Simple Summary
The use of decapods (such as lobsters and crabs) and cephalopods (such as octopuses and cuttlefish) by humans for food, experimentation and education (e.g., in zoos and aquariums) is on the increase. Growing evidence that these species have feelings and can experience emotions has highlighted the need for a tool to monitor the welfare of these species in captivity. This study adapted a welfare monitoring tool, the Animal Welfare Assessment Grid, that has been successfully used with a variety of mammal and bird species, for use with decapods and cephalopods. This tool was then trialed at a zoological institution (Marwell Zoo, UK) and, for the first time, a public aquarium (National Marine Aquarium, UK), with the intention of showing how data collected on invertebrates in a zoological environment can be both efficiently and easily applied to implement positive welfare. This study highlights how evaluating the welfare impact of management processes using animal-based indicators can lead to improved welfare outcomes.
Abstract
Consumer demand for invertebrates is on the rise as their numbers in the wild dwindle. However, with the growing conservation efforts of modern zoos and aquariums, and evidence from over 300 studies showing that invertebrates are capable of sentience, public interest, and moral concern for welfare of invertebrates have increased. The challenge for zoos and aquariums is in developing an objective and repeatable method for evaluating welfare that can be applied to invertebrates in zoological collections. Recently introduced into zoological collection management is the Animal Welfare Assessment Grid (AWAG). The AWAG helps to identify negative and positive welfare states, through assessing animal- and environmental-based indicators to make changes that lead to a better quality of life. Originally developed for the assessment of laboratory primates, the system has been successfully adapted to assess a variety of taxa across different environments, facilitated by the development of cloud-based software. In this study, the AWAG has been adapted to assess the cumulative lifetime experience of captive decapods and cephalopods at two different institutions, Marwell Zoo and National Marine Aquarium. This study has provided further evidence that the AWAG is adaptable and demonstrates the first time any objective scoring system has been successfully adapted for use in invertebrates. Through graphical representation, the results show that the AWAG identifies changes in welfare scores that can be attributed to specific events and can be used to predict the future vulnerability of species to welfare changes and suggest alternative management methods. This monitoring tool provides a versatile method of implementing practical welfare monitoring in zoos and aquariums.
1. Introduction
There is increasing global awareness of the concept of animal welfare, fueled by social media and resulting in growing public concern as evidenced by the increase in production of meat and dairy alternatives, reduction in the use of fur and leather in fashion, and boycotts of the animal entertainment and tourism industries [1,2,3,4]. These welfare concerns have historically been vertebrate-centric, likely resulting from a combination of: (1) our lack of physical similarity with invertebrates and thus our understanding of and ability to empathise with them; (2) invertebrates’ lack of physical characteristics associated with sentience in vertebrates; (3) the ‘disgust response’; and (4) the idea that small brains result in lack of cognition [5,6]. However, a growing body of evidence supporting the notion that some invertebrates do experience pain and suffering is having a profound effect on how the welfare of these species is considered [7].
Following Brexit, where all non-human vertebrates and invertebrates lost legal protection previously afforded to them under EU legislation, the UK government proposed the development of an ‘Animal Welfare (Sentience) Bill’ (from now on referred to as ‘The Bill’). Initally, The Bill planned to recognise all non-human vertebrates as sentient, resulting in all new government policies being required to consider vertebrate animal sentience during their development. Sentience is described by Broom [8] as ‘the ability to feel, perceive and experience’ and is thus inextricably linked with welfare. If an animal is capable of feeling pain and experiencing suffering, then that animal’s welfare can be compromised. Alternatively, their welfare can be positively affected by feelings of happiness, comfort and pleasure. Invertebrates make up 95% of animal life on Earth, and with cephalopod molluscs and decapod crustaceans (from now on referred to as cephalopods and decapods), considered to be the most intelligent and cognitively developed [9], both the public and scientific community argued for the inclusion of these species in The Bill. The extensive evidence gathered by Birch and colleagues [7], supporting sentience in cephalopods and decapods was key to the government’s decision to formally recognise in The Bill ‘any vertebrate other than homo sapiens, any cephalopod mollusc and any decapod crustacean’ [10] as sentient.
Decapod and Cephalopod use by humans, for food, experimentation and education (e.g., in zoos and) is on the increase [11]. For example, 121,000 tonnes of shellfish (including decapods of various species) landed in UK ports in 2020, an increase from 32,000 tonnes 80 years ago [12]. Worldwide cephalopod catches totaled around 3.6 million tonnes a year in 2017 and 2018, and although this is lower than previous years, this is not due to a lack of demand but reduction in stock, leading to plans for the first octopus farm to be opened by Nueva Pescanova in 2023 [13]. This, coupled with the formal acknowledgement, in the form of The Bill, that cephalopods and decapods are sentient, has identified the need for a welfare monitoring tool for these species in captivity.
Animal welfare has no singular definition, however, it is generally considered to be ‘the state of the animal as perceived by the animal itself, with regards to its attempts to cope with its environment’ [14], including its perception of both its physical and psychological health. Animal welfare assessments were initially designed for monitoring farm animal welfare but have since been developed for use with companion, laboratory, and exotic animals, and are becoming essential tools for animal carers due to increasing inclusion of the requirement for high welfare standards to conform to laws and legislation [15,16]. To date, most welfare monitoring tools have been mammal-centric with the gradual adaptation for other vertebrate taxa, but with few developments for invertebrates. This is an understandable consequence of previous lack of consensus regarding the sentience of invertebrates.
The Animal Welfare Assessment Grid (AWAG), a practical animal welfare monitoring tool based on the Five Domains [17], has been successfully trialled with a variety of taxa, including mammals and birds, in a wide range of environments [18,19,20]. Here we are evidencing the success of using the AWAG to objectively assess the welfare of invertebrates, specifically decapods and cephalopods, with the aim to promote the necessity for regular welfare assessment for these species/taxa in captive settings. The number of invertebrates utilised by humans per year vastly outweighs the number of vertebrates, thus the lack of a validated welfare assessment for these could result in untold amounts of suffering.
2. Materials and Methods
2.1. Study Subjects
As the paper aims to ascertain the ease of implementing objective welfare assessment for invertebrates, subjects of this study consisted of three species of decapod; Red-clawed Crayfish Cherax quadricarinatus at Marwell Zoo, UK (MZ), comprising approximately 108 individuals (n = 108), an individual shore-crab Carcinus maenas (n = 1) and a squat-lobster Galathea strigosa (n = 1) both housed at National Marine Aquarium, UK (NMA); and two species of cephalopod; a male cuttlefish Sepia officinalis (n = 1) and a female common octopus Octopus vulgaris (n = 1), both housed at NMA (see Figure 1).
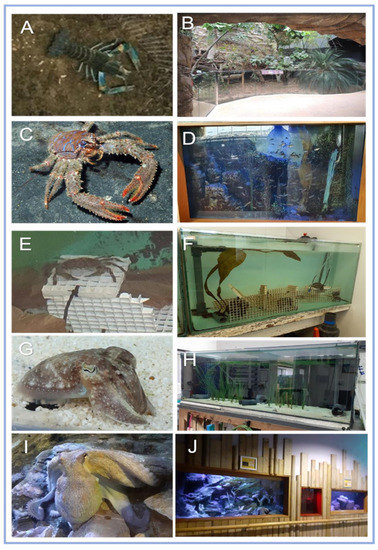
Figure 1.
Study subjects. (A) Red-clawed crayfish Cherax quadricarinatus, housed in (B) Marwell Zoo’s tropical house. (C) NMA’s squat -lobster Galathea strigosa housed in (D) Plymouth Sound 6 tank (PS-6). (E) NMA’s shore-crab Carcinus maenas, initially housed in Temperate Quarantine (TQ) and moved to (F) during the trial period. (G) NMA’s cuttlefish Sepia officinalis housed in (H) PS-3 with two male cuttlefish and NMA’s (I) common octopus Octopus vulgaris, had access to three tanks interconnected by tubes (J). (Photos provided by NMA staff, 2021).
2.2. Experimental Design
The decapod and cephalopod AWAG scoring templates used in this study were adapted from Wolfensohn et al. [19] and Justice et al. [18]. The AWAGs consist of 19 factors for decapod and 21 factors for cephalopod monitoring, divided into four parameters: physical, psychological, environmental, and procedural (described below). Each factor was scored incrementally from 1 to 10, with 1 being the best possible state relative to the health of the individual and 10 being the most detrimental (see [18,20] for methods). For this study, each factor was chosen using validated indicators of welfare identified from previous studies [21,22,23,24,25,26,27,28,29,30,31,32], and input was provided from zoo and aquarium staff and MZ’s veterinarian on current procedural methods for both taxa (shown in Table 1, Table 2, Table 3 and Table 4).

Table 1.
Physical parameter scoring for the AWAG assessment (Underlined—factors only assessed in decapods, Italics—factors assessed in cephalopods only).

Table 2.
Psychological parameter scoring for the AWAG assessment.

Table 3.
Environmental parameter scoring for the AWAG assessment (Underlined—factors assessed in decapods only).

Table 4.
Procedural parameter scoring for the AWAG assessment (Italics—factors assessed in cephalopods only).
The researcher, zoo staff, aquarium staff, and volunteers were trained to score the above species (Figure 1) for one hour daily, or three times a week (due to limited staff) as part of an altered management routine, during the trial period: 18 May to 11 August 2021. The scores were recorded with notes detailing events causing score fluctuations.
The AWAG factors were adapted for both individual and group assessment. Group assessments were carried out by randomizing the individuals observed, to reduce bias and to be representative of all individuals within the group. Using overnight video recording, throughout the trial period, the effect of contingent events was also evaluated in the crayfish enclosure (Figure 1B).
2.2.1. Physical Parameters
Four animal-based factors were assessed within the physical parameter class: general condition, activity level, presence of injury/observable clinical signs, and food intake (Table 1). Apart from minor modifications to factor definitions to account for aquatic conditions, the physical parameter class is similar to that scored by Justice et al. [18]. ‘General condition’ was assessed using visual inspection since zoos currently do not weigh their aquatic invertebrates [21], and in group assessments randomized observations were carried out. ‘Activity level’ was monitored to assess any significant changes as a result of stress or illness (omitting any changes resulting from reproductive activity); this proved useful in highlighting any undetected injury or unfavourable environmental changes; in group assessments of decapod invertebrates, the group was assessed as a whole.
In many aquatic invertebrates, it has been shown that feeding frequency is dependent on water quality (including temperature) [26,33]. Therefore, by monitoring food intake in aquatic species, it is possible to infer the presence of insufficient environmental parameters. This was assessed in both individuals and groups by monitoring the amount of food provided and the quantity of food leftover after a feeding period to establish an estimate of food intake at an individual level.
The cephalopod AWAG includes an alternative factor to ‘Presence of Injury’: observable clinical signs (including excessive inking, discolouration, and wounds). Clinical signs are defined by observations that require veterinary consultation; any ‘Observable clinical signs’ will be an indicator of negative welfare [21].
2.2.2. Psychological Parameters
Four animal-based factors were created within the psychological parameter class with the aim of assessing behavioural abnormalities: natural behaviour, abnormal behaviour, response to social disruption, and routine management (Table 2). With little to no veterinary procedures performed on decapods and cephalopods in zoological collections, and a lack of species welfare requirement information, assessing behavioural abnormalities provides an opportunity to monitor animal health, as behaviour can be observed from afar [28]. ‘Abnormal behaviour(s)’ are defined as behaviours that are distressing and maladaptive, examples of these include: erratic/aggressive behaviour, and ‘spinner’ behavior—the inability to control orientation when swimming and location in the water column, as species have a characteristic place in the water tank [21].
The risk of contra-specific ‘Social disruption’ is relatively high in zoos/aquariums [34,35]. This factor was adapted to assess how well the species coped with the presence of staff. ‘Training’ and ‘Response to catching event’ were omitted from this study as neither apply to the study taxa, instead these factors were replaced by ‘Routine management’, a mandatory form of care in zoological settings (including routine handling, husbandry, transport, and tank cleaning) [32]. This factor allows for monitoring and reviewing the degree of disturbance caused by staff.
2.2.3. Environmental Parameters
Seven factors were assessed within the environmental parameter class: water quality, housing/enclosure, group size, enclosure complexity, nutrition, accessibility, and contingent events (Table 3). ‘Water quality’ is a new indicator, added because of its significant value to aquatic animal welfare assessment. Preferred water quality stipulations are species-specific [21]. Monitoring water quality can implement positive welfare by providing means for growth, reproduction, and obtaining resources (including water temperature, salinity, ammonia concentration, dissolved oxygen concentration, and pH levels), and allows for proactive rather than reactive actions as insufficient water quality will cause stress and disease [21].
‘Housing/enclosure’ is species-specific, and considers the size of the enclosure, lighting, shelter, drainage, noise levels, and substrate, and how these allow behaviours, group size, and structure to replicate that of the natural environment. An excessive group size limits resources and shelter availability; this can increase aggressive behaviour and competitive exclusion, as shown in crayfish [36]. Enclosure complexity is monitored to assess the species engagement with all aspects of its environment. Previous studies question whether we should prioritise reducing states of boredom for cognitive species such as octopuses by focusing on enhancing resources in their enclosures [37].
In the UK the primary purpose of zoos and aquariums is to exhibit and preserve animal life for the purpose of conservation, academia, and public interest [33]. Many of the daily activities related to fulfilling this purpose can impact the welfare of the animals held by these institutions. The impact of such activities is scored under ‘Contingent events’. For example, at the time of this study, some aquariums use decapods as educational aids. This may involve housing decapods in rock pools, removing decapods from the water and allowing children to feel their shells. In some cases, time kept out of water can vary, with a guideline of ‘just a few minutes’ [28]—this has been proven to have a detrimental impact on animal welfare [38].
2.2.4. Procedural Parameters
Five factors were assessed within this parameter class: isolation/restraint, effect of intervention, impact of veterinary procedures, change in daily routine, and sedation/anaesthesia (Table 4). Apart from modifications to account for lack of veterinary interventions in both taxa and the aquatic setting, this section did not differ from the factors scored by Justice et al. [18]. Although rare, close-up clinical examinations of these species require manual restraint in the shallows, or out of the water. In group assessments, scores were based on the percentage of individuals that required examination in comparison to the enclosure group size. Sedation is sometimes required for the examination for larger cephalopod species. Sedation/anaesthesia was only assessed in cephalopods as this is rarely used in clinical examination of decapods and veterinary procedures for these species are infrequent [28].
2.3. Welfare Analysis
The crayfish at MZ were assessed daily. The shore-crab and cuttlefish were scored daily for 37 days and the squat lobster and common octopus at NMA were scored daily for 36 and 38 days, respectively, throughout the 86-day assessment period. For each species, average daily scores were calculated for all factors within each parameter, using the AWAG software. At the end of each day, the average daily parameter scores were plotted on a radar chart to generate a convex polygon for each day. The area of the convex polygon equated to the cumulative welfare assessment score (CWAS), an overall welfare score. Collectively, the daily CWAS scores were used to present the welfare state over the total trial period. Days on which the assessment was not completed were averaged to show trends in the data.
3. Results
3.1. Welfare Observations
Summaries of the AWAG scores, including how individual parameter scores vary over time, daily radar charts and CWAS graphs over the 86-day trial period for each species are shown below. Figure 2 shows the average daily AWAG parameter scores across both taxa for the entire study. General trends for parameter scores remain under a cumulative factor score of 6.00, with high variability across species. There are trends within each taxa group: low (i.e., optimal) average scores for psychological (≤1.20) and procedural (1.00) parameters, and increased (i.e., suboptimal) average scores of physical (≤1.84) and environmental (≤1.51) parameters within the decapod taxa. The cephalopod taxa show low average scores for the environmental (≤1.44) and procedural (≤1.09) parameters but increased physical (≤1.70) and psychological (≤1.73) average parameter scores.
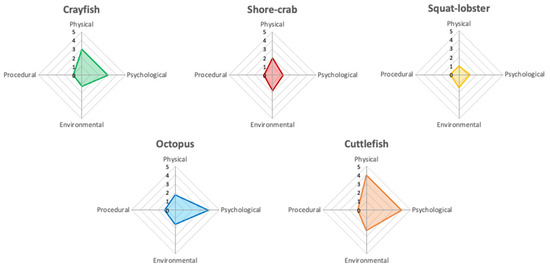
Figure 2.
Averaged animal welfare assessment grids of the decapod (top row) and cephalopod (bottom row) study subjects. The radar charts represent the average scores for physical, psychological, environmental, and procedural parameter class over the study period on a scale from 1 to 10, with 1 being the best possible score and 10 the most detrimental. The axes in the figure are adjusted to increase clarity of the average score for each parameter class for each species. The area of the polygon presented on the radar chart equates to the CWAS value for the complete study period.
The increased physical parameter scores shown across both taxa similarly stem from a change in general condition because of presence of an injury (‘observable clinical signs’ for the cephalopod taxa). Within the decapods, change in physical condition was attributed to a change in the environment (little to no change occurred with the squat lobster over the trial period). Within the cephalopods, the octopus showed an increase in the average score of the psychological parameter when the physical parameter was affected. The cuttlefish was affected by all but the procedural parameter.
The cumulative welfare scores for each species were plotted against time, as shown in Figure 3. Each species displays no similarity in pattern overtime, but similar events occur that result in similar reactions, peaks (i.e., suboptimal) in the welfare score at different intensities.
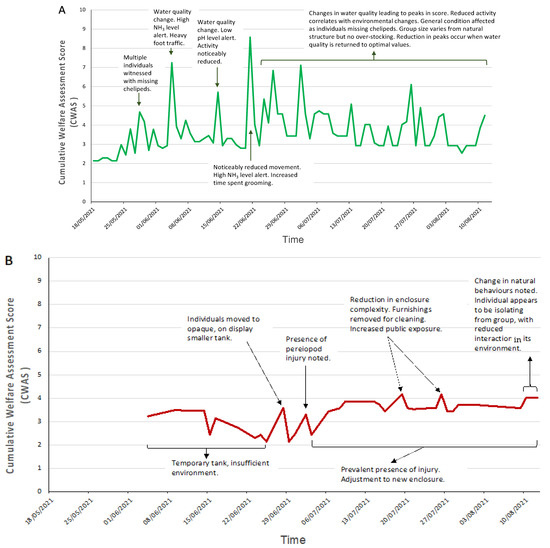
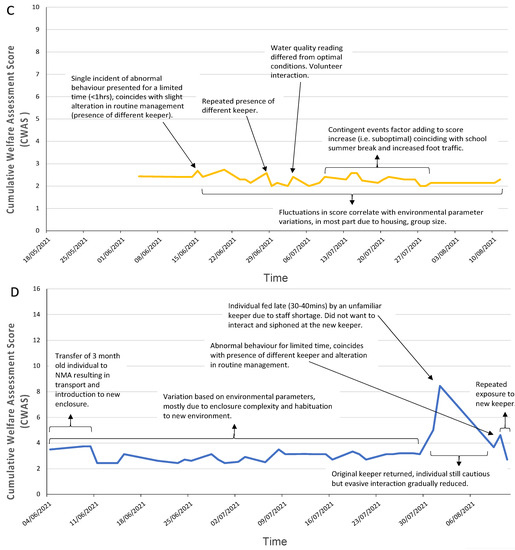
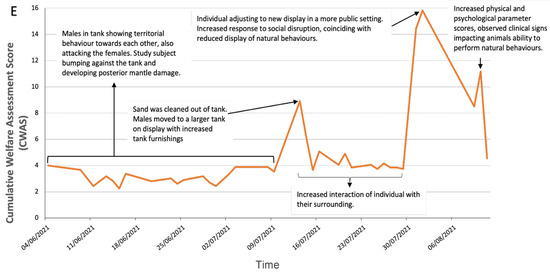
Figure 3.
Daily cumulative welfare assessment scores over time for each of the three decapod species (A) crayfish, (B) shore crab, (C) squat lobster, and the two cephalopod species (D) common octopus and (E) cuttlefish. Annotation of the graphs indicates events that occurred around the time the peak in welfare score was noted (increased value indicates reduction in welfare). A line of general trends is displayed for the days that data were not collected.
3.2. Decapod Cumulative Welfare Lifetime Experience
The CWAS plotted against time for MZs crayfish showed large variation throughout the entirety of the study (mean: 3.68; CWAS range: min 2.18–max 8.58). Figure 3 highlights the events that occurred around the time of the increased scores. Figure 4 shows the breakdown of the CWAS into each of the four parameters. Continual assessment of the crayfish revealed that trends in activity levels and general condition closely matched environmental parameter changes, more specifically water quality and group size changes. The highest average scores presented (7.24, 8.58, 7.12) were a result of the presence of injury and fluctuations in NH3 and/or pH levels.
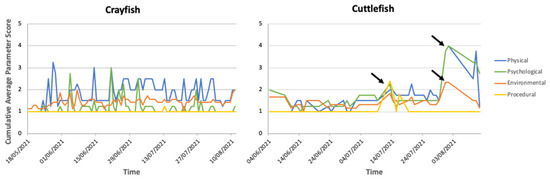
Figure 4.
Daily average parameter welfare assessment scores over time for MZ crayfish. Each line presents one of the four assessed parameters: physical, psychological, environmental, procedural, on a scale of 1 to 10. In the figure, the axes are adjusted based on the range of the daily average parameter scores. The black arrows indicate the noticeable parameter changes between two events that incur greater (i.e., suboptimal) welfare scores presented with the cuttlefish assessment.
The shore crab also presented considerable variation in CWAS over time (mean: 3.35; CWAS range: min 2.14–max 4.17). The high scores in the data (3.58, 4.17) correlate with environmental parameter changes (due in most part to housing and enclosure complexity), as the individual was moved from an off-show holding area to on-show display tank, and physical parameters (due to presence of injury). The score then remained elevated.
The squat lobster average scores remain close to optimal (mean: 2.29; CWAS range: min 2.00–max 2.73) with all parameters scoring below 2, the data retain a similar shape throughout the assessment.
3.3. Cephalopod Cumulative Welfare Lifetime Experience
Scores were taken on 38 days of the trial period for NMA’s common octopus (mean: 3.23; CWAS range: min 2.44–max 8.46). Figure 3D shows that a peak (8.46) in score is attributed to a change of keeper and late feeding. There is a gradual reduction in the welfare score (3.67) when the original keeper returns. Figure 5 highlights the differences in parameter scores of both events.
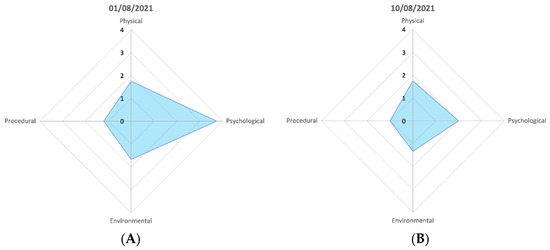
Figure 5.
Individual animal welfare assessment grid of the common octopus, the parameter scores of the two greatest peaks in the data. (A) shows the parameter scores when a change of keeper and late feeding occurred, (B) shows the presence of the same different keeper but a normal feeding time. The shape of the polygon in each is the same but at different magnitudes of change.
Figure 3E shows that the cuttlefish presented higher CWAS scores (mean: 4.7; CWAS range: min 2.44–max 15.82). The data present three substantial peaks (Figure 3B), the first (8.90) is initiated by cleaning of and movement to a larger tank to improve welfare by reducing aggressive behaviour. Mode of transport was not recorded. The second peak (15.82) coincides with an increased public presence. The third peak (11.16) is a result of prolonged presence of posterior mantle burn altering the individual’s behaviour.
4. Discussion
The AWAG was successfully adapted to monitor the welfare of the invertebrate species observed in the study. This is the first time this system has been used to assess invertebrates and, as far as the authors are aware, the first time any objective welfare scoring system has been successfully adapted for use in invertebrates. This is a significant result given the need to monitor the welfare of those invertebrate species evidenced as sentient and consequently included within the new Animal Welfare (Sentience) Bill.
Given the relative paucity of information relating to the needs of invertebrate species to maintain good welfare, the study findings also highlight several key areas relating to the welfare of decapods and cephalopods in captivity. This is well illustrated by how the cumulative welfare score for red-clawed crayfish responds to changes in water quality. The trend produced by the AWAG shows various points where increasing scores (indicating a deterioration in welfare) correlate with deviations in water quality parameters outside of the species preferred range. The sensitivity of crayfish to poor water quality is well documented [39]. The behavioural changes observed during the study, including movement into the shallows or out of the water altogether, are consistent with the response of wild crayfish to poor water quality in the environment [30]. This may indicate a negative welfare impact due to changes in water quality. As a resource-based measure it is not a direct measure of the animal’s welfare state, however it is a reasonable proxy given the difficulties in measuring the direct impact of water quality on the physiology of the crayfish.
These observations and the corresponding change in cumulative welfare score help confirm the validity of using the system for welfare monitoring in this species. These findings also suggest another potential use for the AWAG. Given this example shows the AWAG is capable of detecting changes in welfare due to behaviours observed both in captivity and the wild, it may be possible to use the AWAG as a predictive tool for assessing the welfare of wild animals where parameter values (for example, for water quality) are known. This would be an interesting area for further investigation.
The cumulative welfare score for red-clawed crayfish also reflected changes in group size due to the impact of increased aggression within the group. This aggression was likely to be due to competition over limited resources within the captive environment. This provides valuable information for animal managers to help prevent poor welfare within crayfish colonies. The cumulative welfare score may act as an early indicator of increased competition over resources. Management interventions, such as provision of the lacking resource or reduction in group numbers, can then be made before welfare is significantly compromised. This is also a good example of how the AWAG can be used to assess group welfare and supports the findings of previous studies [18]. When assessing large group sizes, a focus on individuals can be both impractical and detract from group level factors (such as the level of competition within the group) which may have a significant positive or negative impact on welfare. This has potential implications for monitoring the welfare of any colony living species, especially where colonies are comprised of large numbers of individuals.
The cumulative welfare scores for the shore crab highlighted the importance of the physical environment to decapod welfare. This is reflected in the Housing and Environmental Complexity factors under the Environment parameter in the AWAG. Both factors are scored relative to the resources available and complexity in the wild. The cumulative welfare score shows a significant difference between the shore crab off-show holding facilities and the on-show display tank. This highlights the difference in environmental complexity when comparing on-show and off-show areas. This difference is a frequent finding in zoos and aquaria and often occurs due to a heavy emphasis on functionality (such as ease of cleaning) in the historical design of off-show areas. Improving the interactive complexity of off-show areas should contribute to improving overall welfare [40]. Interestingly, there was little variation in the cumulative welfare score for the squat lobster. This suggests that where this species is maintained in a consistent, appropriate environment with minimal intervention or change, good welfare can be achieved.
Scheel [41] suggests that octopuses can recognise individual people and may be able to form a relationship with their carers. The findings of this study also support this assertion, as there are clear changes in the cumulative welfare score which correlate with the presence or absence of familiar people. This has implications for when staff changes, or institutional transfers occur as the absence of a familiar carer may be detrimental to welfare. Similarly, the findings also suggest that human interaction with octopus in captivity may be a source of positive welfare. This is consistent with findings in other vertebrate species and perhaps more evidence for sentience [42]. The cumulative welfare scores for cuttlefish reflected aggressive behaviour due to competition over territory. The negative welfare impact seen here, relating to competition over resources, is similar to the observation made for the red-clawed crayfish. This reinforces the importance of ensuring an appropriate level of resource availability for all individuals held in group situations or where an individual perceives competition from a co-terminus species or human carer. Transfer to another enclosure also resulted in a negative welfare impact. This procedure is analogous to the transportation of vertebrate species between different holding areas or institutions, a process previously highlighted as having a negative impact on animal welfare [43]. The welfare impact of transportation of sentient invertebrates would be another area worthy of further investigation and evaluation. Interestingly, an improvement in welfare was noted when the cuttlefish was introduced to the new enclosure, suggesting that activities such as exploration of complex environments may be beneficial to the welfare of this species.
Several limitations of using the AWAG were noted as a result of the study. The number of cephalopods used in the study is too low to be confident that the system works in all cases. However, the information is included here given its importance due to the lack of data for cephalopod welfare assessment. Next, it was noted that the scoring system assumes that signs of fear in response to an aversive stimulus, in this case moving away or hiding from keepers during routine events, suggests a negative welfare impact, when it may be an indicator of better welfare than those that do not move or hide, possibly because of physical impairment, however in the scores recorded such an impact of physical impairment on behaviour was not seen. Additionally, when monitoring at group level some factors had to be estimated for practical reasons, for example food intake. Finally, care needs to be taken not to assume a direct link between cumulative welfare score and environmental parameters for all species, for example some crayfish are capable of tolerating changes in water quality.
As found in previous studies, the findings show that the AWAG can be used in different institutional settings. Although the system has been used in several zoos, the authors believe this is the first trial of the system in a public aquarium. As others have also noted though, the system cannot be used to compare different taxa or institutions due to the difference in factors scored [18]. However, the flexibility of the system allows different sources of information to be used to generate cumulative welfare scores. This, combined with the availability of user-friendly software, makes the AWAG practical to use for continuous monitoring by animal carers.
5. Conclusions
To conclude, this study has shown that the AWAG can be successfully adapted and applied to decapods and cephalopods in zoo and aquaria environments, presenting for the first time an objective scoring system for use in invertebrates. The AWAG can easily identify changes in welfare scores that can be attributed to specific events, thus presenting a practical method of assessing the welfare of invertebrates. The importance of this monitoring tool is that it highlights changes in cumulative welfare trends, providing evidence for prompt management interventions that can promote the positive welfare of species in zoological collections.
With invertebrates, insects in particular, being hailed as the ‘food of the future’, and the growing evidence for sentience, it is crucial that we continue to expand our methods for accurately assessing invertebrate welfare.
Author Contributions
Conceptualization, D.F.; methodology, T.M.N. and D.F.; formal analysis, T.M.N.; investigation, T.M.N.; writing—original draft preparation, T.M.N., D.F., S.J.S. and W.S.M.J.; writing—review and editing, T.M.N., D.F., W.S.M.J., S.W. and S.J.S.; supervision, D.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Formal review was not required by Imperial College London due to all data collection being purely observational. The work has been through and cleared by Marwell Zoo’s ethical review process.
Informed Consent Statement
Not applicable, as this research did not involve humans.
Data Availability Statement
Both adapted AWAG templates and all data gathered and used are available at: [38]-hNtlAf5abqx8aS7JZda?dl=0; Software used in this study is available at: https://zoo.awag.org.uk/ (accessed on 20 May 2022).
Acknowledgments
The authors would like to thank the staff at Marwell Zoo: Dan Garrick, Ali Reynolds, Kimberley Goodfield, Tom Wells and Robert Haines, for supporting and facilitating data collection. We would also like to thank our collaborators at National Marine Aquarium: Heather Williams, Alix Sterling, Sarah Kunzig, Peggy Holliday, Andrew McFarlane, and Sabine Graf for providing scores for both decapods and cephalopods.
Conflicts of Interest
The authors declare no conflict of interest.
References
- AHDB. Understanding Consumers Attitudes to Animal Welfare|AHDB. 2021. Available online: https://ahdb.org.uk/news/consumer-insight-understanding-consumers-attitudes-to-animal-welfare (accessed on 10 January 2022).
- Broom, D.M. Animal Welfare: An Aspect of Care, Sustainability, and Food Quality Required by the Public. J. Vet. Med. Educ. 2010, 37, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Shaheer, I.; Carr, N.; Insch, A. Rallying support for animal welfare on Twitter: A tale of four destination boycotts. Tour. Recreat. Res. 2021, 10, 1–15. [Google Scholar] [CrossRef]
- Aji, A.P. The role of social media in shaping the animal protection movement in Indonesia. J. Studi Komun. Indones. J. Commun. Stud. 2019, 3, 389. [Google Scholar] [CrossRef]
- Mikhalevich, I.; Powell, R. Minds without spines: Evolutionarily inclusive animal ethics. Anim. Sentience 2020, 5, 1. [Google Scholar] [CrossRef]
- Proctor, H. Animal Sentience: Where Are We and Where Are We Heading? Animals 2012, 2, 628–639. [Google Scholar] [CrossRef]
- Birch, J.; Burn, C.; Schnell, A.; Browning, H.; Crump, A. Review of the Evidence of Sentience in Cephalopod Molluscs and Decapod Crustaceans; LSE Consulting: London, UK, 2021. [Google Scholar]
- Broom, D.M. Sentience and Animal Welfare; CABI: Wallingford, UK, 2014. [Google Scholar]
- Winlow, W.; Di Cosmo, A. Editorial: Sentience, Pain, and Anesthesia in Advanced Invertebrates. Front. Physiol. 2019, 10. [Google Scholar] [CrossRef]
- Ares, E. Animal Welfare (Sentience) Bill; House of Commons Library, UK Parliament: London, UK, 2022. Available online: https://researchbriefings.files.parliament.uk/documents/CBP-9423/CBP-9423.pdf (accessed on 21 May 2022).
- O’Brien, C.E.; Roumbedakis, K.; Winkelmann, I.E. The Current State of Cephalopod Science and Perspectives on the Most Critical Challenges Ahead From Three Early-Career Researchers. Front. Physiol. 2018, 9. [Google Scholar] [CrossRef]
- UK Sea Fisheries Annual Statistics Report 2020—GOV.UK. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1020837/UK_Sea_Fisheries_Statistics_2020_-_AC_checked.pdf (accessed on 21 May 2022).
- Marshall, C. The World’s First Octopus Farm-Should it Go Ahead? BBC News: London, UK, 2021. [Google Scholar]
- Broom, D.M. The scientific assessment of animal welfare. Appl. Anim. Behav. Sci. 1988, 20, 5–19. [Google Scholar] [CrossRef]
- Hawkins, P.; Morton, D.B.; Burman, O.; Dennison, N.; Honess, P.; Jennings, M.; Lane, S.; Middleton, V.; Roughan, J.V.; Wells, S.; et al. A guide to defining and implementing protocols for the welfare assessment of laboratory animals: Eleventh report of the BVAAWF/FRAME/RSPCA/UFAW Joint Working Group on Refinement. Lab. Anim. 2011, 45, 1–13. [Google Scholar] [CrossRef]
- Prescott, M.J.; Leach, M.C.; Truelove, M.A. Harmonisation of welfare indicators for macaques and marmosets used or bred for research. F1000 Res. 2022, 11, 272. [Google Scholar] [CrossRef]
- Mellor, D.; Beausoleil, N. Extending the “Five Domains” model for animal welfare assessment to incorporate positive welfare states. Anim. Welf. 2015, 24, 241–253. [Google Scholar] [CrossRef]
- JJustice, W.S.M.; O’Brien, M.F.; Szyszka, O.; Shotton, J.; Gilmour, J.E.M.; Riordan, P.; Wolfensohn, S. Adaptation of the animal welfare assessment grid (AWAG) for monitoring animal welfare in zoological collections. Vet. Rec. 2017, 181, 143. [Google Scholar] [CrossRef]
- Wolfensohn, S.; Shotton, J.; Bowley, H.; Davies, S.; Thompson, S.; Justice, W. Assessment of Welfare in Zoo Animals: Towards Optimum Quality of Life. Animals 2018, 8, 110. [Google Scholar] [CrossRef]
- Brouwers, S.; Duchateau, M.J. Feasibility and validity of the Animal Welfare Assessment Grid to monitor the welfare of zoo-housed gorillas Gorilla gorilla gorilla. J. Zoo Aquar. Res. 2021, 9, 208–217. [Google Scholar] [CrossRef]
- Fiorito, G.; Affuso, A.; Basil, J.; Cole, A.; De Girolamo, P.; D’Angelo, L.; Dickel, L.; Gestal, C.; Grasso, F.; Kuba, M.; et al. Guidelines for the Care and Welfare of Cephalopods in Research—A consensus based on an initiative by CephRes, FELASA and the Boyd Group. Lab. Anim. 2015, 49, 1–90. [Google Scholar] [CrossRef]
- Rosewarne, P.J.; Mortimer, R.J.G.; Dunn, A.M. Size-dependent impacts of the endangered white-clawed crayfish (Austropotamobius pallipes) (Lereboullet) on the littoral community. Knowl. Manag. Aquat. Ecosyst. 2013, 6, 1–10. [Google Scholar] [CrossRef]
- Wood, C.M.; Rogano, M.S. Physiological Responses to Acid Stress in Crayfish (Orconectes): Haemolymph Ions, Acid–Base Status, and Exchanges with the Environment. Can. J. Fish. Aquat. Sci. 1986, 43, 1017–1026. [Google Scholar] [CrossRef]
- Wood, T.C.; Smiley, P.C.S.; Gillespie, R.B.; Gonzalez, J.M.; King, K.W. Injury frequency and severity in crayfish communities as indicators of physical habitat quality and water quality within agricultural headwater streams. Environ. Monit. Assess. 2020, 192, 1–17. [Google Scholar] [CrossRef]
- Mariappan, P.; Balasundaram, C.; Schmitz, B. Decapod crustacean chelipeds: An overview. J. Biosci. 2000, 25, 301–313. [Google Scholar] [CrossRef]
- Stumpf, L.; Greco, L.S.L. Compensatory Growth in Juveniles of Freshwater Redclaw Crayfish Cherax quadricarinatus Reared at Three Different Temperatures: Hyperphagia and Food Efficiency as Primary Mechanisms. PLoS ONE 2015, 10, e0139372. [Google Scholar] [CrossRef]
- Meade, M.E.; Watts, S.A. Toxicity of ammonia, nitrite, and nitrate to juvenile Australian crayfish, Cherax quadricarinatus. Oceanogr. Lit. Rev. 1996, 9, 946. [Google Scholar]
- Smith, S.J.; (Marwell Wildlife, Winchester, UK). Personal communication, 2022.
- Carder, G. A preliminary investigation into the welfare of lobsters in the UK. Anim. Sentience 2017, 2, 19. [Google Scholar] [CrossRef]
- Peay, S. Guidance on Habitat for White-Clawed Crayfish; Environment Agency Technical Report W1-067/TR 2002 Species Recovery Programme for English Nature, Kendal, UK and Environment Agency, Wallingford, UK. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/290346/sw1-067-tr-e-e.pdf (accessed on 21 May 2022).
- Ponte, G.; Andrews, P.; Galligioni, V.; Pereira, J.; Fiorito, G. Cephalopod Welfare, Biological and Regulatory Aspects: An EU Experience. In The Welfare of Invertebrate Animals; Carere, C., Mather, J., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 209–228. [Google Scholar] [CrossRef]
- Queensland Government. Redclaw Crayfish Aquaculture. 1995. Available online: https://www.business.qld.gov.au/_designs/content/guide-printing2?parent=56963&SQ_DESIGN_NAME=print_layout (accessed on 20 August 2021).
- Fitzgibbon, Q.P.; Simon, C.J.; Smith, G.G.; Carter, C.G.; Battaglene, S.C. Temperature dependent growth, feeding, nutritional condition and aerobic metabolism of juvenile spiny lobster, Sagmariasus verreauxi. Comp. Biochem. Physiol. Mol. Integr. Physiol. 2017, 207, 13–20. [Google Scholar] [CrossRef]
- Ward, S.J.; Melfi, V. Keeper-Animal Interactions: Differences between the Behaviour of Zoo Animals Affect Stockmanship. PLoS ONE 2015, 10, e0140237. [Google Scholar] [CrossRef] [PubMed]
- Carlstead, K. A comparative approach to the study of Keeper–Animal Relationships in the zoo. Zoo Biol. 2009, 28, 589–608. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, H.H., III. Decapoda, 2nd ed.; Academic Press: Cambridge, MA, USA, 2001; p. 955. [Google Scholar]
- Carere, C.; Mather, J.A. Why Invertebrate Welfare? In The Welfare of Invertebrate Animals; Carere, C., Mather, J., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–5. [Google Scholar] [CrossRef]
- Elwood, R.W.; Barr, S.; Patterson, L. Pain and stress in crustaceans? Appl. Anim. Behav. Sci. 2009, 118, 128–136. [Google Scholar] [CrossRef]
- Svobodová, J.; Douda, K.; Štambergová, M.; Picek, J.; Vlach, P.; Fischer, D. The relationship between water quality and indigenous and alien crayfish distribution in the Czech Republic: Patterns and conservation implications. Aquat. Conserv. Mar. Freshw. Ecosyst. 2012, 22, 776–786. [Google Scholar] [CrossRef]
- EAZA Animal Welfare Webinar–Sabrina Brando & Jon Coe–Confronting Back-of-House Traditions. 2021. Available online: www.youtube.com/watch?v=G3_Rv4M9wRA (accessed on 21 May 2022).
- Scheel, D. Octopuses in wild and domestic relationships. Soc. Sci. Inf. 2018, 57, 403–421. [Google Scholar] [CrossRef]
- Smith, J.J. Human–animal relationships in zoo-housed orangutans (P. abelii) and gorillas (G. g. gorilla): The effects of familiarity. Am. J. Primatol. 2014, 76, 942–955. [Google Scholar] [CrossRef]
- Laaksonen, S.; Jokelainen, P.; Pusenius, J.; Oksanen, A. Is transport distance correlated with animal welfare and carcass quality of reindeer (Rangifer tarandus tarandus)? Acta Vet. Scand. 2017, 59, 17. [Google Scholar] [CrossRef][Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).