No Morphological Integration of Dorsal Profiles in the Araucanian Horse (Colombia)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
- (a)
- Those specimens that contradicted any of the inclusion criteria that identify them as being pure Araucanian.
- (b)
- Photographs of low sharpness to distinguish homologous landmarks or images with obvious distortions due to enlargement or deformation.
- (c)
- Individuals who presented obvious morphopathological signs.
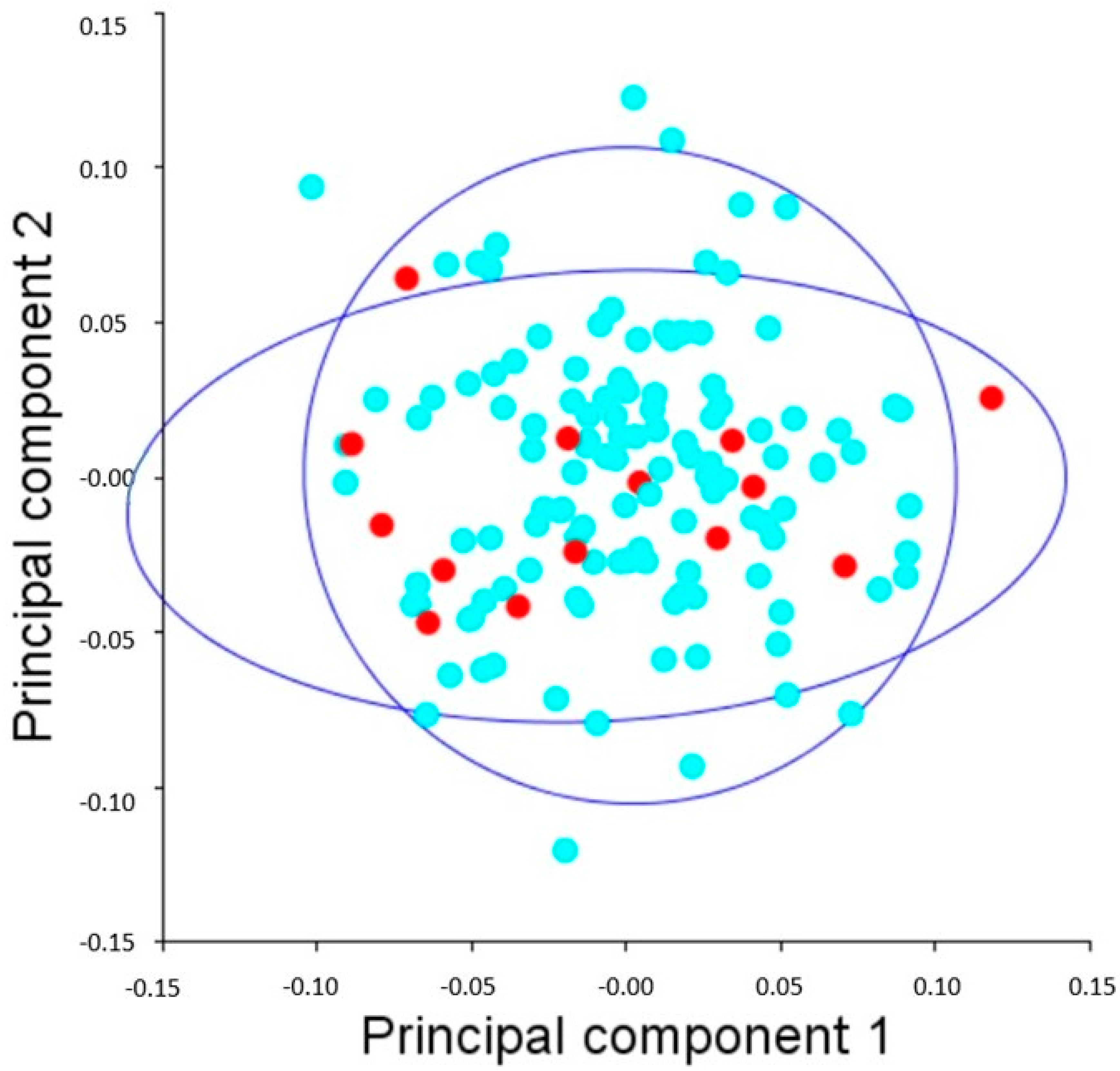
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sañudo, C. Valoración Morfológica de Los Animales Domésticos; Minist. de Medio Ambiente y Medio Rural y Marino: Madrid, Spain, 2009. [Google Scholar]
- Maśko, M.; Wierzbicka, M.; Zdrojkowski, Ł.; Jasinski, T.; Sikorska, U.; Pawlinski, B.; Domino, M. Comparison of Donkey, Pony, and Horse Dorsal Profiles and Head Shapes Using Geometric Morphometrics. Animals 2022, 12, 931. [Google Scholar] [CrossRef]
- Adams, D.C.; Rohlf, F.J.; Slice, D.E. A field comes of age: Geometric morphometrics in the 21st century. Hystrix 2013, 24, 7–14. [Google Scholar]
- Bookstein, F.L. Morphometric Tools for Landmark Data: Geometry and Biology; Cambridge University Press: Cambridge, UK, 1991. [Google Scholar]
- Adams, D.C.; Rohlf, F.J.; Dennis, E.S.E. Geometric morphometrics: Ten years of progress following the ‘revolution’. Ital. J. Zool. 2004, 71, 5–16. [Google Scholar] [CrossRef]
- Zelditch, M.L.; Swiderski, D.L.; Sheets, H.D.; Fink, W.L. Geometric Morphometrics for Biologists: A Primer; Elsevier Academic Press: Bostón, MA, USA, 2004; p. 416. [Google Scholar]
- Gunza, P.; Mitteroecker, P. Semilandmarks: A method for quantifying curves and surfaces. Hystrix 2013, 24, 103–109. [Google Scholar]
- Jojić, V.; Budinski, I.; Blagojević, J.; Vujošević, M. Mandibular and Cranial Modularity in the Greater Horseshoe Bat Rhinolophus ferrumequinum (Chiroptera: Rhinolophidae). Hystrix 2015, 26, 163–165. [Google Scholar]
- Heck, L.; Wilson, L.A.B.; Evin, A.; Stange, M.; Sánchez-Villagra, M.R. Shape variation and modularity of skull and teeth in domesticated horses and wild equids. Front. Zool. 2018, 15, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Romaniuk, A. Functional and Phylogenetic Aspect in Modularity of Palearctic Mustelids (Carnivora, Mustelidae) Mandible. Vestn. Zool. 2018, 52, 165–176. [Google Scholar] [CrossRef][Green Version]
- Segura, V.; Cassini, G.H.; Prevosti, F.J.; Andrade, M.F. Integration or Modularity in the Mandible of Canids (Carnivora: Canidae): A Geometric Morphometric Approach. J. Mammal. Evol. 2020, 28, 145–157. [Google Scholar] [CrossRef]
- Adams, D.C. Evaluating Modularity in Morphometric Data: Challenges with the RV Coefficient and a New Test Measure. Methods Ecol. Evol. 2016, 7, 565–572. [Google Scholar] [CrossRef]
- Püschel, T. Modularidad e Integración Morfológica en Cráneos Humanos: Un Enfoque Morfométrico Geométrico. Int. J. Morphol. 2014, 32, 299–304. [Google Scholar] [CrossRef][Green Version]
- Klingenberg, C.P. Studying morphological integration and modularity at multiple levels: Concepts and analysis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 33–35. [Google Scholar] [CrossRef] [PubMed]
- Salamanca, C.A.; Monroy, N.; Parés-Casanova, P.M.; Crosby, R.A. Aporte a la evaluación para la preservación del Caballo Criollo Araucano en Colombia. Zootec. Trop. 2015, 33, 317–325. [Google Scholar]
- Salamanca, C.A.; Parés-Casanova, P.M.; Crosby, R.A.; Monroy, N. Biometric analysis of araucano criollo horse. Arch. Zootec. 2017, 66, 267–278. [Google Scholar] [CrossRef]
- Salamanca-Carreño, A.; Jordana, J.; Crosby-Granados, R.A.; Bentez-Molano, J.; Parés-Casanova, P.M. Lineal discrimination of horses and mules. A sympatric case from arauca, Colombia. Animals 2020, 10, 679. [Google Scholar] [CrossRef]
- Rohlf, F.J. The Tps Series of Software. Hystrix Ital. J. Mamm. 2015, 26, 9–12. [Google Scholar]
- Klingenberg, C.P. MorphoJ: An Integrated Software Package for Geometric Morphometrics. Mol. Ecol. Res. 2011, 11, 353–357. [Google Scholar] [CrossRef]
- Klingenberg, C.P. Morphometric integration and modularity in configurations of landmarks: Tools for evaluating a priori hypotheses. Evol. Dev. 2009, 11, 405–421. [Google Scholar] [CrossRef]
- Goswami, A.; Polly, P.D. Methods for studying morphological integration and modularity. Paleontol. Soc. Pap. 2010, 16, 213–243. [Google Scholar] [CrossRef]
- Goodwin, D.; Levine, M.; McGreevy, P.D. Preliminary investigation of morphological differences between ten breeds of horses suggests selection for paedomorphosis. J. Appl. Anim. Welf. 2008, 11, 204–212. [Google Scholar] [CrossRef]
- Carter, R.A.; Geor, R.J.; Staniar, W.B.; Cubitt, T.A.; Harris, P.A. Apparent adiposity assessed by standardised scoring systems and morphometric measurements in horses and ponies. Vet. J. 2009, 179, 204–210. [Google Scholar] [CrossRef]
- Maśko, M.; Wierzbicka, M.; Zdrojkowski, Ł.; Jasiński, T.; Pawliński, B.; Domino, M. Characteristics of the Donkey’s Dorsal Profile in Relation to Its Functional Body Condition Assessment. Animals 2021, 11, 3095. [Google Scholar] [CrossRef] [PubMed]
- Sénèque, E.; Lesimple, C.; Morisset, S.; Hausberger, M. Could posture reflect welfare state? A study using geometric morphometrics in riding school horses. PLoS ONE 2019, 14, e0211852. [Google Scholar] [CrossRef] [PubMed]
- Sénèque, E.; Morisset, S.; Lesimple, C.; Hausberger, M. Testing optimal methods to compare horse postures using geometric morphometrics. PLoS ONE 2018, 13, e0204208. [Google Scholar] [CrossRef] [PubMed]
- Tallet, C.; Senèque, E.; Megnin, C.; Morisset, S.; Val-Laillet, D.; Meunier-Salaun, M.-C.; Fureix, C.; Hausberger, M. Assessing walking posture with geometric morphometrics: Effects of rearing environment in pigs. Appl. Anim. Behav. Sci. 2016, 174, 32–41. [Google Scholar] [CrossRef]
- Valle, E.; Raspa, F.; Giribaldi, M.; Barbero, R.; Bergagna, S.; Antoniazzi, S.; Mc Lean, A.K.; Minero, M.; Cavallarin, L. A functional approach to the body condition assessment of lactating donkeys as a tool for welfare evaluation. Peer J. 2017, 5, e3001. [Google Scholar] [CrossRef]
- Veneziano, A.; Meloro, C.; Irish, J.D.; Stringer, C.; Profico, A.; De Groote, I. Neuromandibular integration in humans and chimpanzees: Implications for dental and mandibular reduction in Homo. Am. J. Phys. Anthropol. 2018, 167, 84–96. [Google Scholar] [CrossRef]
- Parés-Casanova, P.M. Age-dependent mandibular asymmetries in domestic pigs. Research 2014, 1, 4–7. [Google Scholar] [CrossRef]
- Parés-Casanova, P.M.; Cabello, M. Patterns of mandibular asymmetries in two types of companion rabbits. J. Vet. Medic. Ser. C Anat. Histol. Embryol. 2020, 49, 227–232. [Google Scholar] [CrossRef]

| Semi-Markers | Minimal RV | Coefficient RV | Number of Partitions with an RV Less than or Equal to the a Priori Hypothesis |
|---|---|---|---|
| Neck–back | 0.475759 | 0.475667 | 7 |
| Neck–croup | 0.269920 | 0.269920 | 2 |
| Croup–back | 0.318913 | 0.432302 | 371 |
| Semi-Markers | PLS1 | PLS2 | PLS3 | PLS4 |
|---|---|---|---|---|
| Neck–back | 87.871 | 4.729 | 3.398 | 1.549 |
| Neck–croup | 80.483 | 9.666 | 7.643 | 1.059 |
| Croup–back | 91.742 | 3.727 | 2.153 | 1.318 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salamanca-Carreño, A.; Parés-Casanova, P.M.; Rangel-Pachón, D.E.; Bentez-Molano, J.; Vélez-Terranova, O.M. No Morphological Integration of Dorsal Profiles in the Araucanian Horse (Colombia). Animals 2022, 12, 1731. https://doi.org/10.3390/ani12131731
Salamanca-Carreño A, Parés-Casanova PM, Rangel-Pachón DE, Bentez-Molano J, Vélez-Terranova OM. No Morphological Integration of Dorsal Profiles in the Araucanian Horse (Colombia). Animals. 2022; 12(13):1731. https://doi.org/10.3390/ani12131731
Chicago/Turabian StyleSalamanca-Carreño, Arcesio, Pere M. Parés-Casanova, David Eduardo Rangel-Pachón, Jannet Bentez-Molano, and Oscar Mauricio Vélez-Terranova. 2022. "No Morphological Integration of Dorsal Profiles in the Araucanian Horse (Colombia)" Animals 12, no. 13: 1731. https://doi.org/10.3390/ani12131731
APA StyleSalamanca-Carreño, A., Parés-Casanova, P. M., Rangel-Pachón, D. E., Bentez-Molano, J., & Vélez-Terranova, O. M. (2022). No Morphological Integration of Dorsal Profiles in the Araucanian Horse (Colombia). Animals, 12(13), 1731. https://doi.org/10.3390/ani12131731







