Virulence Factors and Antimicrobial Resistance Profile of Escherichia Coli Isolated from Laying Hens in Italy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. E. coli Isolation and Identification
2.3. Somatic Antigen Identification
2.4. Antimicrobial Susceptibility Testing
2.5. APEC Genes Detection
2.6. Statistical Analysis
3. Results
3.1. Necropsy
3.2. Somatic Antigen
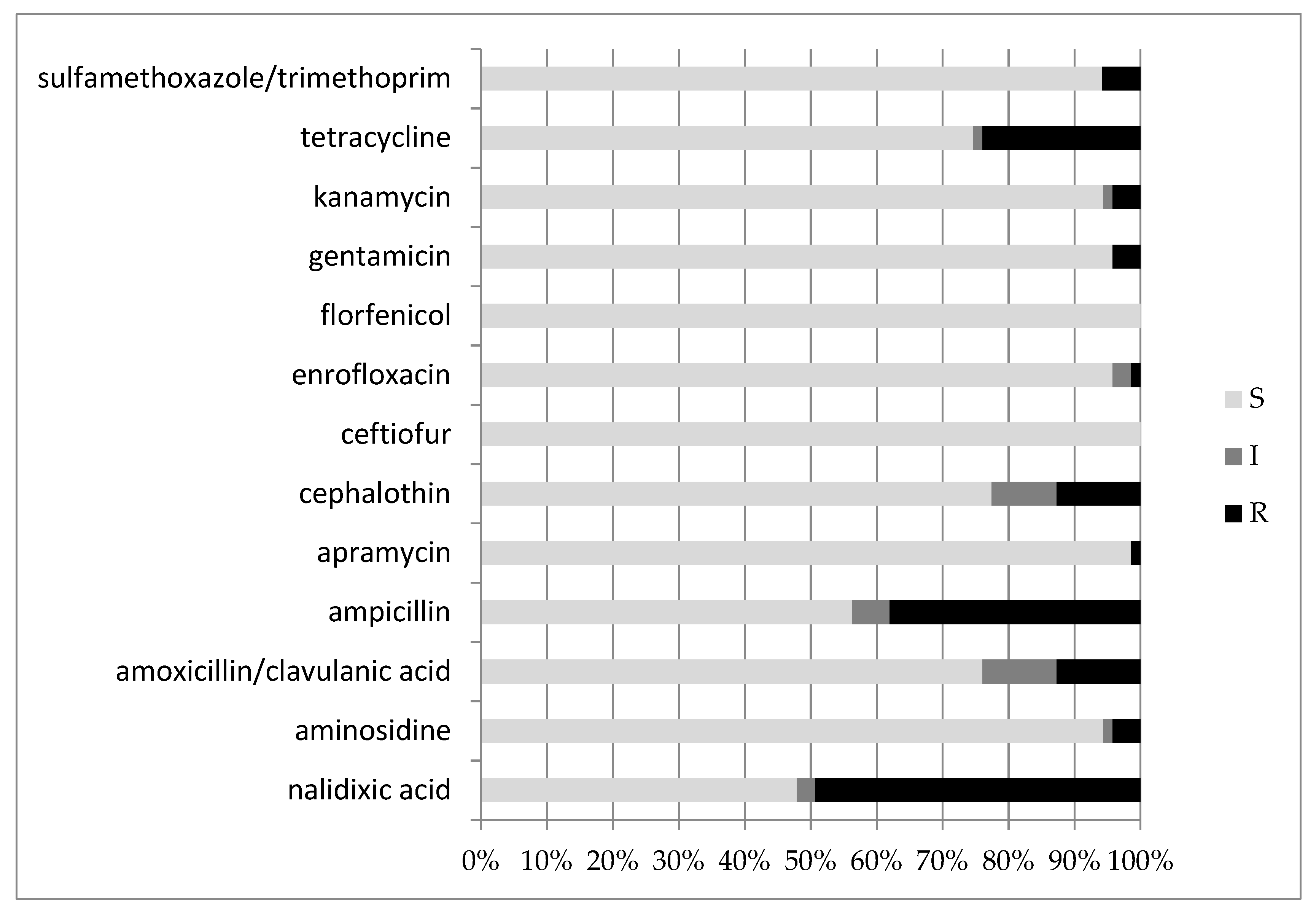
3.3. Antimicrobial Susceptibility Testing
3.4. Virulence Gene Detection
3.5. Serogroups, Virulence Factors and Multidrug Resistance Correlation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barnes, H.J.; Nolan, L.K.; Vaillancourt, J.P. Colibacillosis. In Diseases of Poultry, 12th ed.; Saif, J.M., Ed.; Blackwell Publishing: Oxford, UK, 2009; pp. 691–737. [Google Scholar]
- Wilczynski, J.; Stepien-Pysniak, D.; Wystalska, D.; Wernicki, A. Molecular and Serological Characteristics of Avian Pathogenic Escherichia coli Isolated from Various Clinical Cases of Poultry Colibacillosis in Poland. Animals 2022, 12, 1090. [Google Scholar] [CrossRef] [PubMed]
- Camarda, A. Infezioni da Escherichia Coli. In Manuale di Patologia Aviare, 1st ed.; Le Point Veterinarie SRL: Milano, Italy, 2009; pp. 77–83. [Google Scholar]
- Hafez, H.M.; Attia, Y.A. Challenges to the Poultry Industry: Current Perspectives and Strategic Future after the COVID-19 Outbreak. Front. Vet. Sci. 2020, 7, 516. [Google Scholar] [CrossRef]
- Al-Marri, T.; Al-Marri, A.; Al-Zanbaqi, R.; Al Ajmi, A.; Fayez, M. Multidrug resistance, biofilm formation, and virulence genes of Escherichia coli from backyard poultry farms. Vet. World 2021, 14, 2869–2877. [Google Scholar] [CrossRef] [PubMed]
- Mughini-Gras, L.; van Pelt, W.; van der Voort, M.; Heck, M.; Friesema, I.; Franz, E. Attribution of human infections with Shiga toxin-producing Escherichia coli (STEC) to livestock sources and identification of source-specific risk factors, The Netherlands (2010–2014). Zoonoses Public Health 2018, 65, e8–e22. [Google Scholar] [CrossRef]
- Sgariglia, E.; Aconiti Mandolini, N.; Napoleoni, M.; Medici, L.; Fraticelli, R.; Conquista, M.; Gianfelici, P.; Staffolani, M.; Fisichella, S.; Capuccella, M.; et al. Antibiotic resistance pattern and virulence genes in avian pathogenic Escherichia coli (APEC) from different breeding systems. Vet. Ital. 2019, 55, 26–33. [Google Scholar]
- Xu, X.; Sun, Q.; Zhao, L. Virulence Factors and Antibiotic Resistance of Avian Pathogenic Escherichia Coli in Eastern China. J. Vet. Res. 2019, 63, 317–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ECDC. Surveillance of Antimicrobial Resistance in Europe 2018; Annual Report of the European Antimicrobical Resistance Surveillance Network (EARS-Net) 2018; ECDC: Solna, Sweden, 2019. [Google Scholar]
- Mehat, J.W.; van Vliet, A.H.M.; La Ragione, R.M. The Avian Pathogenic Escherichiacoli (APEC) pathotype is comprised of multiple distinct, independent genotypes. Avian Pathol. 2021, 50, 402–416. [Google Scholar] [CrossRef] [PubMed]
- Dho-Moulin, M.; Fairbrother, J.M. Avian pathogenic Escherichia coli (APEC). Vet. Res. 1999, 30, 299–316. [Google Scholar]
- Schouler, C.; Schaeffer, B.; Brée, A.; Mora, A.; Dahbi, G.; Biet, F.; Oswald, E.; Mainil, J.; Blanco, J.; Moulin-Schouleur, M. Diagnostic Strategy for Identifying Avian Pathogenic Escherichia coli Based on Four Patterns of Virulence Genes. J. Clin. Microbiol. 2012, 50, 1673–1678. [Google Scholar] [CrossRef] [Green Version]
- Delannoy, S.; Schouler, C.; Souillard, R.; Yousfi, L.; Le Devendec, L.; Lucas, C.; Bougeard, S.; Keita, A.; Fach, P.; Galliot, P.; et al. Diversity of Escherichia coli strains isolated from day-old broiler chicks, their environment and colibacillosis lesions in 80 flocks in France. Vet. Microbiol. 2021, 252, 108923. [Google Scholar] [CrossRef]
- Koutsianos, D.; Athanasiou, L.V.; Mossialos, D.; Franzo, G.; Cecchinato, M.; Koutoulis, K.C. Investigation of Serotype Prevalence of Escherichia coli Strains Isolated from Layer Poultry in Greece and Interactions with Other Infectious Agents. Vet. Sci. 2022, 9, 152. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.B.; Yoon, M.Y.; Ha, J.S.; Seo, K.W.; Noh, E.B.; Son, S.H.; Lee, Y.J. Molecular characterization of avian pathogenic Escherichia coli from broiler chickens with colibacillosis. Poult. Sci. 2020, 99, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Goudarztalejerdi, A.; Mohammadzadeh, A.; Varmaziar Najafi, S.; Nargesi, F.; Joudari, S. Serogrouping, phylotyping, and virulence genotyping of commensal and avian pathogenic Escherichia coli isolated from broilers in Hamedan, Iran. Comp. Immunol. Microbiol. Infect. Dis. 2020, 73, 101558. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Thuan, N.K.; Tam, N.T.; Huyen Trang, C.T.; Khanh, N.P.; Bich, T.N.; Taniguchi, T.; Hayashidani, H.; Lien Khai, L.T. Prevalence and Genetic Relationship of Predominant Escherichia coli Serotypes Isolated from Poultry, Wild Animals, and Environment in the Mekong Delta, Vietnam. Vet. Med. Int. 2021, 11, 6504648. [Google Scholar] [CrossRef]
- Circella, E.; Pennelli, D.; Tagliabue, S.; Camarda, A. Virulence-associated genes in avian pathogenic Eschierichia coli from laying hens in Apulia, southern Italy. Br. Poult. Sci. 2012, 53, 465–470. [Google Scholar] [CrossRef]
- Blanco, J.; Blanco, M. Presencia y características de los E. Coli enterotoxigénicos, necrotizantes y verotoxigénicos en el ganado bovino en Galicia (VIII y IX). In Escherichia coli Enterotoxigénicos, Necrotoxigénicos y Verotoxigénicos de Origen Humano y Bovino. Patogénesis, Epidemiología y Diagnóstico Microbiológico; Servicio de Publicaciones de la Diputación Provincial de Lugo: Lugo, Spain, 1993; pp. 177–290. [Google Scholar]
- Ewing, W.H. Edwards and Ewing’s Identification of Enterobacteriaceae, 4th ed.; The Genus Escherichia; Elsevier: New York, NY, USA, 1986; pp. 93–134. [Google Scholar]
- Orskov, F.; Orskov, I. Serotyping of Escherichia coli. In Methods in Microbiology; Bergan, T., Ed.; Accademic Press: London, UK, 1984; Volume 14, pp. 43–112. [Google Scholar]
- Sojka, W.J. Escherichia coli in Domestic Animals and Poultry; Review Series no. 7 of the Commonwealth Bureau of Animal Health; Commonwealth Agricultural Bureau: Wallingford, UK, 1965; pp. 184–214. [Google Scholar]
- Clinical and Laboratory Standard Institute. CLSI VET 01. In Performance Standards for Antimicrobial Disk and Diluition Susceptibility Tests for Bacteria Isolated from Animals, 5th ed.; CLSI: Wayne, PA, USA, 2018. [Google Scholar]
- Clinical and Laboratory Standard Institute. CLSI supplement M100. Performance Standards for Antimicrobial Susceptibility Testing, 28th ed.; CLSI: Wayne, PA, USA, 2018. [Google Scholar]
- Clinical and Laboratory Standard Institute. CLSI. VET 01S. In Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals, 5th ed.; CLSI: Wayne, PA, USA, 2020. [Google Scholar]
- Clinical and Laboratory Standard Institute. CLSI M311-A3. In Performance Standards for Antimicrobial Disk and Diluition Susceptibility Tests for Bacteria Isolated from Animals, 3rd ed.; Approved Standards; Number 8; CLSI: Wayne, PA, USA, 2008; Volume 28. [Google Scholar]
- CRAB (Centro di Referenza Nazionale per l’Antibiotico Resistenza). Molecole Prototipo e Loro Equivalenti In Vitro—Revisione 5. 2021. Available online: https://www.izslt.it/crab/wp-content/uploads/sites/8/2021/03/Molecole-prototipo-e-loro-equivalenti-in-vitro.pdf (accessed on 15 June 2022).
- White, D. Laboratory Methodologies for Bacterial Antimicrobial Susceptibility Testing Cap.2.1.1. In OIE Manual; 2019; Available online: https://www.woah.org/fileadmin/Home/eng/Health_standards/tahm/2.01.01_ANTIMICROBIAL.pdf (accessed on 14 June 2022).
- Guabiraba, R.; Schouler, C. Avian colibacillosis: Still many black holes. FEMS Microbiol. Lett. 2015, 362, fnv118. [Google Scholar] [CrossRef]
- Rodriguez-Siek, K.E.; Giddings, C.W.; Doetkott, C.; Johnson, T.J.; Nolan, L.K. Characterizing the APEC pathotype. Vet. Res. 2005, 36, 241–256. [Google Scholar] [CrossRef] [Green Version]
- Cordoni, G.; Woodward, M.J.; Wu, H.; Alanazi, M.; Wallis, T.; La Ragione, R.M. Comparative genomics of European avian pathogenic E. coli (APEC). BMC Genom. 2016, 17, 960. [Google Scholar] [CrossRef] [Green Version]
- De Carli, S.; Ikuta, N.; Lehmann, F.K.M.; da Silveira, V.P.; de Melo Predebon, G.; Fonseca, A.S.K.; Lunge, V.R. Virulence gene content in Escherichia coli isolates from poultry flocks with clinical signs of colibacillosis in Brazil. Poult. Sci. 2015, 94, 2635–2640. [Google Scholar] [CrossRef]
- Johnson, T.J.; Wannemuehler, Y.; Doetkott, C.; Johnson, S.J.; Rosenberger, S.C.; Nolan, L.K. Identification of Minimal Predictors of Avian Pathogenic Escherichia coli Virulence for Use as a Rapid Diagnostic Tool. J. Clin. Microbiol. 2008, 46, 3987–3996. [Google Scholar] [CrossRef] [Green Version]
- Azam, M.; Mohsin, M.; Sajjad-Ur-Rahman; Saleemi, M.K. Virulence-associated genes and antimicrobial resistance among avian pathogenic Escherichia coli from colibacillosis affected broilers in Pakistan. Trop. Anim. Health Prod. 2019, 51, 1259–1265. [Google Scholar] [CrossRef] [PubMed]
- Parreira, V.R.; Gyles, C.L. A Novel Pathogenicity Island Integrated Adjacent to the thrW tRNA Gene of Avian Pathogenic Escherichia coli Encodes a Vacuolating Autotransporter Toxin. Infect. Immun. 2003, 71, 5087–5096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakhshi, M.; Fatahi Bafghi, M.; Astani, A.; Ranjbar, V.R.; Zandi, H.; Vakili, M. Antimicrobial Resistance Pattern of Escherichia coli Isolated from Chickens with Colibacillosis in Yazd, Iran. J. Food Qual. Hazards Control 2017, 4, 74–78. [Google Scholar]
- Matin, M.A.; Islam, M.A.; Khatun, M.M. Prevalence of colibacillosis in chickens in greater Mymensingh district of Bangladesh. Vet. World 2017, 10, 29–33. [Google Scholar] [CrossRef] [Green Version]
- Shrestha, A.; Bajracharya, A.M.; Subedi, H.; Turha, R.S.; Kafle, S.; Sharma, S.; Neupane, S.; Chaudhary, D.K. Multi-drug resistance and extended spectrum beta lactamase producing Gram negative bacteria from chicken meat in Bharatpur Metropolitan, Nepal. BMC Res. Notes 2017, 10, 574. [Google Scholar] [CrossRef] [Green Version]
- Subedi, M.; Luitel, H.; Devkota, B.; Bhattarai, R.K.; Phuyal, S.; Panthi, P.; Shrestha, A.; Chaudhary, D.K. Antibiotic resistance pattern and virulence genes content in avian pathogenic Escherichia coli (APEC) from broiler chickens in Chitwan, Nepal. BMC Vet. Res. 2018, 14, 113. [Google Scholar] [CrossRef]
- Yang, H.; Chen, S.; White, D.G.; Zhao, S.; McDermott, P.; Walker, R.; Meng, J. Characterization of Multiple-Antimicrobial-Resistant Escherichia coli Isolates from Diseased Chickens and Swine in China. J. Clin. Microbiol. 2004, 42, 3483–3489. [Google Scholar] [CrossRef] [Green Version]
- Vandemaele, F.; Vereecken, M.; Derijcke, J.; Goddeeris, B. Incidence and antibiotic resistance of pathogenic Escherichia coli among poultry in Belgium. Vet. Rec. 2002, 151, 355–356. [Google Scholar] [CrossRef]
- Younis, G.; Awad, A.; Mohamed, N. Phenotypic and genotypic characterization of antimicrobial susceptibility of avian pathogenic Escherichia coli isolated from broiler chickens. Vet. World 2017, 10, 1167–1172. [Google Scholar] [CrossRef] [Green Version]
- Yassin, A.K.; Gong, J.; Kelly, P.; Lu, G.; Guardabassi, L.; Wei, L.; Han, X.; Qiu, H.; Price, S.; Cheng, D.; et al. Antimicrobial resistance in clinical Escherichia coli isolates from poultry and livestock, China. PLoS ONE 2017, 12, e0185326. [Google Scholar] [CrossRef] [Green Version]
- WHO (World Health Organization). Critically Important Antimicrobials for Human Medicine. 6th Revision, 2018. 2019. Available online: https://res.mdpi.com/data/mdpi_references_chicago_guide-update-v6.pdf (accessed on 15 June 2022).
- Ievy, S.; Islam, M.S.; Sobur, M.A.; Talukder, M.; Rahman, M.B.; Khan, M.F.R.; Rahman, M.T. Molecular Detection of Avian Pathogenic Escherichia coli (APEC) for the First Time in Layer Farms in Bangladesh and Their Antibiotic Resistance Patterns. Microorganisms 2020, 8, 1021. [Google Scholar] [CrossRef] [PubMed]
- Kathayat, D.; Lokesh, D.; Ranjit, S.; Rajashekara, G. Avian Pathogenic Escherichia coli (APEC): An Overview of Virulence and Pathogenesis Factors, Zoonotic Potential, and Control Strategies. Pathogens 2021, 10, 467. [Google Scholar] [CrossRef] [PubMed]
- Tarabees, R.; Gafar, K.M.; El-Sayed, M.S.; Shehata, A.A.; Ahmed, M. Effects of Dietary Supplementation of Probiotic Mix and Prebiotic on Growth Performance, Cecal Microbiota Composition, and Protection Against Escherichia coli O78 in Broiler Chickens. Probiot. Antimicrob. Proteins 2019, 11, 981–989. [Google Scholar] [CrossRef] [PubMed]

| n. of Band in Positive Control Lane | Amplified Gene | Expected Product Size |
|---|---|---|
| 1 | astA | 111 bp |
| 2 | iss | 309 bp |
| 3 | irp2 | 413 bp |
| 4 | pap C | 501 bp |
| 5 | cvi/cva | 598 bp |
| 6 | iucD | 693 bp |
| 7 | tsh | 824 bp |
| 8 | vat | 978 bp |
| E. coli Strains | ||||
|---|---|---|---|---|
| Genes | Negative | Positive | ||
| astA | 67 | 94% | 4 | 6% |
| iss | 7 | 10% | 64 | 90% |
| irp2 | 21 | 30% | 50 | 70% |
| papC | 61 | 86% | 10 | 14% |
| cvi/cva | 21 | 30% | 50 | 70% |
| iucD | 11 | 15% | 60 | 85% |
| tsh | 26 | 37% | 45 | 63% |
| vat | 46 | 65% | 25 | 35% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gambi, L.; Rossini, R.; Menandro, M.L.; Franzo, G.; Valentini, F.; Tosi, G.; D’Incau, M.; Fiorentini, L. Virulence Factors and Antimicrobial Resistance Profile of Escherichia Coli Isolated from Laying Hens in Italy. Animals 2022, 12, 1812. https://doi.org/10.3390/ani12141812
Gambi L, Rossini R, Menandro ML, Franzo G, Valentini F, Tosi G, D’Incau M, Fiorentini L. Virulence Factors and Antimicrobial Resistance Profile of Escherichia Coli Isolated from Laying Hens in Italy. Animals. 2022; 12(14):1812. https://doi.org/10.3390/ani12141812
Chicago/Turabian StyleGambi, Lorenzo, Rachele Rossini, Maria Luisa Menandro, Giovanni Franzo, Francesco Valentini, Giovanni Tosi, Mario D’Incau, and Laura Fiorentini. 2022. "Virulence Factors and Antimicrobial Resistance Profile of Escherichia Coli Isolated from Laying Hens in Italy" Animals 12, no. 14: 1812. https://doi.org/10.3390/ani12141812
APA StyleGambi, L., Rossini, R., Menandro, M. L., Franzo, G., Valentini, F., Tosi, G., D’Incau, M., & Fiorentini, L. (2022). Virulence Factors and Antimicrobial Resistance Profile of Escherichia Coli Isolated from Laying Hens in Italy. Animals, 12(14), 1812. https://doi.org/10.3390/ani12141812







