Effect of Folic Acid Supplements on Progesterone Profile and Blood Metabolites of Heat-Stressed Holstein Cows during the Early Stage of Pregnancy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Management and Experimental Design
2.2. Reproductive Management and Experimental Procedures
2.3. Estimation of Progesterone Profile and Serum Folates
2.4. Estimation of Blood Metabolites and Biochemical Indices
2.5. Metrological Data
2.6. Statistical Analysis
- Yijk: An observation of each trait.
- μ: The overall mean.
- Ti: The fixed effect of treatment ith (1, 2, 3).
- Pj: The fixed effect of parity jth.
- eijk: The random effect of error.
3. Results
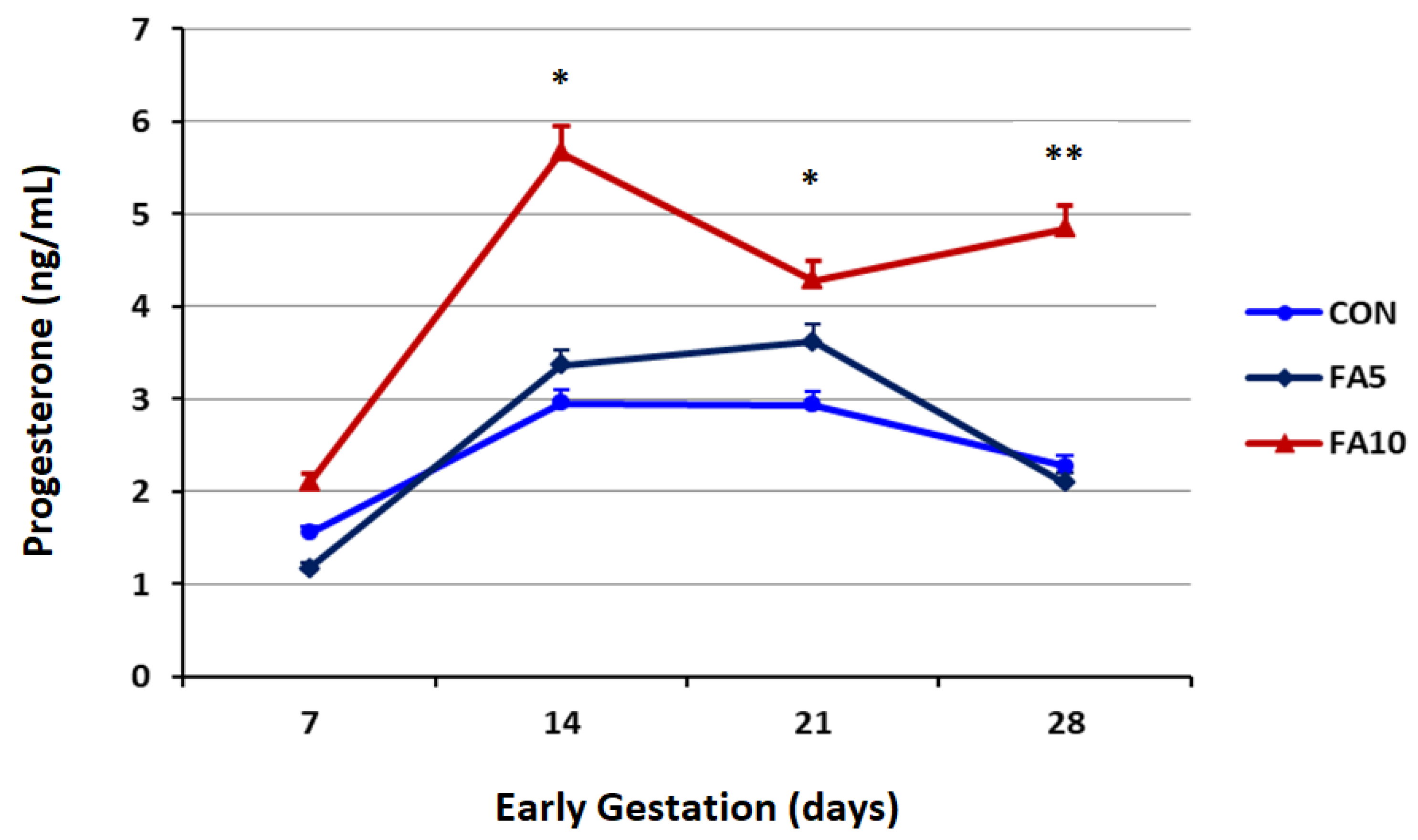
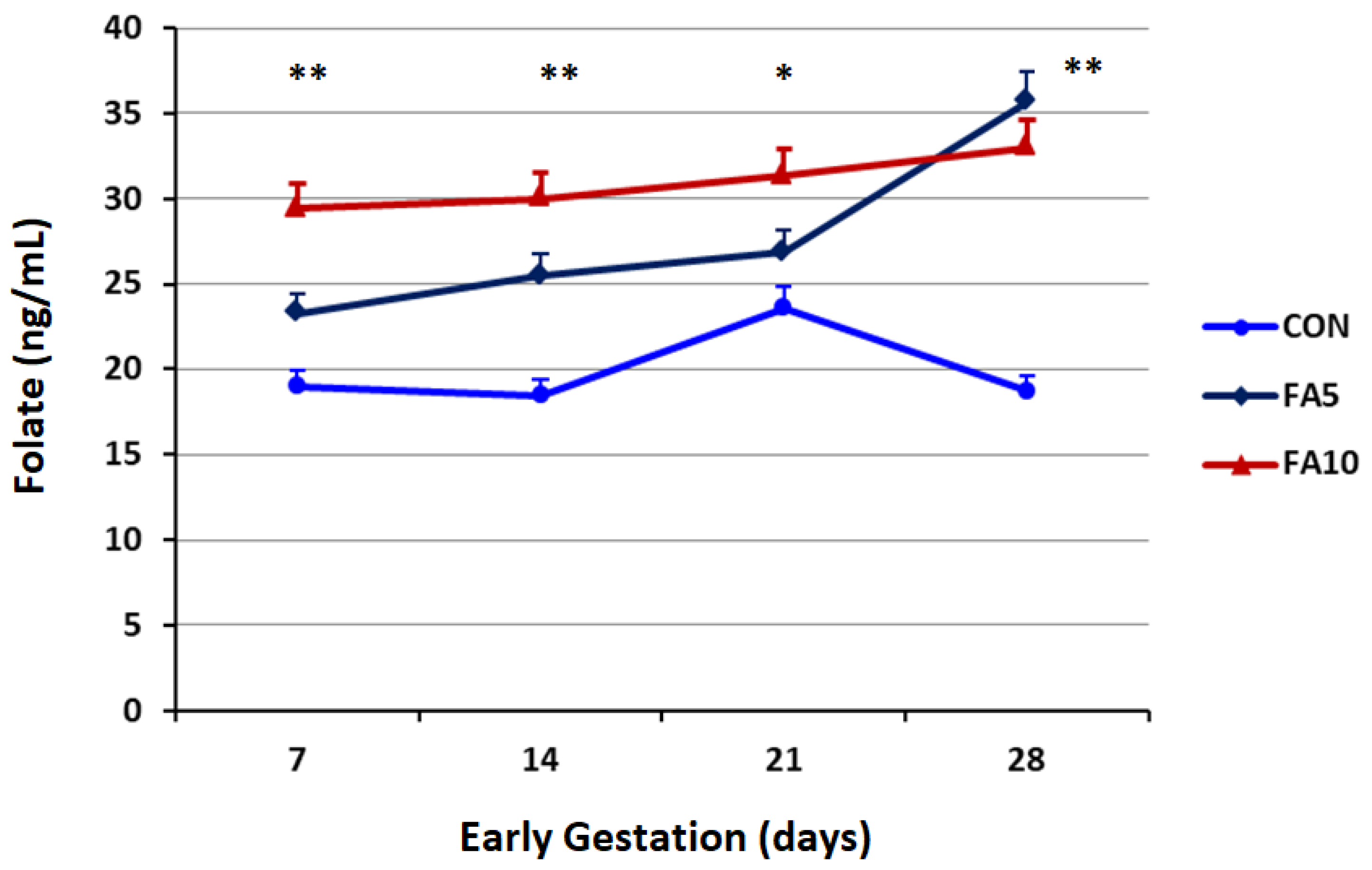
3.1. Progesterone Profile and Serum Folates
3.2. Blood Metabolites and Biochemical Indices
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Darwash, A.O.; Lamming, G.E.; Woolliams, J.A. Estimation of genetic variation in the interval from calving to postpartum ovulation of dairy cows. J. Dairy Sci. 1997, 80, 1227–1234. [Google Scholar] [CrossRef]
- Sturman, H.; Oltenacu, E.A.B.; Foote, R.H. Importance of inseminating only cows in estrus. Theriogenology 2000, 53, 1657–1668. [Google Scholar] [CrossRef]
- El-Tarabany, M.S.; El-Tarabany, A.A. Impact of thermal stress on the efficiency of ovulation synchronization protocols in Holstein cows. Anim. Reprod. Sci. 2015, 160, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Wolfenson, D.; Roth, Z.; Merdan, R. Impaired reproduction in heat-stressed dairy cattle: Basic and applied aspects. Anim. Reprod. Sci. 2000, 60–61, 535–547. [Google Scholar] [CrossRef]
- Jordan, E.R. Effects of heat stress on reproduction. J. Dairy Sci. 2003, 86, E104–E114. [Google Scholar] [CrossRef]
- Hansen, P.J.; Aréchiga, C.F. Strategies for managing reproduction in the heat-stressed dairy cow. J. Anim. Sci. 1999, 77 (Suppl. 2), 36–50. [Google Scholar] [CrossRef]
- Choi, S.-W.; Mason, J.B. Folate and carcinogenesis: An integrated scheme. J. Nutr. 2000, 130, 129–132. [Google Scholar] [CrossRef]
- Pogribny, I.P.; Karpf, A.R.; James, S.R.; Melnyk, S.; Han, T.; Tryndyak, V.P. Epigenetic alterations in the brains of Fisher 344 rats induced by long-term administration of folate/methyl-deficient diet. Brain Res. 2008, 1237, 25–34. [Google Scholar] [CrossRef]
- Khan, M.Z.; Khan, A.; Xiao, J.; Dou, J.; Liu, L.; Yu, Y. Overview of folic acid supplementation alone or in combination with vitamin B12 in dairy cattle during periparturient period. Metabolites 2020, 10, 263. [Google Scholar] [CrossRef]
- Menzies, K.K.; Lefèvre, C.; Sharp, J.A.; Macmillan, K.L.; Sheehy, P.A.; Nicholas, K.R. A novel approach identified the FOLR1 gene, a putative regulator of milk protein synthesis. Mamm. Genome 2009, 20, 498–503. [Google Scholar] [CrossRef]
- Schwab, E.C.; Schwab, C.G.; Shaver, R.D.; Girard, C.L.; Putnam, D.E.; Whitehouse, N.L. Dietary forage and nonfiber carbohydrate contents influence B-vitamin Intake, duodenal flow, and apparent ruminal synthesis in lactating dairy cows. J. Dairy Sci. 2006, 89, 174–187. [Google Scholar] [CrossRef]
- Ghoshal, K.; Li, X.; Datta, J.; Bai, S.; Pogribny, I.; Pogribny, M.; Huang, Y.; Young, D.; Jacob, S.T. A folate and methyl-deficient diet alters the expression of DNA methyltransferases and methyl CpG binding proteins involved in epigenetic gene silencing in livers of F344 rats. J. Nutr. 2006, 136, 1522–1527. [Google Scholar] [CrossRef] [PubMed]
- Santschi, D.E.; Chiquette, J.; Berthiaume, R.; Martineau, R.; Matte, J.J.; Mustafa, A.F.; Girard, C.L. Effects of the forage to concentrate ratio on B-vitamin concentrations in different ruminal fractions of dairy cows. Can. J. Anim. Sci. 2005, 85, 389–399. [Google Scholar] [CrossRef]
- Collier, R.J.; Dahl, G.E.; VanBaale, M.J. Major advances associated with environmental effects on dairy cattle. J. Dairy Sci. 2006, 89, 1244–1253. [Google Scholar] [CrossRef]
- Girard CLMatte, J.J.; Tremblay, G.F. Serum folates in gestating and lactating dairy cows. J. Dairy Sci. 1989, 72, 3240–3246. [Google Scholar] [CrossRef]
- Girard, C.L.; Matte, J.J. Dietary supplements of folic acid during lactation: Effects on the performance of dairy cows. J. Dairy Sci. 1998, 81, 1412–1419. [Google Scholar] [CrossRef]
- Duplessis, M.; Girard, C.L.; Santschi, D.E.; Laforest, J.P.; Durocher, J.; Pellerin, D. Effects of folic acid and vitamin B12 supplementation on culling rate, diseases, and reproduction in commercial dairy herds. J. Dairy Sci. 2014, 97, 2346–2354. [Google Scholar] [CrossRef]
- Czeizel, A.E.; Vargha, P. Periconceptional folic acid/multivitamin supplementation and twin pregnancy. Am. J. Obstet. Gynecol. 2004, 191, 790–794. [Google Scholar] [CrossRef]
- Gagnon, A.; Khan, D.R.; Sirard, M.A.; Girard, C.L.; Laforest, J.P.; Richard, F.J. Effects of intramuscular administration of folic acid and vitamin B12 on granulosa cells gene expression in postpartum dairy cows. J. Dairy Sci. 2015, 98, 7797–7809. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Dairy Cattle, 7th ed.; National Academies Press: Washington, DC, USA, 2001. [Google Scholar]
- Kendall, P.E.; Webster, J.R. Season and physiological status affects the circadian body temperature rhythm of dairy cows. Livest. Sci. 2009, 125, 155–160. [Google Scholar] [CrossRef]
- El-Tarabany, M.S.; Atta, M.A.; Emara, S.S.; Mostafa, M.M. Folic acid and flaxseed oil supplements in Ossimi ewes: Effect on body weight changes, progesterone profile, blood chemistry, and litter traits. Trop. Anim. Health Prod. 2020, 52, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Wolfenson, D.; Sonego, H.; Bloch, A.; Shaham-Albalancy, A.; Kaim, M.; Folman, Y.; Meidan, R. Seasonal differences in progesterone production by luteinized bovine thecal and granulosa cells. Domest. Anim. Endocrinol. 2002, 22, 81–90. [Google Scholar] [CrossRef]
- Wolfenson, D.; Roth, Z. Impact of heat stress on cow reproduction and fertility. Anim. Front. 2019, 9, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Starbuck, M.J.; Dailey, R.A.; Inskeep, E.K. Factors affecting retention of early pregnancy in dairy cattle. Anim. Reprod. Sci. 2004, 84, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Koyama, H.; Ikeda, S.; Sugimoto, M.; Kume, S. Effects of folic acid on the development and oxidative stress of mouse embryos exposed to heat stress. Reprod. Domest. Anim. 2012, 47, 921–927. [Google Scholar] [CrossRef]
- Shin, J.-H.; Shiota, K. Folic acid supplementation of pregnant mice suppresses heat-induced neural tube defects in the offspring. J. Nutr. 1999, 129, 2070–2073. [Google Scholar] [CrossRef][Green Version]
- Mann, G.E.; Lamming, G.E. The influence of progesterone during early pregnancy in cattle. Reprod. Domen. Anim. 1999, 34, 269–274. [Google Scholar] [CrossRef]
- Girard, C.L.; Matte, J.J.; Tremblay, G.F. Gestation and lactation of dairy cows: A role for folic acid? J. Dairy Sci. 1995, 78, 404–411. [Google Scholar] [CrossRef]
- Li, H.Q.; Liu, Q.; Wang, C.; Yang, Z.M.; Guo, G.; Huo, W.J.; Pei, C.X.; Zhang, Y.L.; Zhang, S.L.; Wang, H.; et al. Effects of dietary supplements of rumen-protected folic acid on lactation performance, energy balance, blood parameters and reproductive performance in dairy cows. Anim. Feed Sci. Technol. 2016, 213, 55–63. [Google Scholar] [CrossRef]
- Graulet, B.; Matte, J.J.; Desrochers, A.; Doepel, L.; Palin, M.F.; Girard, C.L. Effects of dietary supplements of folic acid and vitamin B12 on metabolism of dairy cows in early lactation. J. Dairy Sci. 2007, 90, 3442–3455. [Google Scholar] [CrossRef]
- De Koster, J.D.; Opsomer, G. Insulin resistance in dairy cows. Vet. Clin. Food Anim. Pract. 2013, 29, 299–322. [Google Scholar] [CrossRef] [PubMed]
- Wathes, D.C.; Cheng, Z.; Chowdhury, W.; Fenwick, M.A.; Fitzpatrick, R.; Morris, D.G.; Patton, J.; Murphy, J.J. Negative energy balance alters global gene expression and immune responses in the uterus of postpartum dairy cows. Physiol. Genom. 2009, 39, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Herdt, T.H. Variability characteristics and test selection in herd-level nutritional metabolic profle testing. Vet. Clin. N. Am. Food Anim. Pract. 2000, 16, 387–403. [Google Scholar] [CrossRef]
- Li, H.Q.; Wang, B.; Li, Z.; Luo, H.L.; Wang, Y.J.; Zhang, C.; Jian, L.Y.; Gao, Y.F.; Lu, W.; Liu, M.; et al. Effects of rumen-protected folic acid addition in maternal and post-weaning diets on growth performance, total tract digestibility, ruminal fermentation and blood metabolites in lambs. Anim. Feed Sci. Technol. 2020, 260, 114364. [Google Scholar] [CrossRef]
- Duplessis, M.; Lapierre, H.; Pellerin, D.; Laforest, J.P.; Girard, C.L. Effects of intramuscular injections of folic acid, vitamin B12, or both, on lactational performance and energy status of multiparous dairy cows. J. Dairy Sci. 2017, 100, 4051–4064. [Google Scholar] [CrossRef] [PubMed]
- Rastani, R.R.; Lobos, N.E.; Aguerre, M.J.; Grummer, R.R.; Wattiaux, M.A. Relationships between Blood Urea Nitrogen and Energy Balance or Measures of Tissue Mobilization in Holstein Cows During the Periparturient Period. Profess Anim. Sci. 2006, 22, 382–385. [Google Scholar] [CrossRef]
- Xu, C.; Wang, Z.; Liu, G.W.; Li, X.B.; Xie, G.H.; Xia, C.; Zhang, H.Y. Metabolic characteristic of the liver of dairy cows during ketosis based on comparative proteomics. Asian-Aust. J. Anim. Sci. 2008, 21, 1003–1008. [Google Scholar] [CrossRef]
- Blüher, S.; Kratzsch, J.; Kiess, W. Insulin-like growth factor I, growth hormone and insulin in white adipose tissue. Best Pract. Res. Clin. Endocrinol. Metab. 2005, 19, 577–587. [Google Scholar] [CrossRef]
- Zhao, G.Y.; Sun, Y.B. Effects of volatile fatty acids on IGF-I, IGFBP-3, GH, insulin and glucagon in plasma, and IGF-I and IGFBP-3 in different tissues of growing sheep nourished by total intragastric infusions. Asian-Aust. J. Anim. Sci. 2010, 23, 366–371. [Google Scholar] [CrossRef]
- Smith, J.M.; Amburgh, M.E.V.; Diaz, M.C.; Lucy, M.C.; Bauman, D.E. Effect of nutrient intake on the development of the somatotropic axis and its responsiveness to GH in Holstein bull calves. J. Anim. Sci. 2002, 80, 1528–1537. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, Z.; Li, H.; Luo, H.; Blair, H.T.; Jian, L.; Diao, Z. Effect of dietary folic acid supplementation during pregnancy on blood characteristics and milk composition of ewes. Animals 2020, 10, 433. [Google Scholar] [CrossRef] [PubMed]
- Petitclerc, D.; Dumoulin, P.; Ringuet, H.; Matte, J.; Girard, C. Plane of nutrition and folic acid supplementation between birth and four months of age on mammary development of dairy heifers. Can. J. Anim. Sci. 1999, 79, 227–234. [Google Scholar] [CrossRef]
- Haugejorden, G.; Waage, S.; Dahl, E.; Karlbert, K.; Beckers, J.F.; Ropstad, E. Pregnancy associated glycoproteins (PAG) in postpartum cows, ewes, goats and their offspring. Theriogenology 2006, 66, 1976–1984. [Google Scholar] [CrossRef] [PubMed]
- Pohler, K.G.; Pereira, M.H.C.; Lopes, F.R.; Lawrence, J.C.; Keisler, D.H.; Smith, M.F.; Vasconcelos, J.L.M.; Green, J.A. Circulating concentrations of bovine pregnancy-associated glycoproteins and late embryonic mortality in lactating dairy herds. J. Dairy Sci. 2016, 99, 1584–1594. [Google Scholar] [CrossRef] [PubMed]
- Ayad, A.; Sousa, N.M.; Sulon, J.; Hornick, J.L.; Watts, J.; Lopez-Gatius, F.; Iguer-Ouada, M.; Beckers, J.F. Influence of progesterone concentrations on secretory functions of trophoblast and pituitary during the first trimester of pregnancy in dairy cattle. Theriogenology 2007, 67, 1503–1511. [Google Scholar] [CrossRef]
- Ricci, A.; Carvalho, P.D.; Amundson, M.C.; Fourdraine, R.H.; Vincenti, L.; Fricke, P.M. Factors associated with pregnancy-associated glycoprotein (PAG) levels in plasma and milk of Holstein cows during early pregnancy and their effect on the accuracy of pregnancy diagnosis. J. Dairy Sci. 2015, 98, 2502–2514. [Google Scholar] [CrossRef]
- Nanas, I.; Chouzouris, T.M.; Dovolou, E.; Dadouli, K.; Stamperna, K.; Kateri, I.; Barbagianni, M.; Amiridis, G.S. Early embryo losses, progesterone and pregnancy associated glycoproteins levels during summer heat stress in dairy cows. J. Therm. Biol. 2021, 98, 102951. [Google Scholar] [CrossRef]
| Item | Dry Matter Basis (%) |
|---|---|
| Crude Protein | 17.96 |
| NDF | 26.32 |
| Cellulose | 14.93 |
| Hemicellulose | 8.78 |
| Lignin | 2.62 |
| Crude Fat | 5.1 |
| Ash | 10.72 |
| TDN | 71.74 |
| DE (Mcal/kg) | 3.23 |
| ME (Mcal/kg | 2.58 |
| Experimental Weeks | Air Temperature, °C | Humidity, % | THI |
|---|---|---|---|
| 1st week | 37 | 75 | 81 |
| 2nd week | 37 | 73 | 82 |
| 3rd week | 36 | 76 | 81 |
| 4th week | 36 | 75 | 80 |
| Parameter | Gestation (Week) | Experimental Groups | ||||
|---|---|---|---|---|---|---|
| 1 CON | 2 FA5 | 3 FA10 | 4 SEM | p-Value | ||
| Glucose, mmol/L | 2nd week | 2.46 b | 2.35 b | 2.90 a | 0.18 | 0.041 |
| 4th week | 2.55 | 2.16 | 2.22 | 0.16 | 0.118 | |
| Urea, mmol/L | 2nd week | 4.52 | 4.85 | 4.46 | 0.28 | 0.856 |
| 4th week | 5.21 a | 4.17 b | 4.45 ab | 0.33 | 0.028 | |
| Total protein, g/dL | 2nd week | 7.27 | 6.73 | 5.91 | 0.75 | 0.622 |
| 4th week | 4.44 | 6.56 | 6.97 | 0.67 | 0.087 | |
| Albumin, g/dL | 2nd week | 4.56 a | 3.96 a | 2.16 b | 0.25 | 0.010 |
| 4th week | 3.65 | 3.29 | 4.57 | 0.32 | 0.134 | |
| Globulin, g/dL | 2nd week | 3.33 | 2.56 | 2.88 | 0.27 | 0.404 |
| 4th week | 3.28 b | 2.99 b | 4.20 a | 0.23 | 0.049 | |
| Cholesterol, mmol/L | 2nd week | 8.63 | 8.99 | 9.12 | 0.36 | 0.496 |
| 4th week | 8.54 | 9.14 | 8.06 | 0.32 | 0.405 | |
| Triglycerides, mmol/L | 2nd week | 5.31 | 6.38 | 5.36 | 0.32 | 0.095 |
| 4th week | 6.97 | 6.57 | 6.27 | 0.34 | 0.677 | |
| 5 HDL, mmol/L | 2nd week | 4.06 | 2.61 | 4.24 | 0.22 | 0.053 |
| 4th week | 3.95 | 3.72 | 5.68 | 0.28 | 0.209 | |
| 6 LDL, mmol/L | 2nd week | 3.30 | 3.51 | 2.90 | 0.17 | 0.488 |
| 4th week | 3.09 | 3.11 | 2.22 | 0.15 | 0.404 | |
| Parameter | Gestation (Week) | Experimental Groups | ||||
|---|---|---|---|---|---|---|
| 1 CON | 2 FA5 | 3 FA10 | 4 SEM | p-Value | ||
| Cortisol, μg/dL | 2nd week | 4.13 | 3.20 | 3.26 | 0.31 | 0.189 |
| 4th week | 3.92 | 3.16 | 4.29 | 0.24 | 0.323 | |
| 5 GH, pg/mL | 2nd week | 0.280 | 0.250 | 0.254 | 0.040 | 0.889 |
| 4th week | 0.220 b | 0.310 a | 0.303 a | 0.033 | 0.020 | |
| 6 IGF-1, pg/mL | 2nd week | 396 b | 301 b | 786 a | 30.4 | 0.040 |
| 4th week | 349 c | 486 b | 729 a | 31.7 | 0.001 | |
| Parameter | Gestation (Week) | Experimental Groups | ||||
|---|---|---|---|---|---|---|
| 1 CON | 2 FA5 | 3 FA10 | 4 SEM | p-Value | ||
| 5 NEFA, μmol/L | 2nd week | 173.5 a | 130.5 b | 109.0 b | 6.92 | 0.020 |
| 4th week | 100.9 a | 96.3 ab | 82.2 b | 4.15 | 0.035 | |
| 6 BHB, mmol/L | 2nd week | 0.535 a | 0.405 ab | 0.377 b | 0.04 | 0.013 |
| 4th week | 0.349 | 0.379 | 0.374 | 0.03 | 0.069 | |
| 7 PAG, ng/mL | 2nd week | 1.82 b | 2.42 b | 5.21 a | 0.19 | 0.005 |
| 4th week | 3.23 b | 2.44 b | 5.93 a | 0.18 | 0.001 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kilany, A.A.; El-Darawany, A.-H.A.; El-Tarabany, A.A.; Al-Marakby, K.M. Effect of Folic Acid Supplements on Progesterone Profile and Blood Metabolites of Heat-Stressed Holstein Cows during the Early Stage of Pregnancy. Animals 2022, 12, 1872. https://doi.org/10.3390/ani12151872
Kilany AA, El-Darawany A-HA, El-Tarabany AA, Al-Marakby KM. Effect of Folic Acid Supplements on Progesterone Profile and Blood Metabolites of Heat-Stressed Holstein Cows during the Early Stage of Pregnancy. Animals. 2022; 12(15):1872. https://doi.org/10.3390/ani12151872
Chicago/Turabian StyleKilany, Abdelrahman A., Abdel-Halim A. El-Darawany, Akram A. El-Tarabany, and Khaled M. Al-Marakby. 2022. "Effect of Folic Acid Supplements on Progesterone Profile and Blood Metabolites of Heat-Stressed Holstein Cows during the Early Stage of Pregnancy" Animals 12, no. 15: 1872. https://doi.org/10.3390/ani12151872
APA StyleKilany, A. A., El-Darawany, A.-H. A., El-Tarabany, A. A., & Al-Marakby, K. M. (2022). Effect of Folic Acid Supplements on Progesterone Profile and Blood Metabolites of Heat-Stressed Holstein Cows during the Early Stage of Pregnancy. Animals, 12(15), 1872. https://doi.org/10.3390/ani12151872





