2.1. Animals, Husbandry and Housing
The study groups in this research consisted of both rhesus macaques (Macaca mulatta) and cynomolgus macaques (Macaca fascicularis) from the breeding colonies of the BPRC. All procedures, husbandry and housing performed in this study were in accordance with the Dutch laws on animal experimentation and the regulations for animal handling as described in European directive 2010/63/EU. BPRC is accredited by AAALAC International. Before the start of this observational study, approval was obtained by the institutional animal welfare body (IvD 022A).
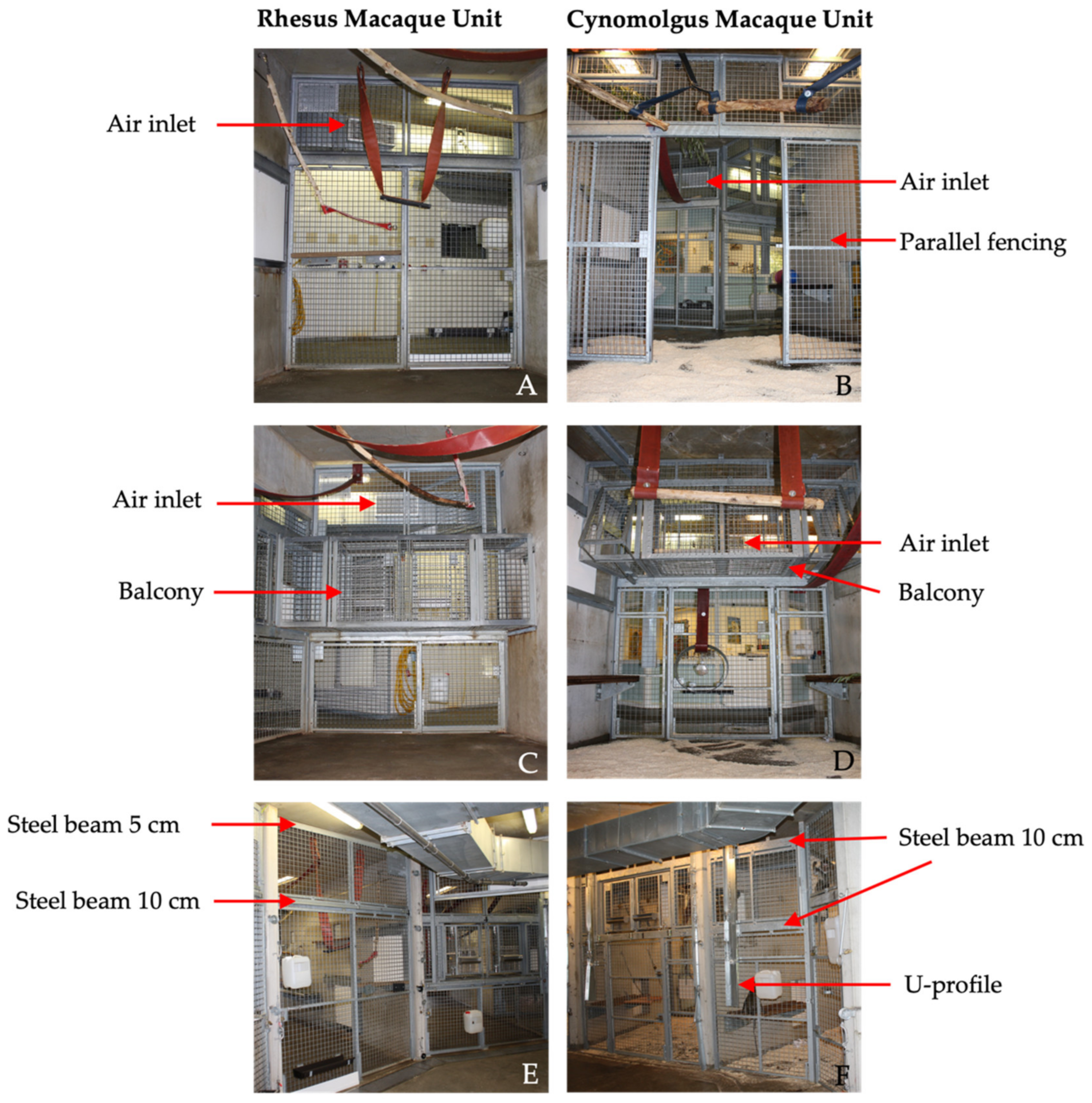
In this study, four groups were selected based on housed species, occupancy rate and comparability regarding the location inside the units. To evaluate the IAQ for the two macaque species housed at the BPRC, a rhesus macaques unit (RMU) and cynomolgus macaques unit (CMU) were selected. These units consisted of two separate animal rooms, and each room consisted of a passageway for the caretakers and the animal enclosures. These indoor enclosures were divided into compartments by concrete walls with passages for the animals.
The indoor enclosures were 2.85 m high and consisted of two (CMU) or three (RMU) connected compartments, with a floor surface of 25 m
2 each. A single enclosure housed a multi-generational group consisting of males and females. The animal details are summarized in
Table 1. In the RMU, two of the three compartments were directly connected to the outdoor enclosures, whereas both compartments in the CMU were connected to the outdoor enclosures. The indoor and outdoor enclosures were freely accessible for the animals by passing hatches with flexible strip curtains that separated the areas.
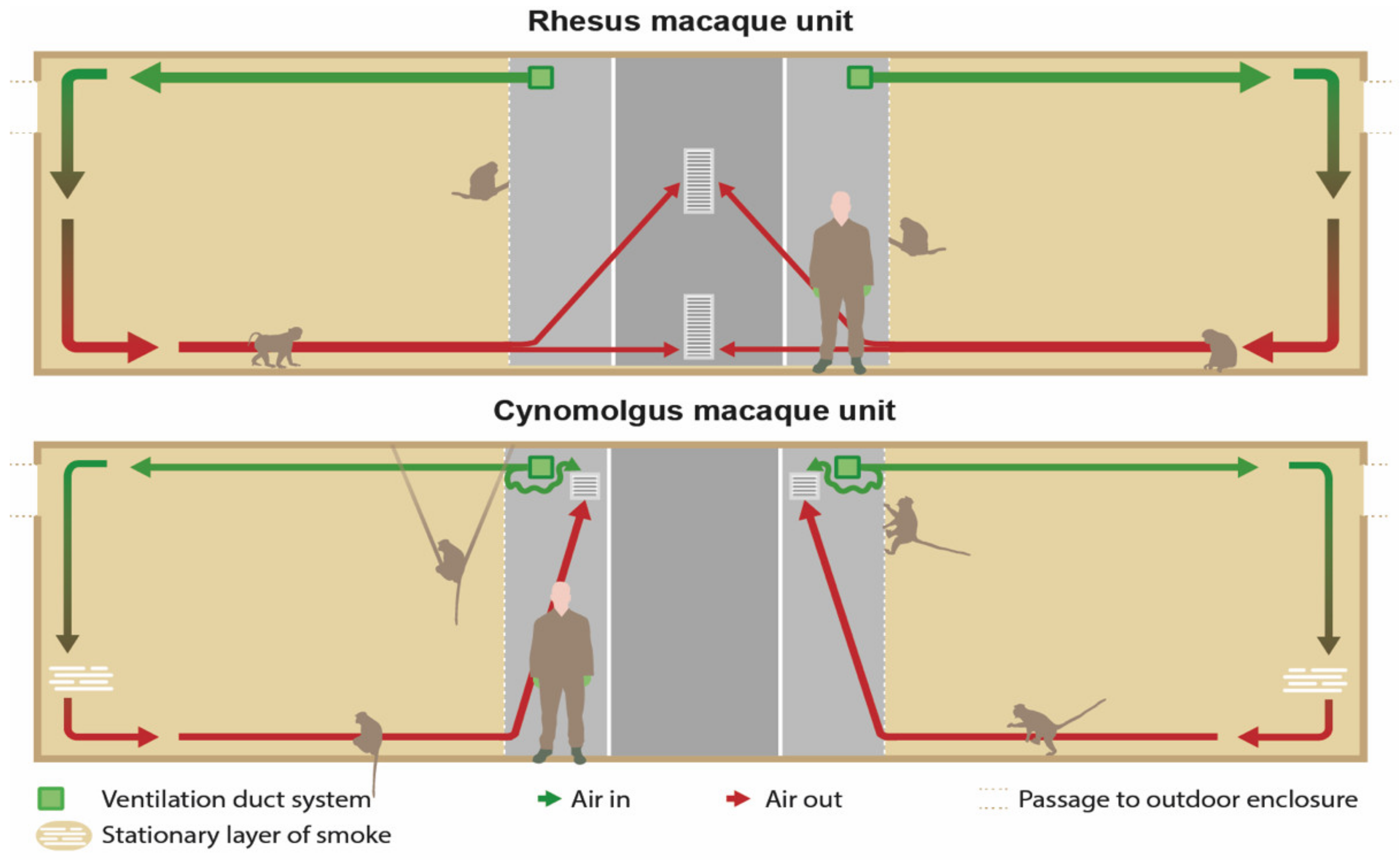
In both units, the front of the enclosures consisted of galvanized steel fencing with 5 × 5 cm spot-welded mesh wire. Although the size and height of the compartments were identical in both units, there were some differences in the design of the front fencing. First, the balcony was located on different heights for the RMU and CMU. Second, the design and location of the sliding doors differed between the units, including the location and size of support beams. Third, in CMU, an additional parallel fence was present 2.5 m from the front. Last, in CMU, a U-profile (4 × 15 cm) was secured on the front fence to prevent the cynomolgus macaques from touching the control wires of the indoor animal passages (
Figure 1).
The floors in the indoor enclosures were provided with wood fiber bedding (Lignocel® 3–4, JRS, Rosenberg, Germany). Standard environmental enrichment in these enclosures consisted of several climbing structures, beams, fire hoses, car tires and sitting platforms to stimulate natural behavior and free access to the outdoor enclosures. Drinking water was ad libitum available via automatic water dispensers. The animals were fed commercial monkey pellets (Ssniff, Soest, Germany) and daily limited amounts of vegetables, fruits or grain mixtures were offered.
Cleaning and enrichment was performed according to standardized protocols. The bedding of the indoor enclosures were cleaned out weekly. High-pressure water cleaning, including disinfection (Anistel Surface disinfectant, Tristel Solutions Limited, Cambridgeshire, UK), was performed monthly. Following disinfection, the enclosures were rinsed with clean water, and the floor was wiped dry. After allowing for a 30–40 min air dry period, approximately 31 kg of wood fiber was provided as bedding in each compartment after each cleaning procedure. Subsequently, enrichment items were provided, e.g., cardboard boxes filled with shredded paper and some mixed grains [
16]. During the study, all groups received the same enrichment items.
Outdoor air entered through the air handling unit BD-7 (VBW clima automatic, Gdynia, Poland) on RMU and LBK 02 AH AT4 (AL-KO luchttechniek b.v. Roden, Netherlands) on CMU and flowed through Hi -FLO -HFGS F7 ISO 16890 ePM1 70% filters (Camfil, Ede, Netherlands) into the duct system of the air ventilation system. The filters prevent 70% of particles of <1 μm in size from passing. The duct system was attached directly under the ceiling of the corridors in the animal rooms. In front of each compartment, one ventilation inlet was located, provided with a vent grille (22 × 60 cm) to guide the airflow into the enclosures.
In the RMU, the exhausts (40 × 80 cm) were located in the middle of the wall opposite to the enclosures and right under the ceiling and above the floor (
Figure 2 and
Figure 3). The ventilation exhausts (31 × 31 cm) in the CMU were located on the left and right side of each room right under the ceiling, opposite to the cages as well. A ventilation rate of six air changes per hour was considered sufficient due to the large cubic capacity of the animal rooms and the relatively low occupancy rates. Furthermore, the outdoor enclosures were freely accessible to the animals during both day and night.
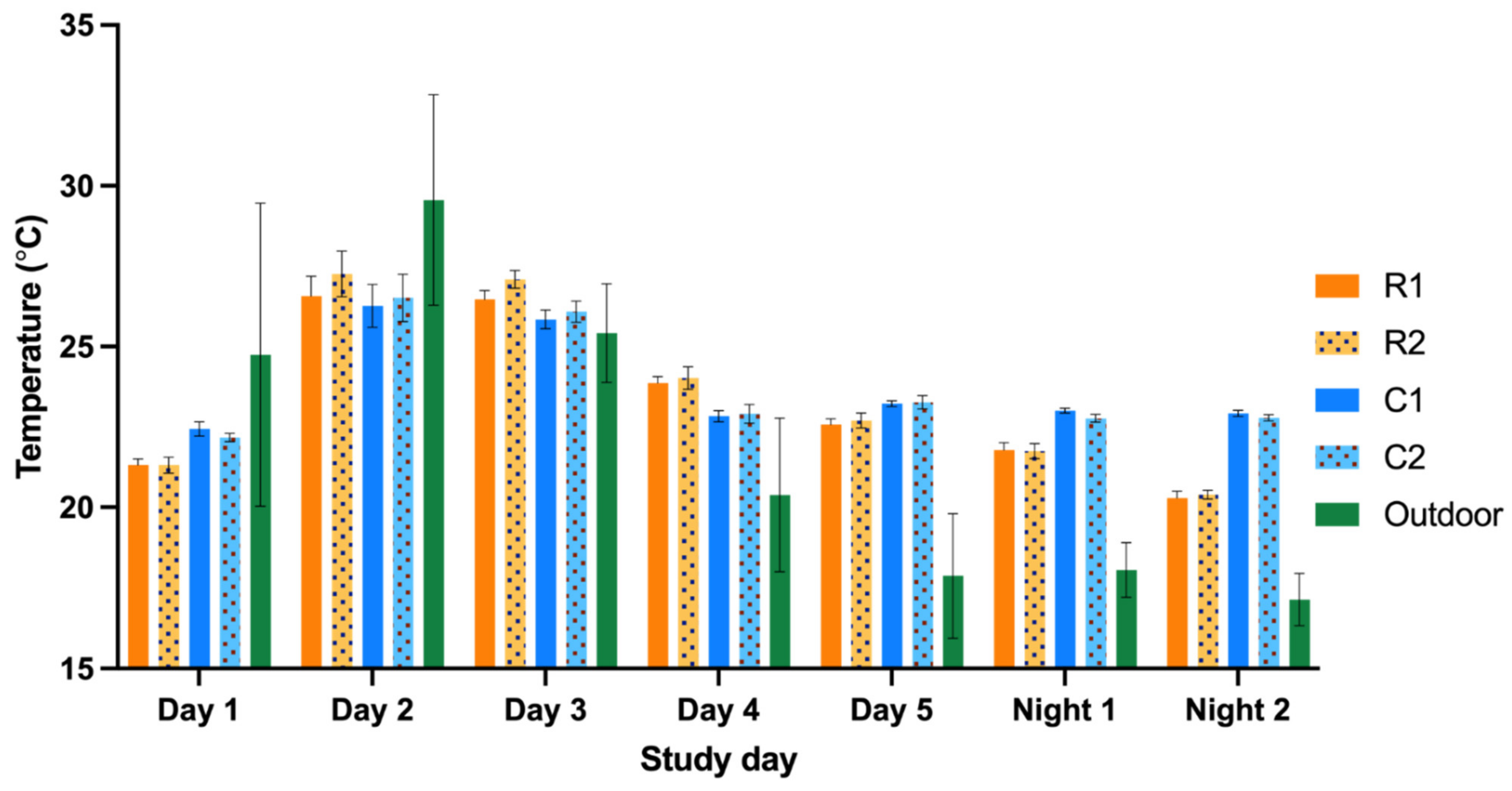
The minimum indoor temperature was controlled by heating the air inside the air handling unit and a radiant heating system inside the walls of the compartments. The minimum indoor temperature was set to 18 °C in the RMU and 21 °C in the CMU, respectively. Due to the maritime climate in the Netherlands combined with the accessibility to the outdoor enclosures, the units were designed without a cooling and humidity control system.
2.2. Study Design
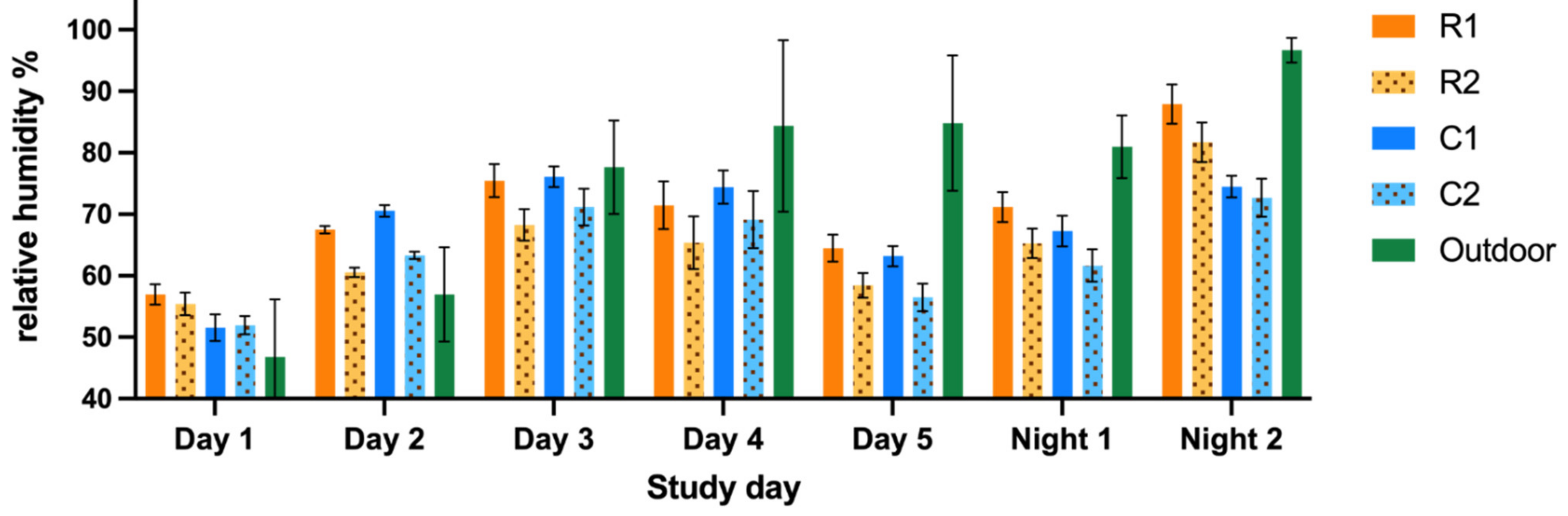
The study was performed from July to September 2020. The equipment was placed, i.e., the air was sampled, in two indoor compartments of each study group (
Figure 2). Dust, endotoxin and ammonia samples were simultaneously obtained for five days for approximately six hours a day (435 ± 12 min). The study days were selected based on the cleaning schedules of the animal rooms (
Appendix A). A similar interval of days after cleaning was aimed for; however, this interval was not synchronized between the units.
To correct for the variability in the natural occupancy rate in the compartments during the day, two night measurements were included. Camera surveillance confirmed that the animals stayed indoors during the nights. The night samples were obtained for approximately 10 h a night (625 ± 155 min).
The temperature and relative humidity were measured alongside the previously mentioned parameters with a recording interval of 10 min. All measurements were performed simultaneously in RMU and CMU. The fungal sampling was performed on one separate day.
To protect the measuring equipment against the inquisitive macaques, a cage construction with a mesh wire of 5 × 5 cm, with an additional mesh wire of 1 × 1 cm around the equipment, was designed ensuring a free airflow (
Figure 4). The construction measured 42.5 × 37.5 × 125.0 cm and was secured to the ceiling. The air quality was sampled approximately 1.6 m above the cage floor and 1.25 m from the ceiling in the middle of two compartments of the indoor enclosures (
Figure 1).
During the study days, animal activities were ad libitum live observed and recorded by two observers in four sessions of 30 min for each group. Two sessions were performed in the morning and two in the afternoon. The observers were randomly assigned to a unit and to a group to start the first observation, and subsequent group observations were alternately performed. The defined and noted activities were: (1) foraging, (2a) play terrestrial, (2b) play arboreal, (3) rest and (4) aggressive interaction (
Table 2). The activities were selected based on their potential to influence IAQ parameters, e.g., manipulation of the wood fiber bedding could potentially increase the measured dust concentration.
The number of animals present in the two compartments and the activities were recorded with a sample interval of five minutes. During the observational sessions, mothers with their offspring in the ventro–ventral position were counted as one animal. In addition, the numbers of sneezes were scored as a non-invasive health indicator for IAQ simultaneously with the animal activities.
2.3. Sampling Techniques
2.3.1. Dust and Endotoxin
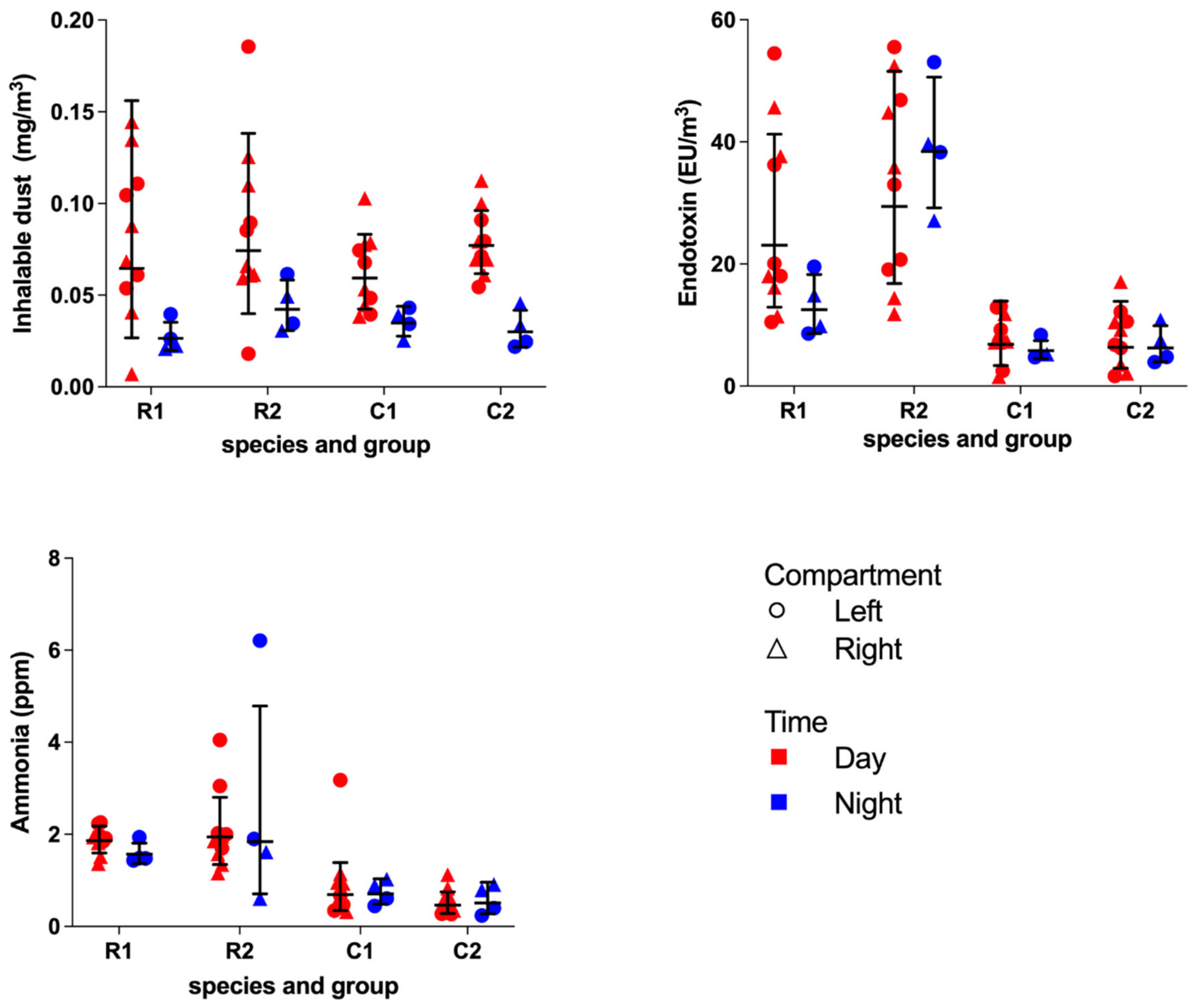
Inhalable dust was collected on 37 mm Whatman
® GF/A glass microfiber filters (Whatman International Ltd., Maidstone, UK) using Gillian GilAir-5 pumps (Gillian, Sensidyne, Clearwater, FL, USA) connected with a flexible tube a conical inhalable sampler (CIS) sampling head (JS Holdings, Stevenage, UK) in which the filter was mounted. Inhalable dust particles perched on the filter after activating the GilAir-5 pump. During all experimental days, a control filter was present. At the start of the sampling day, the pumps were calibrated at a flow rate of 3.5 L/min using a rotameter (Brooks Instruments, Hatfield, Pennsylvania) and repeated at the end of a sampling day. Immediately after collection, dust filters were stored at −20 °C until further processing. The dust concentrations (mg/m
3) were assessed as described previously [
17]. All filters were pre- and post-weighed at the same time in an acclimated room on an analytical balance with 0.01 mg readability. The acclimated room maintained a constant temperature, humidity and pressure.
The endotoxin unit concentration (EU/m
3) was assessed as described earlier [
18]. The filters were placed in sterile 50 mL Greiner
® tubes (Greiner Bio One, Alphen aan den Rijn, Netherlands) with 4 mL pyrogen-free water containing 0.05% Tween-20. The tubes were placed in an end-over-end roller for one hour and centrifuged for 15 min at 1000×
g. The supernatant was stored at −20 °C in 0.1 mL aliquots. The extracts were analyzed using a kinetic chromogenic Limulus amoebocyte lysate assay (Lonza, Breda, the Netherlands) in a dilution of 1:500. A 13-point standard curve ranging from 25 to 0.006 EU/mL was included in the assay as a reference.
2.3.2. Ammonia
The ammonia concentration (ppm) was assessed with the use of Radiello™
ready-to-use diffuse samplers (Instituti Clinici Scientifici Maugeri, Pavia, Italy) pre-assembled with absorbent cartridges within the diffuse bodies, which binds ammonia in the form of ammonium, as described elsewhere [
19]. The ammonia samplers were protected from urine and fecal contamination by an open bottom plastic casing. Until further processing, the ammonia cartridges were stored in closed zip-lock bags and cooled at 4 °C.
After extraction with 10 mL of deionized water, the samples were analyzed by a chemical colorimetric method based on the Berthelot reaction [
20]. A standard curve ranging from 0.5 to 10 ng/mL ammonium was included in each assay as a reference to determine the amount in the air samples.
2.3.3. Fungal Aerosols
Fungal aerosols were measured by active and passive sampling methods. First, for the active sampling method, D5600 Wuppertal pumps (Gebr. Becker®, Wuppertal, Germany) with a preset flow rate of 28.3 L/min were used. The pumps were connected to a Anderson one-stage 400-hole impactor (SKC Inc. Procare, Groningen, the Netherlands) equipped with Dichloran Glycerol 18% agar plate (DG18) and activated for a duration of 10 min. Second, for the passive sampling method, DG18 agar plates were placed directly in front of the enclosures for 10 min. In addition, to sample potential fungal spores originating from the ventilation system, DG18 agar plates were placed in front of the air inlets for 10 min.
Subsequently, all samples were transported to the laboratory and were incubated at 24 °C. At 24, 48 and 96 h of incubation, the colonies were counted with a colony counter (Gallenkamp
®, Loughborough, UK). The numbers of colonies on the agar plates were corrected by a Positive-Hole Correction table [
21]. Moreover, the fungal colonies were microscopically identified to genus. The results were expressed as the number of colony forming units per cubic meter air (CFU/m³) and colony forming units per plate (CFU/plate) for the active and passive sampled plates, respectively.
2.3.4. Temperature and Relative Humidity
Temperature (°C) and the relative humidity (%) were recorded using EL-MOTE-TH Temperature & Humidity Cloud-Connected Data Loggers (Lascar electronics®, Wiltshire, UK). The left compartments were provided with a datalogger, and one logger was placed outside on the BPRC premises to register the outdoor temperature and humidity, with a recording interval of ten minutes. The means of these recording intervals were calculated and used for further analyses. The dataloggers in the compartments were placed into the same protective boxes as the GilAir-5 pumps. For obvious reasons, the boxes were removed on cleaning days.
2.3.5. Smoke Test
Non-toxic smoke was used to visualize the airflow in the animal enclosures. A pyrotechnic smoke cartridge Miniax KS (Scan-Air, Mill, the Netherlands) was lit in front of every air inlet in the indoor compartments. The distribution and flow of the smoke was recorded with cameras until the smoke was not visible anymore. All animals were locked in their outside enclosure during this test.
Figure 3 shows the expected airflow.
2.3.6. Personal Exposure
One animal caretaker per unit wore a GilAir-5 pump during a regular working day. The pump was attached to a waist belt, and the sampling head was attached to the collar of their coveralls to sample air as close to the mouth region as possible. In addition, caretakers kept a log of the tasks and the duration of these tasks that specific day. The measurements were paused when the caretakers left the unit for coffee and lunch breaks.
2.3.7. Data Analyses
Statistical tests were performed with R studio v4.1.3 and GraphPad prism v8.4.2.
The dust, endotoxin, ammonia and fungal concentrations are presented as the GM with geometric standard deviation (GSD). GSD is defined as a multiplicative factor describing the range in a lognormal distribution used with GM, e.g., GM times or divided by GSD [
22]. The between-unit and group differences for the dust, endotoxin, ammonia, temperature and relative humidity were tested non-parametrically using the Mann–Whitney U test. Non-parametric Spearman’s rank correlations were used to evaluate possible associations between dust, endotoxin, ammonia, temperature and relative humidity, between and within the units.
Due to extreme precipitation during one night measurements, the correlations for temperature and relative humidity were also analyzed excluding night measurements. In addition, we investigated the occupancy rate and total body mass between and within the units and the IAQ parameters/contaminants. Furthermore, possible associations between sneezing, observed animal activity and the influence of days after high-pressure cleaning and the IAQ parameters were investigated. The number of sneezes and activity was evaluated with only the day measurements since the observations were performed during daytime. P values smaller than 0.05 were considered as statistically significant. Due to the limited data regarding fungal aerosols, statistical analysis was not performed.