Spatiotemporal Characteristics of Fish Larvae and Juveniles in the Waters around Taiwan from 2007 to 2019
Abstract
:Simple Summary
Abstract
1. Introduction
- To identify the occurrence probability of primary fish larvae and juveniles in different regions and seasons in the waters around Taiwan.
- To identify the main population structure divisions of important fish larvae and juveniles in Taiwan’s waters.
- To explore the temporal and spatial composition of fish larvae and juveniles in the pelagic, mesopelagic, and demersal zones.
2. Materials and Methods
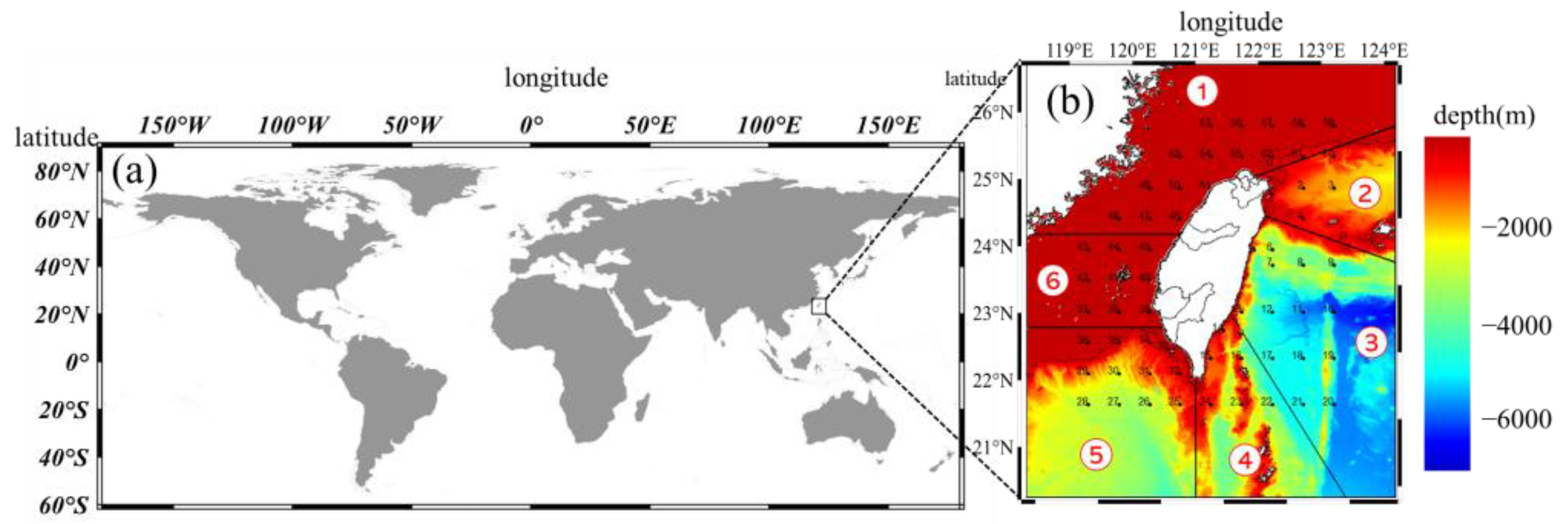
2.1. Data Collection
2.2. Classification and Identification
2.3. Analysis of the Occurrence Probability of Fish Larvae and Juveniles at the Species Level
2.4. Cluster Analysis
2.5. Analysis of Temporal and Spatial Compositional Changes in Fish Larvae and Juveniles in Different Habitat Depth Zones
3. Results
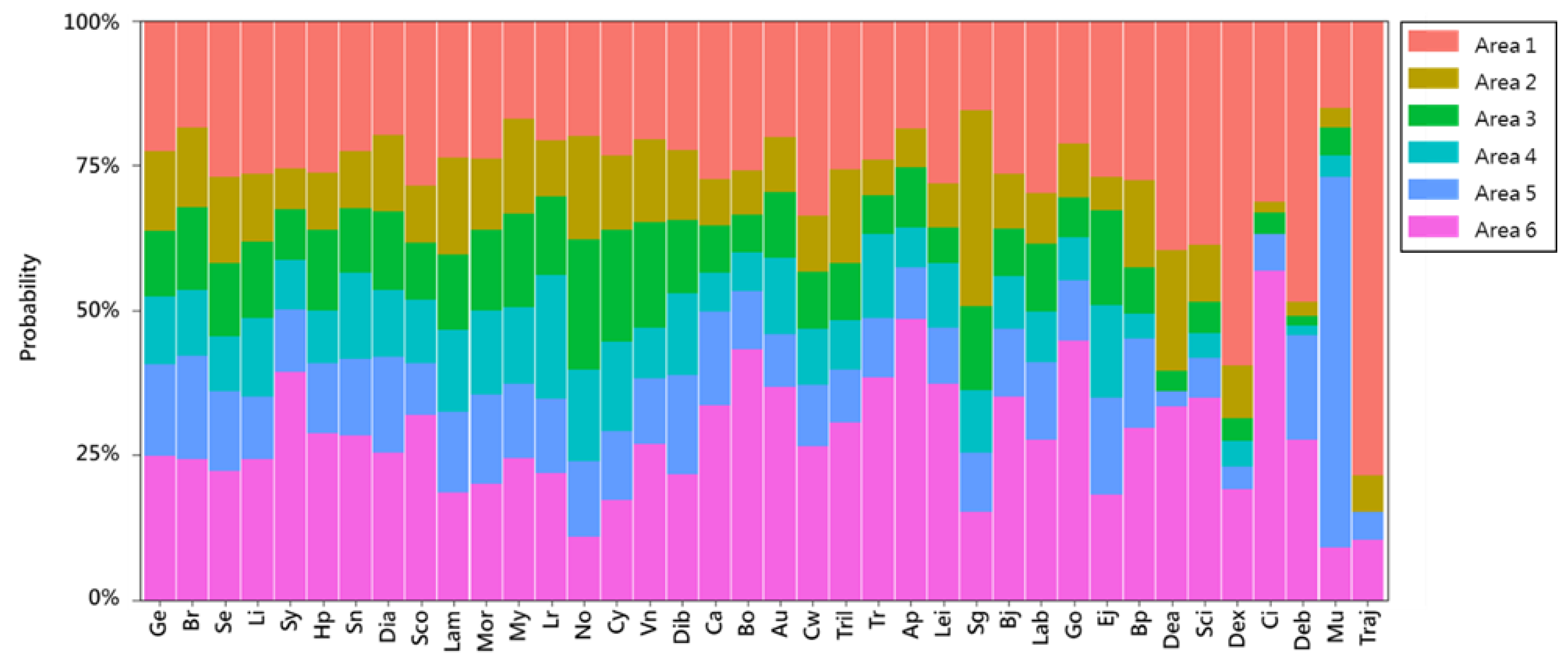
3.1. Cluster Analysis of Fish Larvae and Juveniles
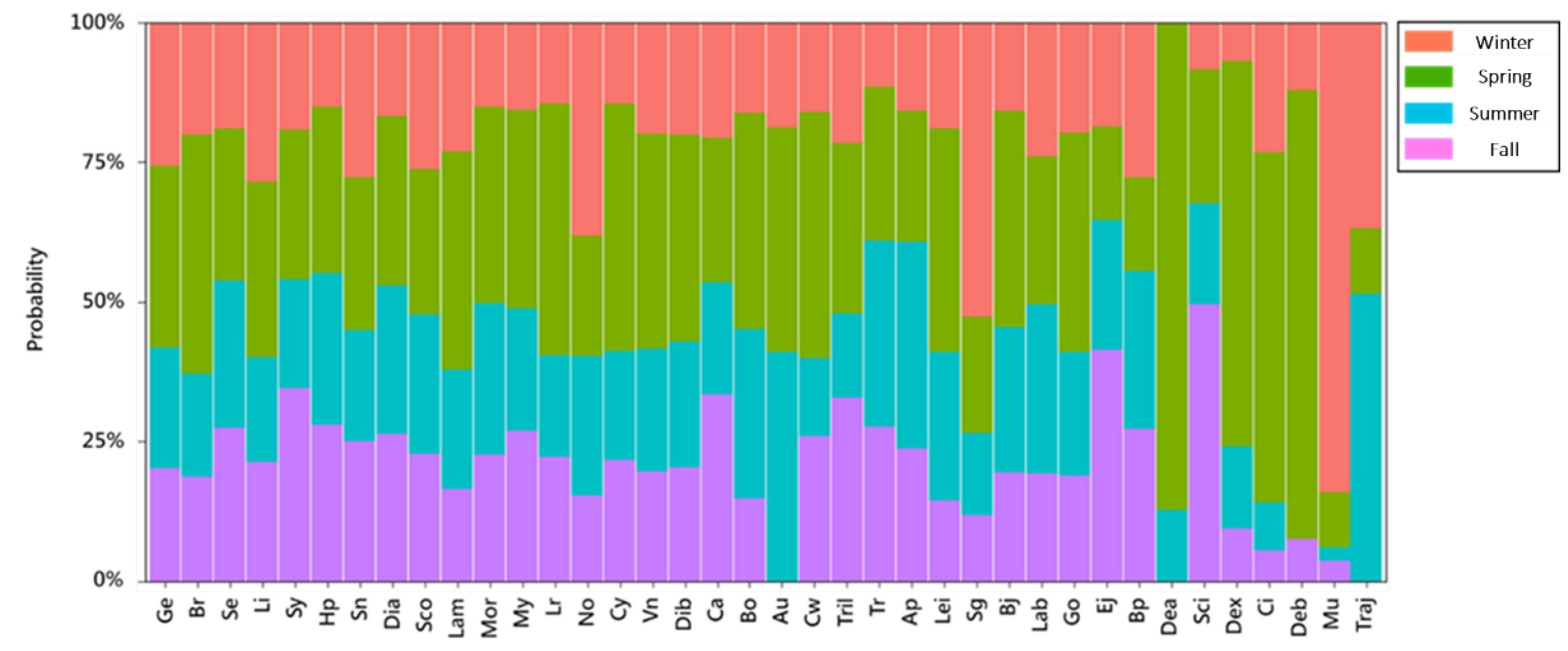
3.2. Temporal and Spatial Variation in Fish Larval and Juvenile Abundances in Different Depth Zones
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Watson, S.A.; Allan, B.J.M.; McQueen, D.E.; Nicol, S.; Parsons, D.M.; Pether, S.M.J.; Pope, S.; Setiawan, A.N.; Smith, N.; Wilson, C.; et al. Ocean warming has a greater effect than acidification on the early life history development and swimming performance of a large circumglobal pelagic fish. Glob. Change Biol. 2018, 24, 4368–4385. [Google Scholar] [CrossRef] [PubMed]
- Houde, E.D. Fish Larvae. In Encyclopedia of Ocean Sciences, 2nd ed.; Steele, J.H., Ed.; Academic Press: Oxford, UK, 2001; pp. 381–391. [Google Scholar]
- Rogers, L.A.; Dougherty, A.B. Effects of climate and demography on reproductive phenology of a harvested marine fish population. Glob. Change Biol. 2019, 25, 708–720. [Google Scholar] [CrossRef] [PubMed]
- Lo, W.T.; Yu, S.F.; Hsieh, H.Y. Hydrographic Processes Driven by Seasonal Monsoon System Affect Siphonophore Assemblages in Tropical-Subtropical Waters (Western North Pacific Ocean). PLoS ONE 2014, 9, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsieh, H.Y.; Yu, S.F.; Lo, W.T. Influence of monsoon-driven hydrographic features on siphonophore assemblages in the Taiwan Strait, western North Pacific Ocean. Mar. Freshw. Res. 2013, 64, 348–358. [Google Scholar] [CrossRef]
- Leis, J.M. Are larvae of demersal fishes plankton or nekton? In Advances in Marine Biology; Southward, A.J., Sims, D.W., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; Volume 51, pp. 57–141. [Google Scholar]
- Sassa, C.; Konishi, Y.; Mori, K. Distribution of jack mackerel (Trachurus japonicus) larvae and juveniles in the East China Sea, with special reference to the larval transport by the Kuroshio Current. Fish. Oceanogr. 2006, 15, 508–518. [Google Scholar] [CrossRef]
- Sassa, C.; Tsukamoto, Y.; Nishiuchi, K.; Konishi, Y. Spawning ground and larval transport processes of jack mackerel Trachurus japonicus in the shelf-break region of the southern East China Sea. Cont. Shelf Res. 2008, 28, 2574–2583. [Google Scholar] [CrossRef]
- Hsieh, H.Y.; Lo, W.T.; Wu, L.J.; Liu, D.C. Larval fish assemblages in the Taiwan Strait, western North Pacific: Linking with monsoon-driven mesoscale current system. Fish. Oceanogr. 2012, 21, 125–147. [Google Scholar] [CrossRef]
- Hsieh, H.Y.; Lo, W.T.; Wu, L.J.; Liu, D.C.; Su, W.C. Monsoon-driven succession of the larval fish assemblage in the East China Sea shelf waters off northern Taiwan. J. Oceanogr. 2011, 67, 159–172. [Google Scholar] [CrossRef]
- Hsieh, H.-Y.; Lo, W.-T.; Wu, L.-J. Community structure of larval fishes from the southeastern Taiwan Strait: Linked to seasonal monsoon-driven currents. Zool. Stud. 2012, 51, 679–691. [Google Scholar]
- Hsieh, F.-J.; Lee, M.-A.; Pan, J.-Y.; Wang, Y.-T.; Lan, K.-W.; Lan, Y.-C. Winter distribution and composition of ichthyoplankton associated with thermal front on the continental shelf of the southern East China Sea. J. Fish. Soc. Taiwan 2007, 34, 69–85. [Google Scholar] [CrossRef]
- Chen, Y.K.; Chen, W.Y.; Wang, Y.C.; Lee, M.A. Winter assemblages of ichthyoplankton in the waters of the East China Sea Shelf and surrounding Taiwan. Fish. Sci. 2016, 82, 755–769. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Chan, J.-W.; Lan, Y.-C.; Yang, W.-C.; Lee, M.-A. Satellite observation of the winter variation of sea surface temperature fronts in relation to the spatial distribution of ichthyoplankton in the continental shelf of the southern East China Sea. Int. J. Remote Sens. 2018, 39, 4550–4564. [Google Scholar] [CrossRef]
- Ohshimo, S.; Sato, T.; Okochi, Y.; Tanaka, S.; Ishihara, T.; Ashida, H.; Suzuki, N. Evidence of spawning among Pacific bluefin tuna, Thunnus orientalis, in the Kuroshio and Kuroshio–Oyashio transition area. Aquat. Living Resour. 2018, 31, 33. [Google Scholar] [CrossRef]
- Tittensor, D.P.; Mora, C.; Jetz, W.; Lotze, H.K.; Ricard, D.; Berghe, E.V.; Worm, B. Global patterns and predictors of marine biodiversity across taxa. Nature 2010, 466, 1098–1101. [Google Scholar] [CrossRef]
- Nielsen, J.M.; Rogers, L.A.; Brodeur, R.D.; Thompson, A.R.; Auth, T.D.; Deary, A.L.; Duffy-Anderson, J.T.; Galbraith, M.; Koslow, J.A.; Perry, R.I. Responses of ichthyoplankton assemblages to the recent marine heatwave and previous climate fluctuations in several Northeast Pacific marine ecosystems. Glob. Change Biol. 2021, 27, 506–520. [Google Scholar] [CrossRef]
- Stiasny, M.H.; Sswat, M.; Mittermayer, F.H.; Falk-Petersen, I.B.; Schnell, N.K.; Puvanendran, V.; Mortensen, A.; Reusch, T.B.H.; Clemmesen, C. Divergent responses of Atlantic cod to ocean acidification and food limitation. Glob. Change Biol. 2019, 25, 839–849. [Google Scholar] [CrossRef] [Green Version]
- Beaugrand, G.; Brander, K.M.; Alistair Lindley, J.; Souissi, S.; Reid, P.C. Plankton effect on cod recruitment in the North Sea. Nature 2003, 426, 661–664. [Google Scholar] [CrossRef]
- Lo-Yat, A.; Simpson, S.D.; Meekan, M.; Lecchini, D.D.; Martinez, E.; Galzin, R. Extreme climatic events reduce ocean productivity and larval supply in a tropical reef ecosystem. Glob. Change Biol. 2011, 17, 1695–1702. [Google Scholar] [CrossRef]
- Pepin, P.; Robert, D.; Bouchard, C.; Dower, J.F.; Falardeau, M.; Fortier, L.; Jenkins, G.P.; Leclerc, V.; Levesque, K.; Llopiz, J.K. Once upon a larva: Revisiting the relationship between feeding success and growth in fish larvae. ICES J. Mar. Sci. 2014, 72, 359–373. [Google Scholar] [CrossRef]
- Morrongiello, J.R.; Walsh, C.T.; Gray, C.A.; Stocks, J.R.; Crook, D.A. Environmental change drives long-term recruitment and growth variation in an estuarine fish. Glob. Change Biol. 2014, 20, 1844–1860. [Google Scholar] [CrossRef]
- Takasuka, A.; Kuroda, H.; Okunishi, T.; Shimizu, Y.; Hirota, Y.; Kubota, H.; Sakaji, H.; Kimura, R.; Ito, S.I.; Oozeki, Y. Occurrence and density of P acific saury C ololabis saira larvae and juveniles in relation to environmental factors during the winter spawning season in the K uroshio C urrent system. Fish. Oceanogr. 2014, 23, 304–321. [Google Scholar] [CrossRef]
- Sogawa, S.; Hidaka, K.; Kamimura, Y.; Takahashi, M.; Saito, H.; Okazaki, Y.; Shimizu, Y.; Setou, T. Environmental characteristics of spawning and nursery grounds of Japanese sardine and mackerels in the Kuroshio and Kuroshio Extension area. Fish. Oceanogr. 2019, 28, 454–467. [Google Scholar] [CrossRef]
- Sassa, C.; Konishi, Y. Late winter larval fish assemblage in the southern East China Sea, with emphasis on spatial relations between mesopelagic and commercial pelagic fish larvae. Cont. Shelf Res. 2015, 108, 97–111. [Google Scholar] [CrossRef]
- Sassa, C.; Takahashi, M. Comparative larval growth and mortality of mesopelagic fishes and their predatory impact on zooplankton in the Kuroshio region. Deep Sea Res. Part I Oceanogr. Res. Pap. 2018, 131, 121–132. [Google Scholar] [CrossRef]
- Akimoto, S.; Kinoshita, S.; Sezaki, K.; Mitani, I.; Watabe, S. Identification of alfonsino and related fish species belonging to the genus Beryx with mitochondrial 16S rRNA gene and its application on their pelagic eggs. Fish. Sci. 2002, 68, 1242–1249. [Google Scholar] [CrossRef]
- Sakamoto, T.T.; Hasumi, H.; Ishii, M.; Emori, S.; Suzuki, T.; Nishimura, T.; Sumi, A. Responses of the Kuroshio and the Kuroshio Extension to global warming in a high-resolution climate model. Geophys. Res. Lett. 2005, 32, 1–4. [Google Scholar] [CrossRef]
- Chen, C.; Wang, G.; Xie, S.-P.; Liu, W. Why Does Global Warming Weaken the Gulf Stream but Intensify the Kuroshio? J. Clim. 2019, 32, 7437–7451. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, X.; He, Y.; Jiang, Y.; Liu, Y.; Xie, Z.; Sun, L.; Liu, Z. Persistent intensification of the Kuroshio Current during late Holocene cool intervals. Earth Planet. Sci. Lett. 2019, 506, 15–22. [Google Scholar] [CrossRef]
- Hu, D.; Wu, L.; Cai, W.; Sen Gupta, A.; Ganachaud, A.; Qiu, B.; Gordon, A.L.; Lin, X.; Chen, Z.; Hu, S.; et al. Pacific western boundary currents and their roles in climate. Nature 2015, 522, 299–308. [Google Scholar] [CrossRef]
- Han, G.; Huang, W. Pacific Decadal Oscillation and Sea Level Variability in the Bohai, Yellow, and East China Seas. J. Phys. Oceanogr. 2008, 38, 2772–2783. [Google Scholar] [CrossRef]
- Andres, M.; Park, J.-H.; Wimbush, M.; Zhu, X.-H.; Nakamura, H.; Kim, K.; Chang, K.-I. Manifestation of the Pacific Decadal Oscillation in the Kuroshio. Geophys. Res. Lett. 2009, 36, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.-R. Interannual modulation of the Pacific Decadal Oscillation (PDO) on the low-latitude western North Pacific. Prog. Oceanogr. 2013, 110, 49–58. [Google Scholar] [CrossRef]
- Wu, C.-R.; Wang, Y.-L.; Lin, Y.-F.; Chao, S.-Y. Intrusion of the Kuroshio into the South and East China Seas. Sci. Rep. 2017, 7, 7895. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Cai, W.; Zhang, L.; Nakamura, H.; Timmermann, A.; Joyce, T.; McPhaden, M.J.; Alexander, M.; Qiu, B.; Visbeck, M. Enhanced warming over the global subtropical western boundary currents. Nat. Clim. Change 2012, 2, 161. [Google Scholar] [CrossRef]
- Miyamoto, H.; Itoh, H.; Okazaki, Y. Temporal and spatial changes in the copepod community during the 1974–1998 spring seasons in the Kuroshio region; a time period of profound changes in pelagic fish populations. Deep Sea Res. Part I Oceanogr. Res. Pap. 2017, 128, 131–140. [Google Scholar] [CrossRef]
- Chiba, S.; Sugisaki, H.; Nonaka, M.; Saino, T. Geographical shift of zooplankton communities and decadal dynamics of the Kuroshio-Oyashio currents in the western North Pacific. Glob. Change Biol. 2009, 15, 1846–1858. [Google Scholar] [CrossRef]
- Sherman, K.; Smith, W.; Morse, W.; Berman, M.; Green, J.; Ejsymont, L. Spawning strategies of fishes in relation to circulation, phytoplankton production, and pulses in zooplankton off the northeastern United States. Mar. Ecol. Prog. Ser. 1984, 18, 1–19. [Google Scholar] [CrossRef]
- Doyle, M.J.; Morse, W.W.; Kendall, A.W., Jr. A comparison of larval fish assemblages in the temperate zone of the northeast Pacific and northwest Atlantic Oceans. Bull. Mar. Sci. 1993, 53, 588–644. [Google Scholar]
- Shannon, L.J.; Nelson, G.; Crawford, R.J.M.; Boyd, A.J. Possible impacts of environmental change on pelagic fish recruitment: Modelling anchovy transport by advective processes in the southern Benguela. Glob. Change Biol. 1996, 2, 407–420. [Google Scholar] [CrossRef]
- Sabatés, A.; Olivar, M.P.; Salat, J.; Palomera, I.; Alemany, F. Physical and biological processes controlling the distribution of fish larvae in the NW Mediterranean. Prog. Oceanogr. 2007, 74, 355–376. [Google Scholar] [CrossRef] [Green Version]
- Ciszewski, K.; Wawrzyniak, W.; Czerniejewski, P. Effect of Artificial Regime Shifts and Biotic Factors on the Intensity of Foraging of Planktivorous Fish. Animals 2022, 12, 17. [Google Scholar] [CrossRef]
- Cuttitta, A.; Quinci, E.M.; Patti, B.; Bonomo, S.; Bonanno, A.; Musco, M.; Torri, M.; Placenti, F.; Basilone, G.; Genovese, S.; et al. Different key roles of mesoscale oceanographic structures and ocean bathymetry in shaping larval fish distribution pattern: A case study in Sicilian waters in summer 2009. J. Sea Res. 2016, 115, 6–17. [Google Scholar] [CrossRef]
- Tiedemann, M.; Fock, H.O.; Döring, J.; Badji, L.B.; Möllmann, C. Water masses and oceanic eddy regulation of larval fish assemblages along the Cape Verde Frontal Zone. J. Mar. Syst. 2018, 183, 42–55. [Google Scholar] [CrossRef]
- Auth, T.D.; Daly, E.A.; Brodeur, R.D.; Fisher, J.L. Phenological and distributional shifts in ichthyoplankton associated with recent warming in the northeast Pacific Ocean. Glob. Change Biol. 2018, 24, 259–272. [Google Scholar] [CrossRef]
- Aoki, I.; Miyashita, K. Dispersal of larvae and juveniles of Japanese anchovy Engraulis japonicus in the Kuroshio Extension and Kuroshio–Oyashio transition regions, western North Pacific Ocean. Fish. Res. 2000, 49, 155–164. [Google Scholar] [CrossRef]
- Mododa, S. Devices of simple plankton apparatus. Mem. Fac. Fish. Hokkaido Univ. 1959, 14, 152–162. [Google Scholar]
- Okiyama, M. An Atlas of the Early Stage Fishes in Japan; Tokai University Press: Tokyo, Japan, 1988. [Google Scholar]
- Chiu, T.-S. The Larvae of Fishes in Taiwan; National Museum of Marine Biology and Aquarium: Pingtung, Taiwan, 1999; p. 296. [Google Scholar]
- Leis, J.M.; Rennis, D.S. The Larvae of Indo-Pacific Coral Reef Fishes; New South Wales University Press: Sydney, Australia, 1983. [Google Scholar]
- Kendall, A., Jr. Early life history stages of fishes and their characters. In Ontogeny and Systematics of Fishes; American Society of Ichthyologists and Herpetologists: Lawrence, KS, USA, 1984. [Google Scholar]
- Shih, N.-T.; Cai, Y.-H.; Ni, I.-H. A concept to protect fisheries recruits by seasonal closure during spawning periods for commercial fishes off Taiwan and the East China Sea. J. Appl. Ichthyol. 2009, 25, 676–685. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.R-project.org/ (accessed on 16 May 2022).
- MacQueen, J. Some methods for classification and analysis of multivariate observations. In Proceedings of the Fifth Berkeley Symposium on Mathematical Statistics and Probability; The Regents of the University of California: Oakland, CA, USA, 1967; pp. 281–297. [Google Scholar]
- Joshi, K.D.; Nalwade, P. Modified K-Means for better initial cluster centres. Int. J. Comput. Sci. Mob. Comput. 2013, 2, 219–223. [Google Scholar]
- Kaufma, L.; Rousseeuw, P. Finding Groups in Data: An Introduction to Cluster Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Shao, K.T. Taiwan Fish Database. Available online: http://fishdb.sinica.edu.tw (accessed on 16 May 2022).
- Wang, Y.-C.; Chen, W.-Y.; Chen, Y.-K.; Kuo, Y.-C.; Lee, M.-A. Winter abundance and species composition of anchovy larvae associated with hydrological conditions in the coastal waters of Tanshui, Taiwan. J. Mar. Sci. Technol. 2018, 26, 18. [Google Scholar] [CrossRef]
- Harrison, P.A.; Berry, P.M.; Simpson, G.; Haslett, J.R.; Blicharska, M.; Bucur, M.; Dunford, R.; Egoh, B.; Garcia-Llorente, M.; Geamana, N.; et al. Linkages between biodiversity attributes and ecosystem services: A systematic review. Ecosyst. Serv. 2014, 9, 191–203. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, H.-Y.; Lo, W.-T.; Wu, L.-J.; Liu, D.-C.; Su, W.-C. Comparison of distribution patterns of larval fish assemblages in the Taiwan Strait between the northeasterly and southwesterly monsoons. Zool. Stud. 2011, 50, 491–505. [Google Scholar]
- Chien, F.C.; Kuo, H.C. On the extreme rainfall of Typhoon Morakot. J. Geophys. Res. Atmos. 2011, 116. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.S.; Yamashita, T.; Hsu, J.R.-C.; Ding, F. Integrated modeling of the dynamic meteorological and sea surface conditions during the passage of Typhoon Morakot. Dyn. Atmos. Ocean. 2013, 59, 1–23. [Google Scholar] [CrossRef]
- Chang, Y.; Chan, J.-W.; Huang, Y.-C.A.; Lin, W.-Q.; Lee, M.-A.; Lee, K.-T.; Liao, C.-H.; Wang, K.-Y.; Kuo, Y.-C. Typhoon-enhanced upwelling and its influence on fishing activities in the southern East China Sea. Int. J. Remote Sens. 2014, 35, 6561–6572. [Google Scholar] [CrossRef]
- Wu, C.-H.; Chen, S.-C.; Feng, Z.-Y. Formation, failure, and consequences of the Xiaolin landslide dam, triggered by extreme rainfall from Typhoon Morakot, Taiwan. Landslides 2014, 11, 357–367. [Google Scholar] [CrossRef] [Green Version]
- Kuo, C.-Y.; Meng, P.-J.; Ho, P.-H.; Wang, J.-T.; Chen, J.-P.; Chiu, Y.-W.; Lin, H.-J.; Chang, Y.-C.; Fan, T.-Y.; Chen, C.A. Damage to the reefs of Siangjiao Bay marine protected area of Kenting National Park, southern Taiwan during typhoon Morakot. Zool. Stud. 2011, 50, 85. [Google Scholar]
- Lawson, K.M.; Hill, J.E. Life history strategies differentiate established from failed non-native freshwater fish in peninsular Florida. Divers. Distrib. 2022, 28, 160–172. [Google Scholar] [CrossRef]
- McLean, M.; Mouillot, D.; Auber, A. Ecological and life history traits explain a climate-induced shift in a temperate marine fish community. Mar. Ecol. Prog. Ser. 2018, 606, 175–186. [Google Scholar] [CrossRef] [Green Version]
- Schaal, S.M.; Lotterhos, K.E. Comparative thermal performance among four young-of-the-year temperate reef fish species. ICES J. Mar. Sci. 2021, 78, 1684–1696. [Google Scholar] [CrossRef]
- Wang, X.X.; Lang, X.M.; Jiang, D.B. Linkage of future regional climate extremes to global warming intensity. Clim. Res. 2020, 81, 43–54. [Google Scholar] [CrossRef]
- Levin, L.A.; Wei, C.L.; Dunn, D.C.; Amon, D.J.; Ashford, O.S.; Cheung, W.W.L.; Colaco, A.; Dominguez-Carrio, C.; Escobar, E.G.; Harden-Davies, H.R.; et al. Climate change considerations are fundamental to management of deep-sea resource extraction. Glob. Change Biol. 2020, 26, 4664–4678. [Google Scholar] [CrossRef] [PubMed]

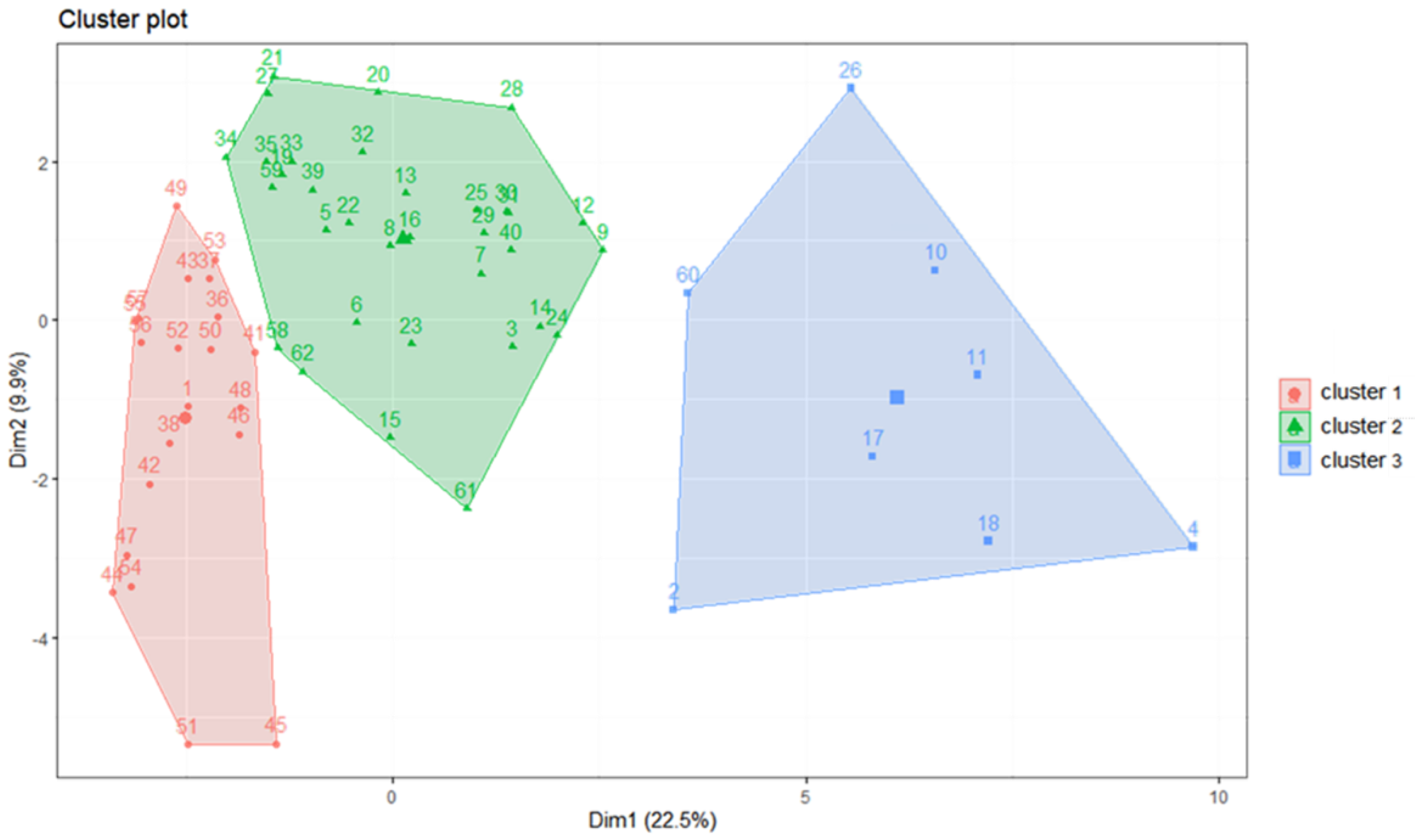
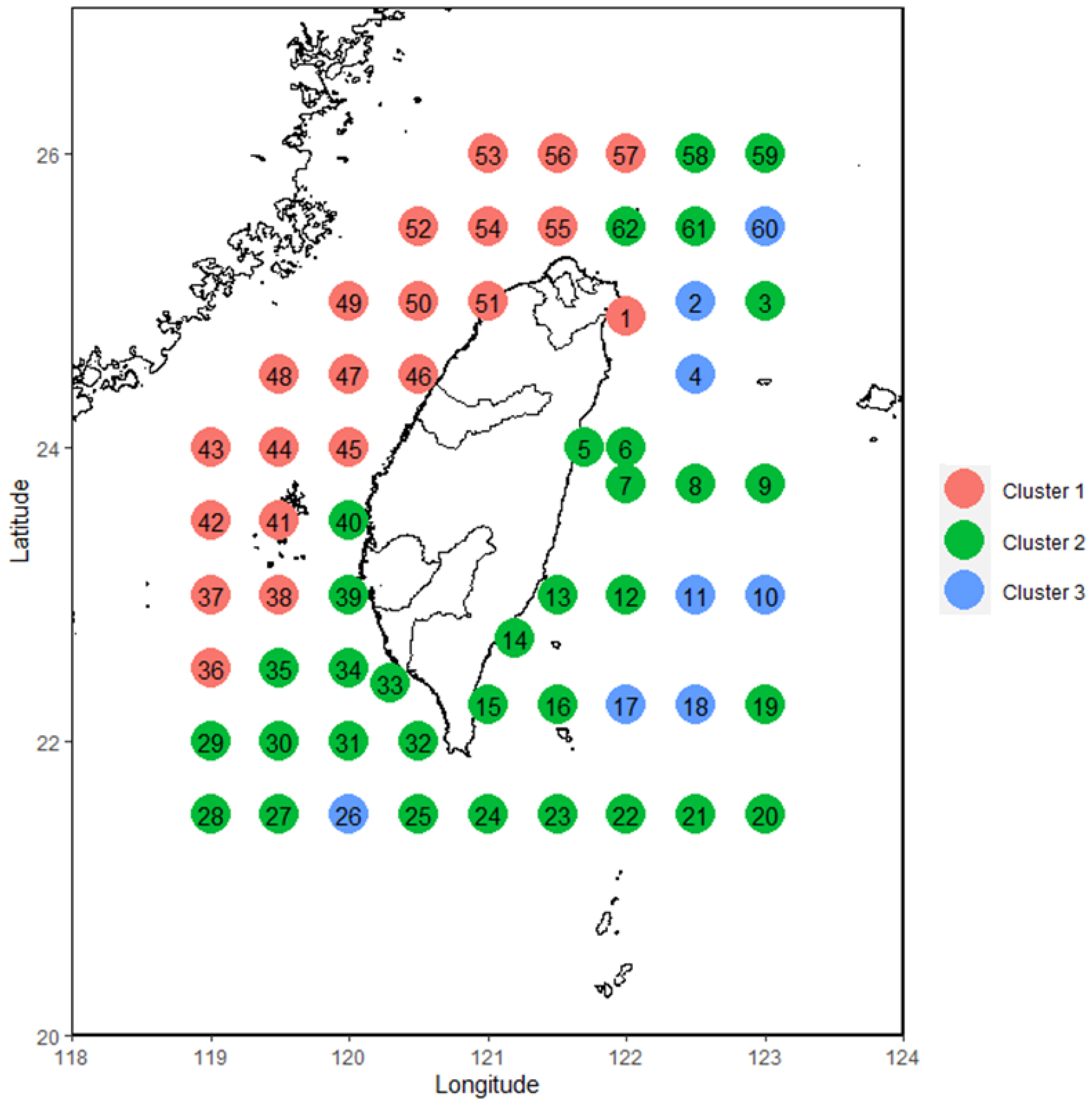
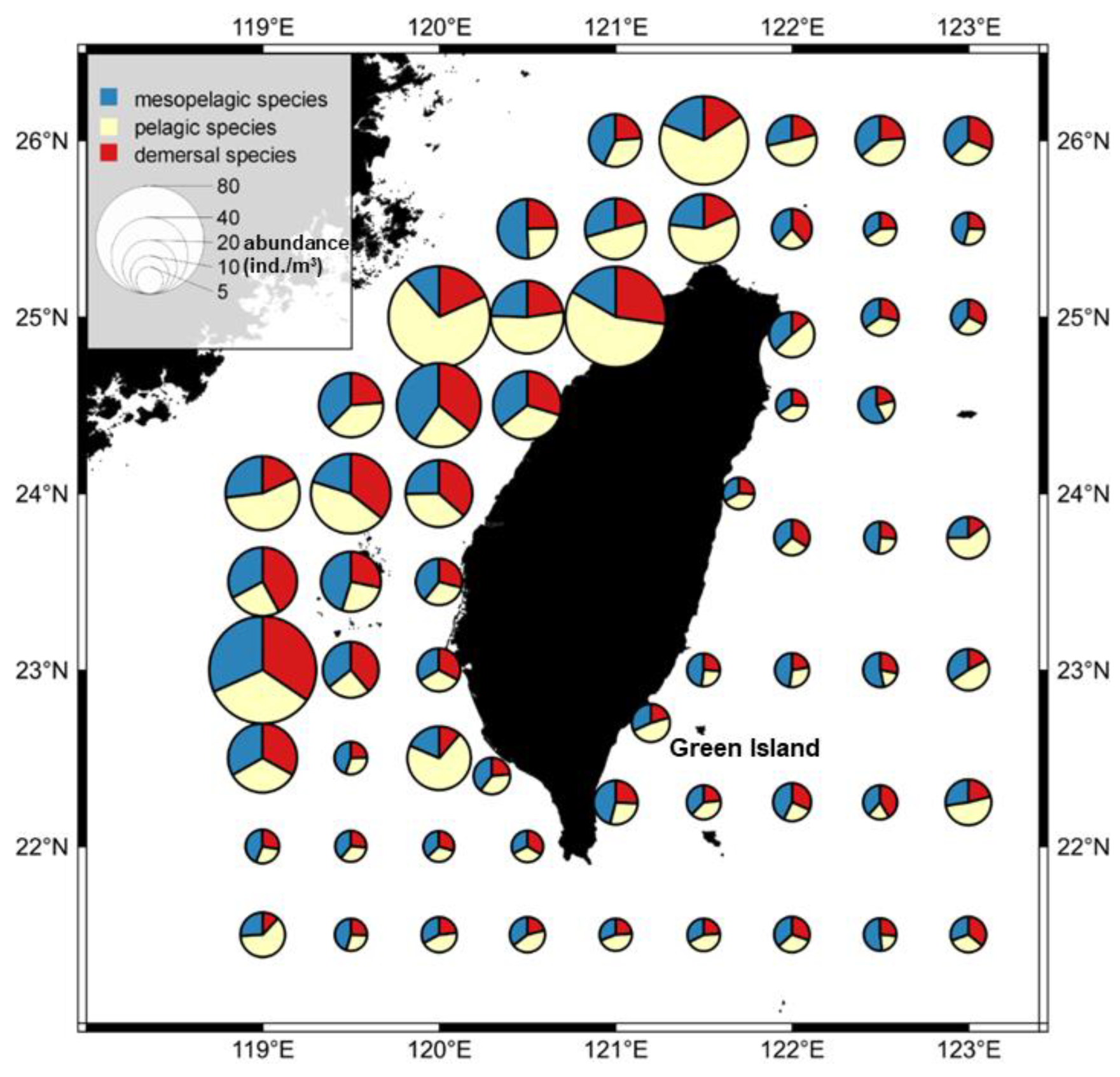
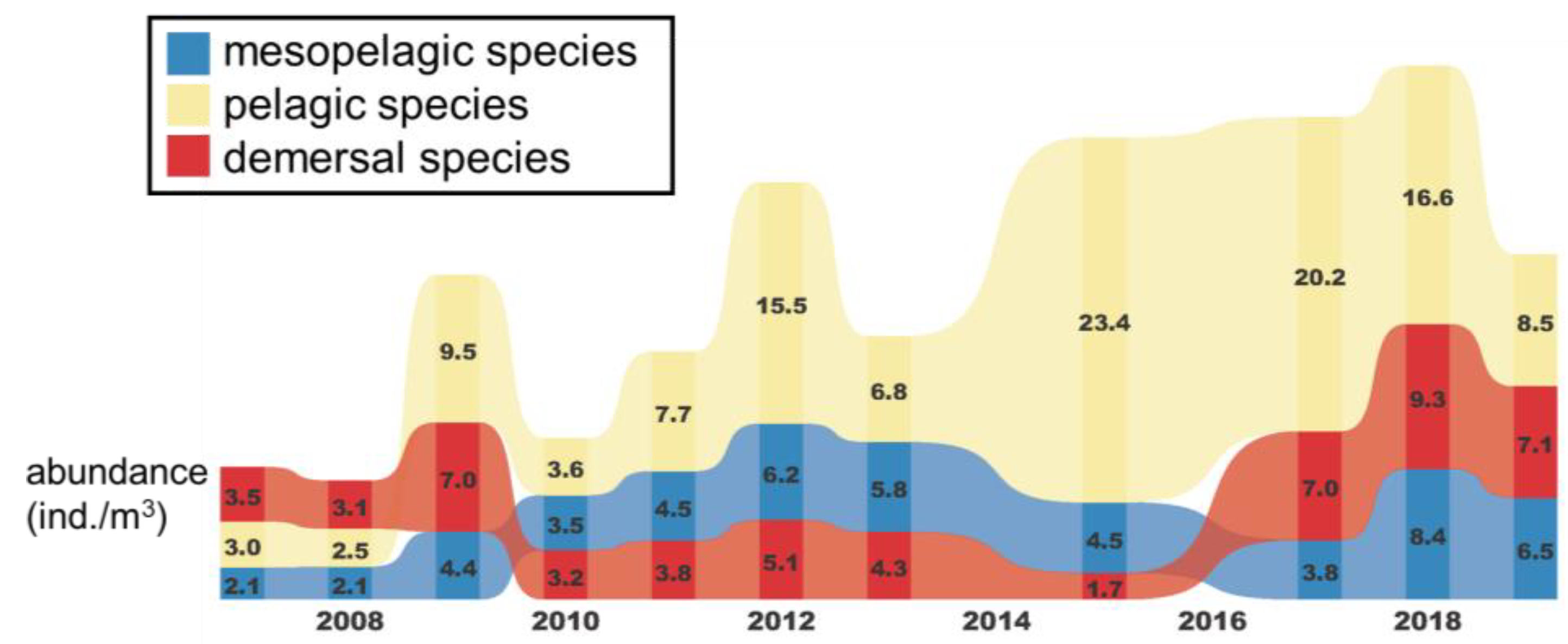
| No | Family | Species | Code | Depth Zone |
|---|---|---|---|---|
| 1 | Carangidae | Trachurus japonicus | Traj | pelagic |
| 2 | Engraulidae | Engraulis japonicus | Ej | pelagic |
| 3 | Trichiuridae | Trichiurus spp. | Tril | pelagic |
| 4 | Acropomatidae | Synagrops spp. | Sy | pelagic |
| 5 | Scombridae | Auxis spp. | Au | pelagic |
| 6 | Carangidae | Decapterus macrosoma | Dea | pelagic |
| 7 | Bramidae | spp. | Br | pelagic |
| 8 | Gempylidae | spp. | Ge | pelagic |
| 9 | Carangidae | Decapterus spp. | Dex | pelagic |
| 10 | Carangidae | Decapterus maruadsi | Deb | pelagic |
| 11 | Mugilidae | spp. | Mu | pelagic |
| 12 | Clupeidae | spp. | Cl | pelagic |
| 13 | Myctophidae | Benthosema pterotum | Bp | mesopelagic |
| 14 | Myctophidae | Ceratoscopelus warmingi | Cw | mesopelagic |
| 15 | Gonostomatidae | Cyclothone alba | Cy | mesopelagic |
| 16 | Paralepididae | Lestrolepis spp. | Lr | mesopelagic |
| 17 | Gonostomatidae | Vinciguerria nimbaria | Vn | mesopelagic |
| 18 | Myctophidae | Myctophum orientale | Mor | mesopelagic |
| 19 | Myctophidae | Hygophum proximum | Hp | mesopelagic |
| 20 | Stomiidae | Stomias nebulosus | Sn | mesopelagic |
| 21 | Myctophidae | Lampanyctus spp. | Lam | mesopelagic |
| 22 | Bregmacerotidae | Bregmaceros spp. | Bj | mesopelagic |
| 23 | Myctophidae | spp. | My | mesopelagic |
| 24 | Myctophidae | Diaphus A group | Dia | mesopelagic |
| 25 | Myctophidae | Diaphus B group | Dib | mesopelagic |
| 26 | Serranidae | spp. | Se | mesopelagic |
| 27 | Gonostomatidae | Sigmops gracilis | Sg | mesopelagic |
| 28 | Synodontidae | Trachinocephalus myops | Tr | demersal |
| 29 | Notosudidae | spp. | No | demersal |
| 30 | Apogonidae | spp. | Ap | demersal |
| 31 | Labridae | spp. | Lab | demersal |
| 32 | Callionymidae | spp. | Ca | demersal |
| 33 | Paralepididae | Lestidium | Li | demersal |
| 34 | Bothidae | spp. | Bo | demersal |
| 35 | Scorpaenidae | spp. | Sco | demersal |
| 36 | Leiognathidae | spp. | Lei | demersal |
| 37 | Gobiidae | spp. | Go | demersal |
| 38 | Sciaenidae | spp. | Sci | demersal |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yen, K.-W.; Pan, C.-I.; Chen, C.-H.; Lien, W.-H. Spatiotemporal Characteristics of Fish Larvae and Juveniles in the Waters around Taiwan from 2007 to 2019. Animals 2022, 12, 1890. https://doi.org/10.3390/ani12151890
Yen K-W, Pan C-I, Chen C-H, Lien W-H. Spatiotemporal Characteristics of Fish Larvae and Juveniles in the Waters around Taiwan from 2007 to 2019. Animals. 2022; 12(15):1890. https://doi.org/10.3390/ani12151890
Chicago/Turabian StyleYen, Kuo-Wei, Chia-I Pan, Chia-Hsiang Chen, and Wei-Hsiang Lien. 2022. "Spatiotemporal Characteristics of Fish Larvae and Juveniles in the Waters around Taiwan from 2007 to 2019" Animals 12, no. 15: 1890. https://doi.org/10.3390/ani12151890
APA StyleYen, K.-W., Pan, C.-I., Chen, C.-H., & Lien, W.-H. (2022). Spatiotemporal Characteristics of Fish Larvae and Juveniles in the Waters around Taiwan from 2007 to 2019. Animals, 12(15), 1890. https://doi.org/10.3390/ani12151890






