Effects of Heavy Metal Exposure from Leather Processing Plants on Serum Oxidative Stress and the Milk Fatty Acid Composition of Dairy Cows: A Preliminary Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling
2.3. Measurement of Heavy Metals
2.4. Oxidative Stress Analysis
2.5. Determination of Fatty Acids
2.6. Data Analysis
3. Results and Discussion
3.1. Heavy Metal Residues in Blood
3.2. Effect of Heavy Metal Exposure on Oxidative Stress in Serum
3.3. Changes in the Fatty Acid Content in Raw Milk from Polluted Areas
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- National Burea of Statistics of China National Data. 2022. Available online: http://www.stats.gov.cn/tjsj/ (accessed on 6 June 2022).
- Zhou, X.; Zheng, N.; Su, C.; Wang, J.; Soyeurt, H. Relationships between Pb, As, Cr, and Cd in individual cows’ milk and milk composition and heavy metal contents in water, silage, and soil. Environ. Pollut. 2019, 255, 113322. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Liu, H.; Qu, X.; Zhou, X.; Gao, Y.; Yang, H.; Zheng, N.; Wang, J. Heavy Metals in Raw Milk and Dietary Exposure Assessment in the Vicinity of Leather-Processing Plants. Biol. Trace Elem. Res. 2020, 199, 3303–3311. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhang, X.; Huang, H. Bi-enzymes treatments attenuate cognitive impairment associated with oxidative damage of heavy metals. R. Soc. Open Sci. 2021, 8, 201404. [Google Scholar] [CrossRef]
- Matović, V.; Buha, A.; Dukić-Ćosić, D.; Bulat, Z. Insight into the oxidative stress induced by lead and/or cadmium in blood, liver and kidneys. Food Chem. Toxicol. 2015, 78, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.Y.; Wang, W.X. A lipidomic approach to understand copper resilience in oyster Crassostrea hongkongensis. Aquat. Toxicol. 2018, 204, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Qu, R.; Feng, M.; Wang, X.; Qin, L.; Wang, C.; Wang, Z.; Wang, L. Metal accumulation and oxidative stress biomarkers in liver of freshwater fish Carassius auratus following in vivo exposure to waterborne zinc under different pH values. Aquat. Toxicol. 2014, 150, 9–16. [Google Scholar] [CrossRef]
- Liu, X.; Wang, W.X. Antioxidant and detoxification responses of oysters Crassostrea hongkongensis in a multimetal-contaminated estuary. Environ. Toxicol. Chem. 2016, 35, 2798–2805. [Google Scholar] [CrossRef]
- Liu, X.; Wang, W.X. Time changes in biomarker responses in two species of oyster transplanted into a metal contaminated estuary. Sci. Total Environ. 2016, 544, 281–290. [Google Scholar] [CrossRef]
- Wang, W.X.; Meng, J.; Weng, N. Trace metals in oysters: Molecular and cellular mechanisms and ecotoxicological impacts. Environ. Sci. Process. Impacts 2018, 20, 892–912. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Xu, Z.; Wang, Y.; Huang, Q. Effect of Cadmiumon Lipid Peroxidation and Activities of Antioxidant Enzymes in Growing Pigs. Boil. Trace Elem. Res. 2006, 110, 251–263. [Google Scholar] [CrossRef]
- Cui, W.; Cao, L.; Liu, J.; Ren, Z.; Zhao, B.; Dou, S. Effects of seawater acidi fi cation and cadmium on the antioxidant defense of flounder Paralichthys olivaceus larvae. Sci. Total. Environ. 2020, 718, 137234. [Google Scholar] [CrossRef] [PubMed]
- Javed, M.; Ahmad, I.; Usmani, N.; Ahmad, M. Studies on biomarkers of oxidative stress and associated genotoxicity and histopathology in Channa punctatus from heavy metal polluted canal. Chemosphere 2016, 151, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, D.; Samanta, L. Multivariate analysis of potential biomarkers of oxidative stress in Notopterus notopterus tissues from Mahanadi River as a function of concentration of heavy metals. Chemosphere 2016, 155, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Giarratano, E.; Gil, M.N.; Marinho, C.H.; Malanga, G. Metals from mine waste as potential cause of oxidative stress in burrowing crab Neohelice granulata from San Antonio bay. Ecotoxicol. Environ. Saf. 2016, 132, 68–76. [Google Scholar] [CrossRef]
- Souza-talarico, J.N.; Marcourakis, T.; Barbosa, F.; Berlanga, S.; Barros, M.; Pineda, D.; Pompéia, S.; Caramelli, P.; Plusquellec, P.; Lupien, S.J.; et al. Association between heavy metal exposure and poor working memory and possible mediation effect of antioxidant defenses during aging. Sci. Total Environ. 2017, 575, 750–757. [Google Scholar] [CrossRef]
- Olisekodiaka, M.J.; Igbeneghu, C.A.; Onuegbu, A.J.; Oduru, R.; Lawal, A.O. Lipid, lipoproteins, total antioxidant status and organ changes in rats administered high doses of cadmium chloride. Med Princ. Pract. 2012, 21, 156–159. [Google Scholar] [CrossRef]
- Alonso, M.L.; Benedito, J.L.; Miranda, M.; Castillo, C.; Hernández, J.; Shore, R.F. Cattle as biomonitors of soil arsenic, copper, and zinc concentrations in Galicia (NW Spain). Arch. Environ. Contam. Toxicol. 2002, 43, 103–108. [Google Scholar] [CrossRef]
- Toman, R. Heavy Metals–Environmental Contaminants and Their Occurrence in Different Types of Milk. Slovak J. Anim. Sci. 2016, 49, 122–131. [Google Scholar]
- Patra, R.C.; Swarup, D.; Naresh, R.; Kumar, P.; Shekhar, P.; Ranjan, R. Cadmium level in blood and milk from animals reared around different polluting sources in India. Bull. Environ. Contam. Toxicol. 2005, 74, 1092–1097. [Google Scholar] [CrossRef]
- Swarup, D.; Patra, R.C.; Naresh, R.; Kumar, P.; Shekhar, P. Blood lead levels in lactating cows reared around polluted localities; Transfer of lead into milk. Sci. Total Environ. 2005, 349, 67–71. [Google Scholar] [CrossRef]
- Mateo, R.; Beyer, W.N.; Spann, J.W.; Hoffman, D.J. Relation of fatty acid composition in lead-exposed mallards to fat mobilization, lipid peroxidation and alkaline phosphatase activity. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2003, 135, 451–458. [Google Scholar] [CrossRef]
- Reglero, M.M.; Taggart, M.A.; Monsalve-González, L.; Mateo, R. Heavy metal exposure in large game from a lead mining area: Effects on oxidative stress and fatty acid composition in liver. Environ. Pollut. 2009, 157, 1388–1395. [Google Scholar] [CrossRef]
- Gurer, H.; Ercal, N. Can antioxidants be beneficial in the treatment of lead poisoning? Free Radic. Biol. Med. 2000, 29, 927–945. [Google Scholar] [CrossRef]
- Hsu, P.C.; Guo, Y.L. Antioxidant nutrients and lead toxicity. Toxicology 2002, 180, 33–44. [Google Scholar] [CrossRef]
- Luo, T.; Shen, M.; Zhou, J.; Wang, X.; Xia, J.; Fu, Z.; Jin, Y. Chronic exposure to low doses of Pb induces hepatotoxicity at the physiological, biochemical, and transcriptomic levels of mice. Environ. Toxicol. 2019, 34, 521–529. [Google Scholar] [CrossRef]
- Hossain, S.; Hussain, J.; Bhowmick, S.; Sarkar, M.; Basunia, M.; Al Mamun, A.; Tanabe, Y.; Matsuzaki, K.; Hashimoto, M.; Shido, O. Docosahexaenoic Acid (DHA, C22:6, ω-3) Composition of Milk and Mammary Gland Tissues of Lactating Mother Rats Is Severely Affected by Lead (Pb) Exposure. Biol. Trace Elem. Res. 2020, 195, 525–534. [Google Scholar] [CrossRef]
- Shailaja, M.; Reddy, Y.S.; Kalakumar, B.D.P.; Brinda, S.A.; Manohar, G.; Kumar, B.D. Lead and trace element levels in milk and blood of buffaloes (Bubalus bubalis) from Hyderabad, India. Bull. Environ. Contam. Toxicol. 2014, 92, 698–702. [Google Scholar] [CrossRef]
- Unger, A.L.; Bourne, D.E.; Walsh, H.; Kraft, J. Fatty Acid Content of Retail Cow’s Milk in the Northeastern United States—What’s in It for the Consumer? J. Agric. Food Chem. 2020, 68, 4268–4276. [Google Scholar] [CrossRef]
- Pawar, M.; Panchasara, H.H.; Gupta, J.P. Compositional and fatty acid analysis of Kankrej cows’ milk. Indian J. Dairy Sci. 2020, 73, 399–402. [Google Scholar] [CrossRef]
- The Goverment of Wuji the Leather Industry in Wuji Country. Available online: http://www.wuji.gov.cn/col/1585720993954/2020/04/08/1586339404372.html (accessed on 6 June 2022).
- Su, C.; Zhang, J.; Li, Z.; Zhao, Q.; Liu, K.; Sun, Y.; Wang, J. Accumulation and Depletion of Cadmium in the Blood, Milk, Hair, Feces, and Urine of Cows during and After Treatment. Biol. Trace Elem. Res. 2017, 175, 122–128. [Google Scholar] [CrossRef]
- Wu, X.; Sun, H.; Xue, M.; Wang, D.; Luo, L.; Liu, J. Serum metabolome profiling revealed potential biomarkers for milk protein yield in dairy cows. J. Proteom. 2018, 184, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Methods in Ruminant Nutrition Research; Modern Education Press: Beijing, China, 2011. [Google Scholar]
- JECFA. Safety Evaluation of Certain Food Additives and Contaminants, Seventy-Third Meeting of the Joint FAO/WHO Expert Committee on Food Additives, Lead; WHO Food Additives Series 64; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- Tahir, M.; Iqbal, M.; Abbas, M.; Tahir, M.A.; Nazir, A.; Iqbal, D.N.; Kanwal, Q.; Hassan, F.; Younas, U. Comparative study of heavy metals distribution in soil, forage, blood and milk. Acta Ecol. Sin. 2017, 37, 207–212. [Google Scholar] [CrossRef]
- Pompilio, C.G.N.; Francisco, C.S.; Tulio, F.D.M.T.M.; Samuel, S.M.S.; Elisa, G.J.F. Heavy metals in blood, milk and cow’s urine reared in irrigated areas with wastewater. Heliyon 2021, 7, e06693. [Google Scholar] [CrossRef]
- Arslan, H.H. Evaluation of the Relationship of Blood Heavy Metal, Trace Element Levels and Antioxidative Metabolism in Cattle Which Are Living Near the Trunk Roads Anayol Yakınında Yaşayan Sığırlarda Kan Ağır Metal, İz Element Seviyeleri ve Antioksidan Metabolizma A. Kafkas. Univ. Vet. Fak. Derg. 2011, 17, 77–82. [Google Scholar]
- Rodríguez-Estival, J.; Barasona, J.A.; Mateo, R. Blood Pb and δ-ALAD inhibition in cattle and sheep from a Pb-polluted mining area. Environ. Pollut. 2012, 160, 118–124. [Google Scholar] [CrossRef]
- Alonso, M.L.; Benedito, J.L.; Miranda, M.; Castillo, C.; Hernández, J.; Shore, R.F. Arsenic, cadmium, lead, copper and zinc in cattle from Galicia, NW Spain. Sci. Total Environ. 2000, 246, 237–248. [Google Scholar] [CrossRef]
- Luna, D.; López-Alonso, M.; Cedeño, Y.; Rigueira, L.; Pereira, V.; Miranda, M. Determination of essential and toxic elements in cattle blood: Serum vs. plasma. Animals 2019, 9, 465. [Google Scholar] [CrossRef]
- Kou, H.; Ya, J.; Gao, X.; Zhao, H. The effects of chronic lead exposure on the liver of female Japanese quail (Coturnix japonica): Histopathological damages, oxidative stress and AMP-activated protein kinase based lipid metabolism disorder. Ecotoxicol. Environ. Saf. 2020, 190, 110055. [Google Scholar] [CrossRef]
- Wang, Z.; Yan, C.; Vulpe, C.D.; Yan, Y.; Chi, Q. Incorporation of in situ exposure and biomarkers response in clams Ruditapes philippinarum for assessment of metal pollution in coastal areas from the Maluan Bay of China. Mar. Pollut. Bull. 2012, 64, 90–98. [Google Scholar] [CrossRef]
- Kanwal, S.; Abbasi, N.A.; Chaudhry, M.J.I.; Ahmad, S.R.; Malik, R.N. Oxidative stress risk assessment through heavy metal and arsenic exposure in terrestrial and aquatic bird species of Pakistan. Environ. Sci. Pollut. Res. 2020, 27, 12293–12307. [Google Scholar] [CrossRef]
- Rodríguez-estival, J.; Martinez-haro, M.; Monsalve-gonzález, L.; Mateo, R. Interactions between endogenous and dietary antioxidants against Pb-induced oxidative stress in wild ungulates from a Pb polluted mining area. Sci. Total Environ. 2011, 409, 2725–2733. [Google Scholar] [CrossRef]
- Hermenean, A.; Damache, G.; Albu, P.; Ardelean, A.; Ardelean, G.; Puiu Ardelean, D.; Horge, M.; Nagy, T.; Braun, M.; Zsuga, M.; et al. Histopatological alterations and oxidative stress in liver and kidney of Leuciscus cephalus following exposure to heavy metals in the Tur River, North Western Romania. Ecotoxicol. Environ. Saf. 2015, 119, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Javed, M.; Ahmad, I.; Usmani, N.; Ahmad, M. Bioaccumulation, oxidative stress and genotoxicity in fish (Channa punctatus) exposed to a thermal power plant effluent. Ecotoxicol. Environ. Saf. 2016, 127, 163–169. [Google Scholar] [CrossRef]
- Hassan, M.Q.; Hadi, R.A.; Al-Rawi, Z.S.; Stohs, S.J. Protective effects of antioxidants against rheumatoid arthritis-Induced lipid peroxidation and glutathione depletion. Res. Commun. Pharmacol. Toxicol. 1998, 3, 105–113. [Google Scholar]
- Hanus, O.; Samkova, E.; Křížova, L.; Hasoňova, L.; Kala, R. Role of fatty acids in milk fat and the influence of selected factors on their variability—A review. Molecules 2018, 23, 1636. [Google Scholar] [CrossRef]
- Georgieva, N. Oxidative stress as a factor of disrupted ecological oxidative balance in biological systems—A review. Bulg. J. Vet. Med. 2005, 8, 1–11. [Google Scholar]
- Lawton, L.J.; Donaldson, W.E. Lead-induced tissue fatty acid alterations and lipid peroxidation. Biol. Trace Elem. Res. 1991, 28, 83–97. [Google Scholar] [CrossRef]
- Lim, S.Y.; Doherty, J.D.; McBride, K.; Miller-Ihli, N.J.; Carmona, G.N.; Stark, K.D.; Salem, N. Lead exposure and (n-3) fatty acid deficiency during rat neonatal development affect subsequent spatial task performance and olfactory discrimination. J. Nutr. 2005, 135, 1019–1026. [Google Scholar] [CrossRef][Green Version]
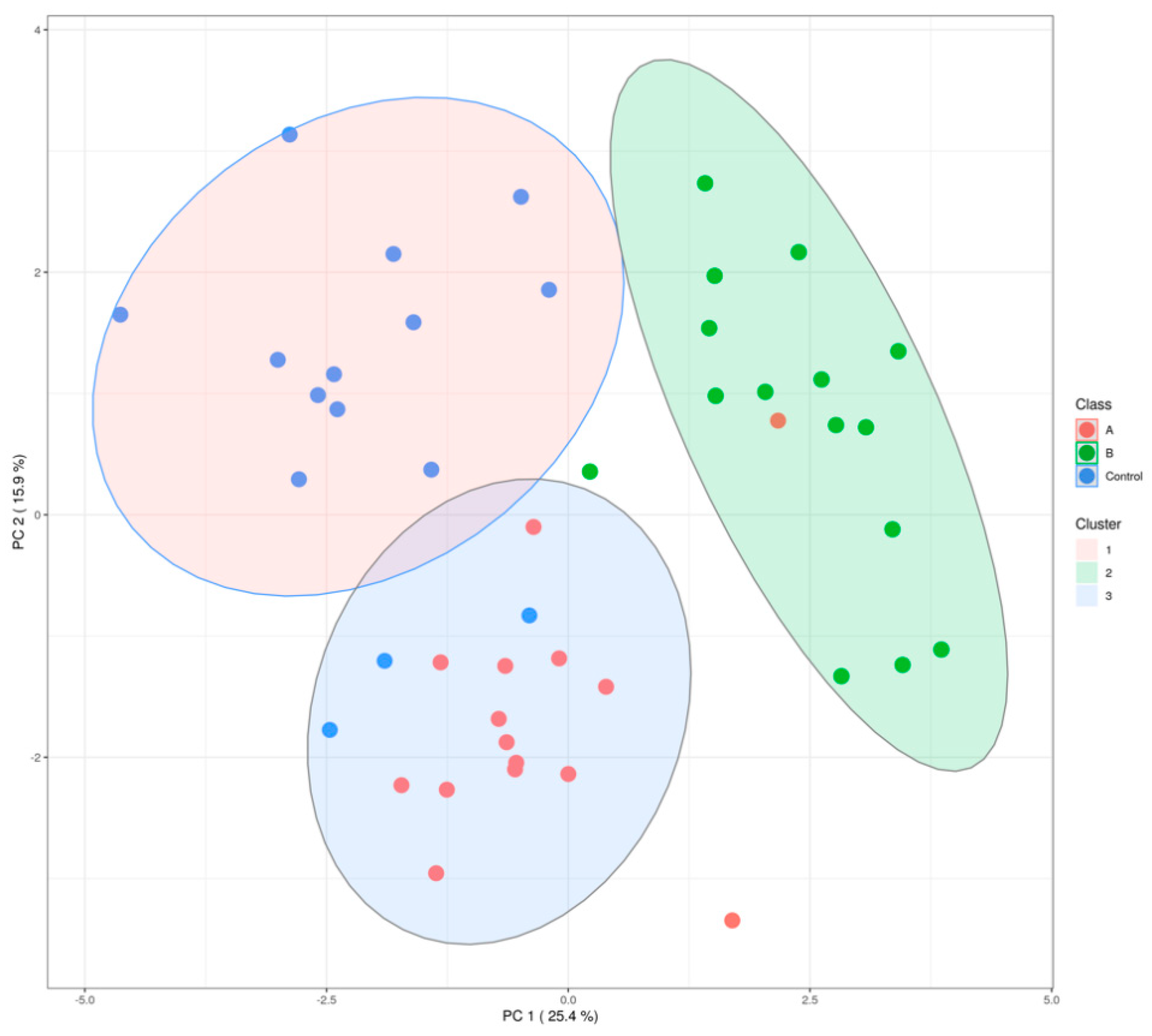
 Unpolluted area
Unpolluted area  polluted area.
polluted area.
 Unpolluted area
Unpolluted area  polluted area.
polluted area.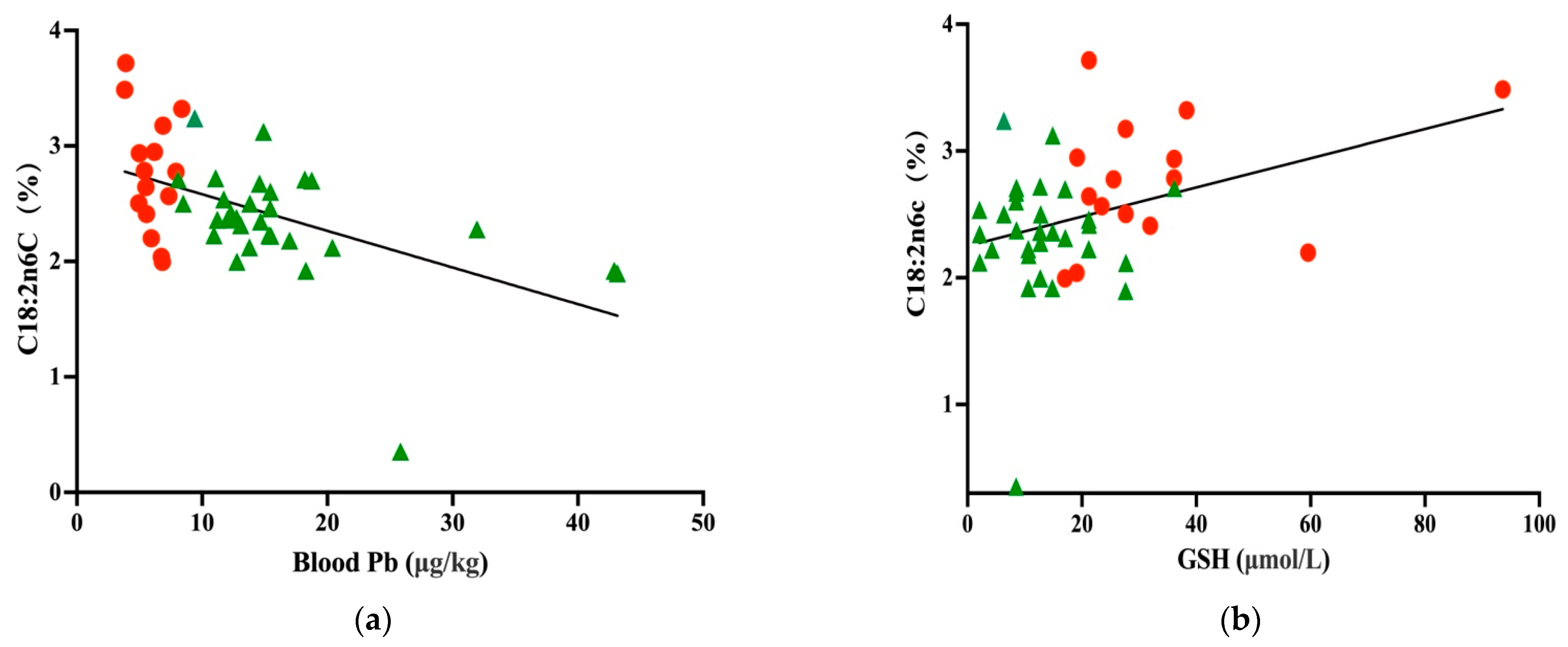
| Metals (μg/L) | Unpolluted (n = 15) | Polluted (n = 15) | p | ||
|---|---|---|---|---|---|
| Mean ± SD | Range | Mean ± SD | Range | ||
| As | 1.25 ± 0.18 | 1.04–1.66 | 1.61±0.28 | 1.19−2.18 | 0.000 ** |
| Pb | 6.25 ± 3.04 | 2.76–12.08 | 16.27±8.63 | 6.48−46.43 | 0.000 ** |
| Cr | 1.55 ± 0.56 | 1.08–2.98 | 2.54±1.51 | 1.16−9.11 | 0.014 * |
| Cd | 0.125 ± 0.043 | 0.07–0.21 | 0.119±0.057 | 0.06−0.26 | 0.702 |
| Parameters | Unpolluted (n = 15) | Polluted (n = 30) |
|---|---|---|
| SOD U/mL | 66.90 ± 8.25 | 69.10 ± 7.99 |
| GST U/mL | 68.81 ± 16.66 a | 52.09 ± 21.16 b |
| GPX U/L | 501.04 ± 58.62 a | 435.29 ± 19.14 b |
| GR ng/mL | 267.96 ± 114.50 | 292.83 ± 134.76 |
| CAT ng/mL | 23.98 ± 6.93 | 21.67 ± 5.66 |
| GSH μmol/L | 33.18 ± 19.91 a | 13.18 ± 7.99 b |
| MT ng/mL | 1458.19 ± 520.18 | 1195.85 ± 426.03 |
| MDA nmol/mL | 28.11 ± 19.11 | 20.23 ± 20.47 |
| Fatty Acids (%) | Unpolluted (n = 15) | Polluted (n = 30) |
|---|---|---|
| C6:0 | 1.96 ± 0.22 | 1.91 ± 0.55 |
| C8:0 | 1.4 ± 0.19 | 1.32 ± 0.52 |
| C10:0 | 8.5 ± 1.52 a | 7.28 ± 2.19 b |
| C12:0 | 4.11 ± 0.71 a | 3.26 ± 0.81 b |
| C13:0 | 0.04 ± 0.06 | 0.05 ± 0.05 |
| C14:0 | 12.25 ± 1.26 a | 11.19 ± 1.39 b |
| C15:0 | 1.23 ± 0.14 a | 1.10 ± 0.18 b |
| C16:0 | 32.01 ± 2.74 a | 35.72 ± 4.54 b |
| C17:0 | 0.82 ± 0.22 | 0.75 ± 0.091 |
| C18:0 | 11.13 ± 1.6 | 10.69 ± 2.08 |
| C20:0 | 0.09 ± 0.08 | 0.10 ± 0.07 |
| C23:0 | 0.19 ± 0.11 | 0.18 ± 0.07 |
| Total saturated fatty acids | 75.98 ± 8.66 | 75.72 ± 9.41 |
| C14:1 | 1.30 ± 0.22 | 1.21 ± 0.31 |
| C15:1 | 0.25 ± 0.17 | 0.21 ± 0.10 |
| C16:1 | 1.58 ± 0.41 | 1.64 ± 0.66 |
| C17:1 | 0.16 ± 0.12 | 0.15 ± 0.09 |
| C18:1n9t | 0.45 ± 0.24 | 0.45 ± 0.29 |
| C18:1n9c | 20.97 ± 2.48 | 21.03 ± 1.63 |
| C20:1 | 0.22 ± 0.20 | 0.15 ± 0.11 |
| The monoene fatty acids | 24.93 ± 7.02 | 24.83 ± 7.20 |
| C18:2n6t | 0.29 ± 0.11 | 0.23 ± 0.11 |
| C18:2n6c | 2.77 ± 0.51 a | 2.35 ± 0.49 b |
| C18:3n6 | 0.19 ± 0.19 | 0.19 ± 0.15 |
| C18:3n3 | 0.35 ± 0.22 | 0.29 ± 0.13 |
| C20:3n3 | 0.23 ± 0.19 | 0.25 ± 0.19 |
| Total poly unsaturated fatty acids | 3.83 ± 1.05 | 3.31 ± 0.89 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, C.; Qu, X.; Gao, Y.; Zhou, X.; Yang, X.; Zheng, N. Effects of Heavy Metal Exposure from Leather Processing Plants on Serum Oxidative Stress and the Milk Fatty Acid Composition of Dairy Cows: A Preliminary Study. Animals 2022, 12, 1900. https://doi.org/10.3390/ani12151900
Su C, Qu X, Gao Y, Zhou X, Yang X, Zheng N. Effects of Heavy Metal Exposure from Leather Processing Plants on Serum Oxidative Stress and the Milk Fatty Acid Composition of Dairy Cows: A Preliminary Study. Animals. 2022; 12(15):1900. https://doi.org/10.3390/ani12151900
Chicago/Turabian StyleSu, Chuanyou, Xueyin Qu, Yanan Gao, Xuewei Zhou, Xue Yang, and Nan Zheng. 2022. "Effects of Heavy Metal Exposure from Leather Processing Plants on Serum Oxidative Stress and the Milk Fatty Acid Composition of Dairy Cows: A Preliminary Study" Animals 12, no. 15: 1900. https://doi.org/10.3390/ani12151900
APA StyleSu, C., Qu, X., Gao, Y., Zhou, X., Yang, X., & Zheng, N. (2022). Effects of Heavy Metal Exposure from Leather Processing Plants on Serum Oxidative Stress and the Milk Fatty Acid Composition of Dairy Cows: A Preliminary Study. Animals, 12(15), 1900. https://doi.org/10.3390/ani12151900






