens, D.R. Rate and extent of digestion. In Quantitative Aspects of Ruminant Digestion and Metabolism; Dijkstra, J., Forbes, J.M., France, J., Eds.; CABI Publishing: Wallingford, UK, 2005; pp. 13–47. [Google Scholar] [CrossRef]
- Agricultural and Food Research Council—AFRC. Energy and Protein Requirements of Ruminants; CAB International: Wallingford, UK, 1993; 176p. [Google Scholar]
- National Research Council—NRC. Nutrient Requirements of Dairy Cattle, 7th ed.; Academy Press: Washington, DC, USA, 2001; 381p. [Google Scholar]
- Valadares Filho, S.C.; Costa e Silva, L.F.; Gionbelli, M.P.; Rotta, P.P.; Marcondes, M.I.; Chizzotti, M.L.; Prado, L.F. Nutrient Requirements of Zebu and Crossbred Cattle BR-CORTE, 3rd ed.; Suprema Gráfica: Viçosa, Brazil, 2016; 314p. [Google Scholar]
- Sampaio, C.B.; Gomes, D.I.; Regadas Filho, J.G.L.; Detmann, E.; Valadares Filho, S.C. Variations among animals when estimating the undegradable fraction of fiber in forage samples. Semin. Ciên. Agrárias 2014, 35, 2739–2748. [Google Scholar] [CrossRef] [Green Version]
- Tajima, K.; Aminov, R.I.; Nagamine, T.; Matsui, H.; Nakamura, M.; Benno, Y. Diet-dependent shifts in the bacterial population of the rumen revealed with real-time PCR. Appl. Environ. Microbiol. 2001, 67, 2766–2774. [Google Scholar] [CrossRef] [Green Version]
- Reis, W.L.S.; Detmann, E.; Batista, E.D.; Rufino, L.M.A.; Gomes, D.I.; Bento, C.B.P.; Mantovani, H.C.; Valadares Filho, S.V. Effects of ruminal and post-ruminal protein supplementation in cattle fed tropical forages on insoluble fiber degradation, activity of fibrolytic enzymes, and the ruminal microbial community profile. Anim. Feed. Sci. Technol. 2016, 218, 1–16. [Google Scholar] [CrossRef]
- Weimer, P.J.; Stevenson, D.M.; Mantovani, H.C.; Man, S.L.C. Host specificity of the ruminal bacterial community in the dairy cow following near-total exchange of ruminal contents. J. Dairy Sci. 2010, 93, 5902–5912. [Google Scholar] [CrossRef]
- Jami, E.; Mizrahi, I. Composition and similarity of bovine rumen microbiota across individual animals. PLoS ONE 2012, 7, e33306. [Google Scholar] [CrossRef] [Green Version]
- Tomich, T.R.; Sampaio, I.B.M. A new strategy for the determination of forage degradability with an in situ technique through the use of one fistulated ruminant. J. Agric. Sci. 2004, 142, 589–593. [Google Scholar] [CrossRef]
- Mehrez, A.Z.; Ørskov, E.R. A study of artificial fibre bag technique for determining the digestibility of feeds in the rumen. J. Agric. Sci. 1977, 88, 645–650. [Google Scholar] [CrossRef]
- Åkerlind, M.; Weisbjerg, M.; Eriksson, T.; Togersen, R.; Udén, P.; Ólafsson, B.L.; Harstad, O.M.; Volden, H. Feed analyses and digestion methods. In NorFor—The Nordic Feed Evaluation System; Volden, H., Ed.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2011; pp. 41–54. [Google Scholar]
- Menezes, A.C.B.; Valadares Filho, S.C.; Rotta, P.P.; Santos, S.A.; Pacheco, M.V.C.; Silva, B.C.; Pucetti, P.; Alhadas, H.M.; Detmann, E.; Caton, J.S. Does microbial nitrogen contamination affect the estimation of crude protein degradability of concentrate feeds? J. Anim. Sci. 2017, 95, 4164–4171. [Google Scholar] [CrossRef] [PubMed]
- Seifried, N.; Steingaß, H.; Rodehutscord, M. In vitro and in situ evaluation of secondary starch particle losses from nylon bags during the incubation of different cereal grains. Anim. Feed. Sci. Technol. 2015, 210, 26–36. [Google Scholar] [CrossRef]
- Trujillo, A.I.; Marichal, M.J.; Carriquiry, M. Comparison of dry matter and neutral detergent fibre degradation of fibrous feedstuffs as determined with in situ and in vitro gravimetric procedures. Anim. Feed. Sci. Technol. 2010, 161, 49–57. [Google Scholar] [CrossRef]
- Machado, P.A.S.; Valadares Filho, S.C.; Detmann, E.; Santos, S.A.; Valadares, R.F.D.; Ducatti, C.; Rotta, P.P.; Costa e Silva, L.F. Development of equations to estimate microbial contamination in ruminal incubation residues of forage produced under tropical conditions using 15N as a label. J. Anim. Sci. 2013, 91, 3836–3846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ford, C.W.; Morrison, I.M.; Wilson, J.R. Temperature effects on lignin, hemicellulose and cellulose in tropical and temperate grasses. Aust. J. Agric. Res. 1979, 30, 621–633. [Google Scholar] [CrossRef]
- Wilson, J.R.; Minson, D.J. Prospects for improving the digestibility and intake of tropical grasses. Trop. Grassl. 1980, 14, 253–259. [Google Scholar]
- Assoumaya, C.; Sauvant, D.; Archimede, H. Etude comparative de l’ingestion et de la digestion des fourrages tropicaux et tempérés. INRA Prod. Anim. 2007, 20, 383–392. [Google Scholar] [CrossRef]
- Khan, P.; Tong, L.; Khan, S.U.; Zhang, C.; Wang, W. Lignin: A defensive shield halting the environmental stresses—A review. Appl. Ecol. Environ. Res. 2022, 20, 1991–2015. [Google Scholar] [CrossRef]
- Detmann, E.; Valadares Filho, S.C.; Pina, D.S.; Henriques, L.T.; Paulino, M.F.; Magalhães, K.A.; Silva, P.A.; Chizzotti, M.L. Prediction of the energy value of cattle diets based on the chemical composition of the feeds under tropical conditions. Anim. Feed. Sci. Technol. 2008, 143, 127–147. [Google Scholar] [CrossRef]
- Vanzant, E.S.; Cochran, R.C.; Titgemeyer, E.C. Standardization of in situ techniques for ruminant feedstuff evaluation. J. Anim. Sci. 1998, 76, 2717–2729. [Google Scholar] [CrossRef] [PubMed]
- Michalet-Doreau, B.; Ould-Bah, M.Y. In vitro and in sacco methods for the estimation of dietary nitrogen degradability in the rumen: A review. Anim. Feed. Sci. Technol. 1992, 40, 57–86. [Google Scholar] [CrossRef]
- Bruttel, P.; Schlink, R. Water Determination by Karl Fischer Titration; Metrohm: Herisau, Switzerland, 2006; 80p. [Google Scholar]
- Detmann, E.; Costa e Silva, L.F.; Rocha, G.C.; Palma, M.N.N.; Rodrigues, J.P.P. Métodos Para Análise de Alimentos; Suprema: Visconde do Rio Branco, Brazil, 2021; 350p. [Google Scholar]
- Machado, M.G.; Detmann, E.; Mantovani, H.C.; Valadares Filho, S.C.; Bento, C.B.B.; Marcondes, M.I.; Assunção, A.S. Evaluation of the length of adaptation period for changeover and crossover nutritional experiments with cattle fed tropical forage-based diets. Anim. Feed. Sci. Technol. 2016, 222, 132–148. [Google Scholar] [CrossRef]
- Nocek, J.E. In situ and other methods to estimate ruminal protein and energy digestibility: A review. J. Dairy Sci. 1988, 71, 2051–2069. [Google Scholar] [CrossRef]
- Detmann, E.; Paulino, M.F.; Valadares Filho, S.C. Avaliação de alimentos ou dietas? In Simpósio de Produção de Gado de Corte; Universidade Federal de Viçosa: Viçosa, Brazil, 2008; pp. 21–52. [Google Scholar]
- Littell, R.C.; Milliken, G.A.; Stroup, W.W.; Wolfinger, R.D.; Schabenberger, O. SAS for Mixed Models, 2nd ed.; SAS Institute Inc.: Cary, NV, USA, 2006; 814p. [Google Scholar]
- Ørskov, E.R.; McDonald, I. The estimation of protein degradability in the rumen from incubation measurements weighted according to rate of passage. J. Agric. Sci. 1979, 92, 499–503. [Google Scholar] [CrossRef] [Green Version]
- Van Milgen, J.; Murphy, M.R.; Berger, L.L. A compartmental model to analyze ruminal digestion. J. Dairy Sci. 1991, 74, 2515–2529. [Google Scholar] [CrossRef]
- Beal, S.L.; Sheiner, L.B. Estimating population kinetics. Crit. Rev. Biomed. Eng. 1982, 8, 195–222. [Google Scholar] [PubMed]
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Autom. Control. 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Casali, A.O.; Detmann, E.; Valadares Filho, S.C.; Pereira, J.C.; Henriques, L.T.; Freitas, S.G.; Paulino, M.F. Influence of incubation time and particles size on indigestible compounds contents in cattle feeds and feces obtained by in situ procedures. Rev. Bras. Zootec. 2008, 37, 335–342. [Google Scholar] [CrossRef] [Green Version]
- Valente, T.N.P.; Detmann, E.; Queiroz, A.C.; Valadares Filho, S.C.; Gomes, D.I.; Figueiras, J.F. Evaluation of ruminal degradation profiles of forages using bags made from different textiles. Rev. Bras. Zootec. 2011, 40, 2565–2573. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Zuidhof, M.J. Estimation of growth parameters using a nonlinear mixed Gompertz model. Poult. Sci. 2004, 83, 847–852. [Google Scholar] [CrossRef] [PubMed]
- Aggrey, S.E. Logistic nonlinear mixed effects model for estimating growth parameters. Poult. Sci. 2009, 88, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Leng, R.A. Factors affecting the utilization of ‘poor-quality’ forages by ruminants particularly under tropical conditions. Nutr. Res. Rev. 1990, 3, 277–303. [Google Scholar] [CrossRef] [PubMed]
- Detmann, E.; Franco, M.O.; Batista, E.D.; Reis, W.L.S.; Valadares Filho, S.C.; Paulino, M.F. Cinética de digestão e passagem da fibra em ruminantes e sua otimização por meio de estratégias nutricionais. In Simpósio Brasileiro de Microbiologia do Rúmen; Universidade Federal do Mato Grosso: Cuiabá, Brazil, 2015. [Google Scholar]
- Zanton, G.I.; Heinrichs, A.J. Evaluation of modeling procedure for fitting in situ feed degradation profiles. J. Anim. Sci. 2009, 87, 2080–2088. [Google Scholar] [CrossRef] [Green Version]
- Casella, G.; Berger, R.L. Statistical Inference, 2nd ed.; Cengage Learning: Belmont, CA, USA, 2001; 660p. [Google Scholar]
- Waldo, D.R.; Smith, L.W.; Cox, E.L. Model of cellulose disappearance from the rumen. J. Dairy Sci. 1972, 55, 125–129. [Google Scholar] [CrossRef]
- Bento, C.P.B.; Azevedo, A.C.; Gomes, D.I.; Batista, E.D.; Rufino, L.M.A.; Detmann, E.; Meantovani, H.C. Effect of protein supplementation on ruminal parameters and microbial community fingerprint of Nellore steers fed tropical forages. Animal 2016, 10, 44–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siegfried, B.; Ruckermann, B.; Stumpf, R.H. Method for determination of organic acids in silage by high performance liquid chromatography. Landwirtsch. Forsch. 1984, 37, 298–304. [Google Scholar]

| Feeds 1 | DM | OM | CP | NDF | Lignin |
|---|---|---|---|---|---|
| g/kg | g/kg DM | ||||
| Sugarcane | 303 | 971 | 35.9 | 515 | 52.1 |
| Corn silage | 287 | 915 | 112 | 373 | 33.1 |
| Tifton hay | 878 | 929 | 114 | 771 | 54.2 |
| Soybean meal | 890 | 940 | 543 | 165 | 2.6 |
| Corn grain | 883 | 988 | 105 | 105 | 2.1 |
| Soybean hulls | 886 | 955 | 197 | 652 | 19.1 |
| Cottonseed meal | 940 | 941 | 465 | 364 | 89.3 |
| Feed Type | Component 1 | Time (h) | p Value 2 |
|---|---|---|---|
| Forage | DM | 0 | 0.888 |
| 240 | 0.209 | ||
| NDF | 240 | 0.308 | |
| Concentrates | DM | 0 | 0.818 |
| 144 | 0.836 | ||
| CP | 0 | 0.868 | |
| 144 | 0.605 |
| Fixed Model 1 | Mixed Model 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Feed | k/λ | ASE | σ2Ԑ | k/λ | ASE | σ2a | p value | σ2Ԑ | Δ(%) |
| Dry Matter | |||||||||
| Sugarcane | 0.0171 | 0.00093 | 1.90 | 0.0169 | 0.00168 | 3.57 × 10−6 | 0.513 | 1.49 | 21.6 |
| Corn silage | 0.0256 | 0.00123 | 4.61 | 0.0257 | 0.00146 | 4.61 × 10−6 | 0.235 | 3.75 | 18.7 |
| Tifton hay | 0.0266 | 0.00126 | 8.74 | 0.0264 | 0.00170 | 9.55 × 10−6 | 0.154 | 6.17 | 29.4 |
| Soybean meal | 0.0492 | 0.00440 | 48.75 | 0.0511 | 0.00607 | 1.30 × 10−4 | 0.313 | 34.50 | 29.2 |
| Corn grain | 0.0492 | 0.00272 | 19.74 | 0.0482 | 0.00392 | 7.20 × 10−5 | 0.219 | 10.53 | 46.7 |
| Soybean hulls | 0.0280 | 0.00198 | 27.11 | 0.0281 | 0.00204 | 6.13 × 10−6 | 0.214 | 23.18 | 14.5 |
| Cottonseed meal | 0.0300 | 0.00230 | 16.88 | 0.0303 | 0.00251 | 6.87 × 10−6 | 0.330 | 14.87 | 11.9 |
| Crude Protein | |||||||||
| Soybean meal | 0.0418 | 0.00392 | 88.28 | 0.0421 | 0.00405 | 2.90 × 10−5 | 0.408 | 73.59 | 16.6 |
| Corn grain | 0.0342 | 0.00335 | 25.71 | 0.0361 | 0.00447 | 4.60 × 10−5 | 0.355 | 19.78 | 23.1 |
| Soybean hulls | 0.0362 | 0.00402 | 19.61 | 0.0360 | 0.00322 | 0 | --- | 19.61 | --- |
| Cottonseed meal | 0.0794 | 0.00537 | 32.73 | 0.0825 | 0.00778 | 1.32 × 10−4 | 0.399 | 27.13 | 17.1 |
| Neutral Detergent Fiber | |||||||||
| Sugarcane | 0.0438 | 0.00161 | 8.47 | 0.0438 | 0.00170 | 1.40 × 10−5 | 0.078 | 6.57 | 22.4 |
| Corn silage | 0.0523 | 0.00186 | 18.46 | 0.0524 | 0.00264 | 2.90 × 10−5 | 0.248 | 13.57 | 26.5 |
| Tifton hay | 0.0588 | 0.00200 | 12.91 | 0.0591 | 0.00320 | 5.20 × 10−5 | 0.143 | 7.93 | 38.6 |
| σ2Ԑ (±10%) | σ2a (±10%) | σ2a (±10%) | σ2a (<0) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Feed | 2 | 3 | 4 | 2 | 3 | 4 | 2 | 3 | 4 | 2 | 3 | 4 |
| Dry Matter | ||||||||||||
| Sugarcane | 0.82 | 0.94 | >0.99 | 0.47 | 0.74 | 0.86 | 0.14 | 0.16 | 0.21 | 0.04 | 0.02 | <0.01 |
| Corn silage | 0.53 | 0.98 | >0.99 | 0.55 | 0.74 | 0.93 | 0.08 | 0.09 | 0.13 | 0.17 | 0.13 | 0.06 |
| Tifton hay | 0.79 | 0.94 | 0.99 | 0.27 | 0.44 | 0.63 | 0.10 | 0.12 | 0.16 | 0.10 | 0.07 | 0.02 |
| Soybean meal | 0.45 | 0.67 | 0.83 | 0.16 | 0.26 | 0.39 | 0.09 | 0.13 | 0.18 | 0.13 | 0.05 | 0.01 |
| Corn grain | 0.51 | 0.70 | 0.92 | 0.18 | 0.27 | 0.39 | 0.13 | 0.17 | 0.28 | 0.05 | 0.02 | <0.01 |
| Soybean hulls | 0.77 | 0.94 | >0.99 | 0.37 | 0.50 | 0.70 | 0.08 | 0.11 | 0.16 | 0.17 | 0.08 | 0.02 |
| Cottonseed meal | 0.64 | 0.99 | >0.99 | 0.29 | 0.44 | 0.67 | 0.05 | 0.08 | 0.12 | 0.25 | 0.17 | 0.07 |
| Crude Protein | ||||||||||||
| Soybean meal | 0.62 | 0.77 | 0.94 | 0.24 | 0.39 | 0.56 | 0.08 | 0.13 | 0.25 | 0.16 | 0.05 | <0.01 |
| Corn grain | 0.14 | 0.13 | 0.16 | 0.22 | 0.25 | 0.30 | 0.00 | 0.00 | 0.00 | 0.49 | 0.49 | 0.50 |
| Cottonseed meal | 0.33 | 0.99 | >0.99 | 0.22 | 0.34 | 0.50 | 0.10 | 0.18 | 0.53 | 0.10 | 0.01 | <0.01 |
| Neutral Detergent Fiber | ||||||||||||
| Sugarcane | 0.89 | 0.99 | >0.99 | 0.26 | 0.66 | 0.83 | 0.08 | 0.10 | 0.13 | 0.14 | 0.10 | 0.05 |
| Corn silage | 0.79 | 0.94 | >0.99 | 0.30 | 0.50 | 0.71 | 0.10 | 0.13 | 0.18 | 0.12 | 0.06 | 0.01 |
| Tifton hay | 0.77 | 0.92 | >0.99 | 0.26 | 0.39 | 0.57 | 0.11 | 0.13 | 0.26 | 0.08 | 0.05 | <0.01 |
| Incubation Times (h) 1 | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Feed | 0 | 3 | 6 | 9 | 12 | 18 | 24 | 30 | 36 | 48 | 72 | 96 | 120 | 144 | 168 | 240 |
| Forages | ||||||||||||||||
| Sugarcane | × | × | × | - | × | - | × | × | - | × | × | - | × | - | - | - |
| Corn silage | × | × | × | - | × | - | × | × | - | × | × | - | × | - | - | - |
| Tifton hay | × | × | × | - | × | - | × | × | - | × | × | - | × | - | - | - |
| Concentrates | ||||||||||||||||
| Soybean meal | × | × | × | n | × | - | × | × | - | × | × | × | - | - | n | n |
| Corn grain | × | × | × | n | × | - | × | × | - | × | × | × | - | - | n | n |
| Soybean hulls | × | × | × | n | × | - | × | × | - | × | × | × | - | - | n | n |
| Cottonseed meal | × | × | × | n | × | - | × | × | - | × | × | × | - | - | n | n |
| Complet Time Set | Minimal Design 1 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Feed | A | B | U | k/λ | σ2Ԑ | A | B | U | k/λ | σ2Ԑ | p Value 2 |
| Dry Matter | |||||||||||
| Sugarcane | 48.5 | 26.1 | - | 0.0171 | 1.90 | 48.7 | 26.9 | - | 0,0159 | 2.48 | 0.151 |
| Corn silage | 47.3 | 39.2 | - | 0.0256 | 4.61 | 47.7 | 38.4 | - | 0.0258 | 5.60 | 0.224 |
| Tifton hay | 21.7 | 54.2 | - | 0.0266 | 8.74 | 22.4 | 54.2 | - | 0.0261 | 11.88 | 0.118 |
| Soybean meal | 32.1 | 69.1 | - | 0.0492 | 48.75 | 31.9 | 72.7 | - | 0.0442 | 42.17 | 0.690 |
| Corn grain | 28.5 | 71.2 | - | 0.0492 | 19.74 | 28.3 | 73.3 | - | 0.0461 | 23.32 | 0.263 |
| Soybean hulls | 22.8 | 76.3 | - | 0.0280 | 27.11 | 23.4 | 78.6 | - | 0.0262 | 26.12 | 0.544 |
| Cottonseed meal | 33.5 | 53.9 | - | 0.0300 | 16.88 | 32.2 | 55.3 | - | 0.0308 | 15.15 | 0.642 |
| Crude Protein | |||||||||||
| Soybean meal | 14.1 | 88.8 | - | 0.0418 | 88.28 | 17.2 | 87.2 | - | 0.0406 | 91.08 | 0.445 |
| Corn grain | 51.7 | 49.5 | - | 0.0342 | 25.71 | 52.7 | 50.6 | - | 0.0314 | 32.44 | 0.190 |
| Soybean hulls | 58.6 | 37.5 | - | 0.0362 | 19.61 | 59.5 | 36.5 | - | 0.0357 | 24.24 | 0.212 |
| Cottonseed meal | 24.7 | 73.3 | - | 0.0794 | 32.73 | 25.0 | 73.8 | - | 0.0770 | 48.64 | 0.069 |
| Neutral Detergent Fiber 3 | |||||||||||
| Sugarcane | - | 46.9 | 53.1 | 0.0438 | 8.47 | - | 47.8 | 52.2 | 0.0448 | 7.58 | 0.656 |
| Corn silage | - | 70.4 | 29.6 | 0.0523 | 18.46 | - | 70.2 | 29.8 | 0.0535 | 20.05 | 0.365 |
| Tifton hay | - | 66.5 | 33.5 | 0.0588 | 12.91 | - | 66.4 | 33.6 | 0.0615 | 16.59 | 0.159 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Assunção, A.d.S.; Silva, T.E.d.; Quirino, D.; Franco, M.d.O.; Detmann, E. Variability among Animals and Incubation Protocols for Ruminant In Situ Degradation Studies with Tropical Feeds. Animals 2022, 12, 1901. https://doi.org/10.3390/ani12151901
Assunção AdS, Silva TEd, Quirino D, Franco MdO, Detmann E. Variability among Animals and Incubation Protocols for Ruminant In Situ Degradation Studies with Tropical Feeds. Animals. 2022; 12(15):1901. https://doi.org/10.3390/ani12151901
Chicago/Turabian StyleAssunção, Amanda de Souza, Tadeu Eder da Silva, Daiana Quirino, Marcia de Oliveira Franco, and Edenio Detmann. 2022. "Variability among Animals and Incubation Protocols for Ruminant In Situ Degradation Studies with Tropical Feeds" Animals 12, no. 15: 1901. https://doi.org/10.3390/ani12151901
APA StyleAssunção, A. d. S., Silva, T. E. d., Quirino, D., Franco, M. d. O., & Detmann, E. (2022). Variability among Animals and Incubation Protocols for Ruminant In Situ Degradation Studies with Tropical Feeds. Animals, 12(15), 1901. https://doi.org/10.3390/ani12151901







