Lafora Disease and Alpha-Synucleinopathy in Two Adult Free-Ranging Moose (Alces alces) Presenting with Signs of Blindness and Circling
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
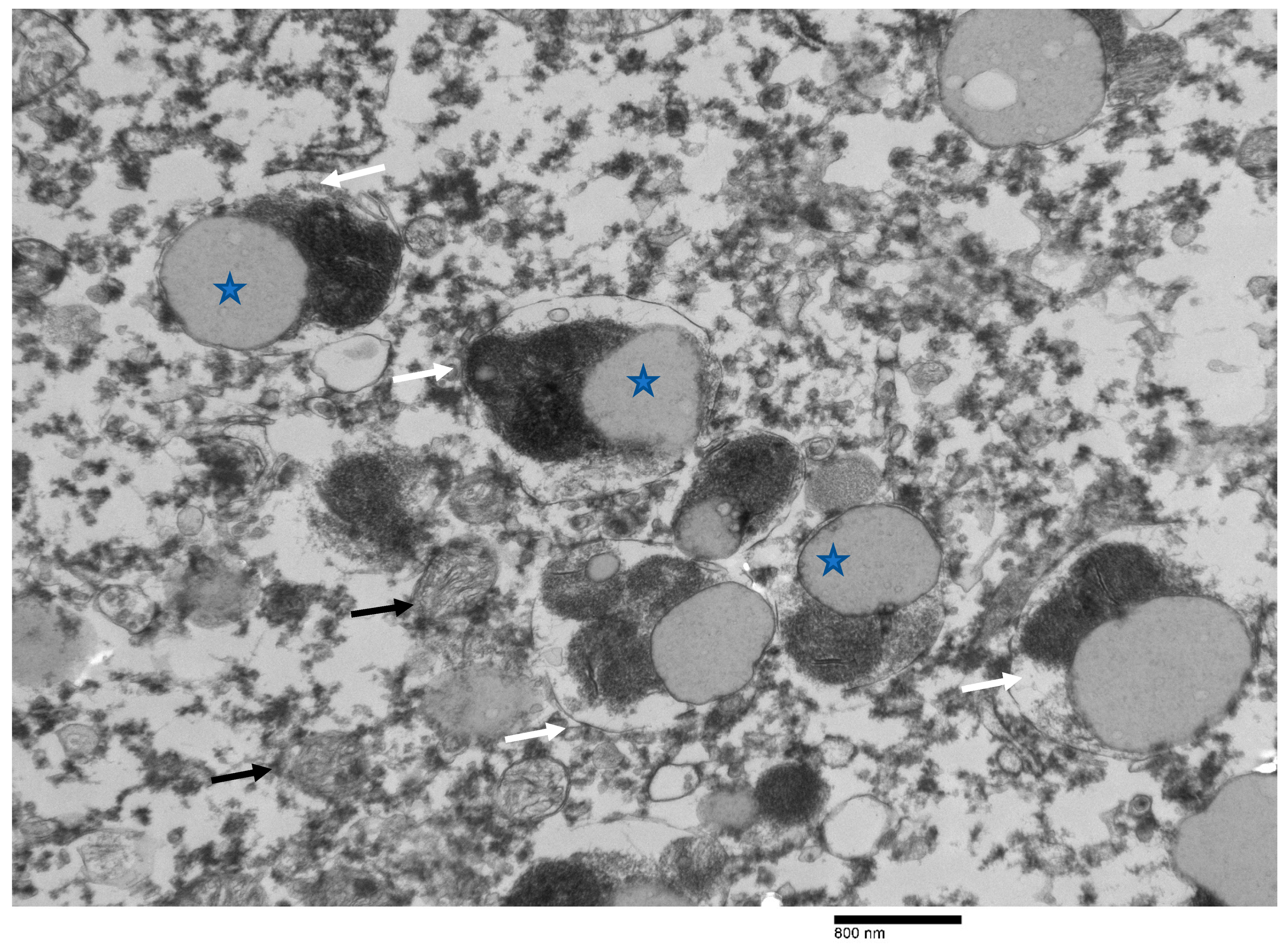
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lafora, G.R. Über das Vorkommen amyloider Körperchen im Innern der Ganglienzellen. Virchows Arch. Pathol. Anat. Physiol. Klin. Med. 1911, 205, 295–303. [Google Scholar] [CrossRef]
- Gentry, M.S.; Guinovart, J.J.; Minassian, B.A.; Roach, P.J.; Serratosa, J.M. Lafora disease offers a unique window into neuronal glycogen metabolism. J. Biol. Chem. 2018, 293, 7117–7125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, E.M.; Bulman, D.E.; Paterson, A.D.; Turnbull, J.; Andermann, E.; Andermann, F.; Rouleau, G.A.; Delgado-Escueta, A.V.; Scherer, S.W.; Minassian, B.A. Genetic mapping of a new Lafora progressive myoclonus epilepsy locus (EPM2B) on 6p22. J. Med. Genet. 2003, 40, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi-Fakhari, D.; Wahlster, L.; Hoffmann, G.F.; Kolker, S. Emerging role of autophagy in pediatric neurodegenerative and neurometabolic diseases. Pediatr. Res. 2014, 75, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, S.; Delgado-Escueta, A.V.; Suzuki, T.; Francheschetti, S.; Riggio, C.; Avanzini, G.; Rabinowicz, A.; Bohlega, S.; Bailey, J.; Alonso, M.E.; et al. Genotype-phenotype correlations for EPM2A mutations in Lafora’s progressive myoclonus epilepsy: Exon 1 mutations associate with an early-onset cognitive deficit subphenotype. Hum. Mol. Genet. 2002, 11, 1263–1271. [Google Scholar] [CrossRef] [Green Version]
- Minassian, B.A.; Ianzano, L.; Delgado-Escueta, A.V.; Scherer, S.W. Identification of new and common mutations in the EPM2A gene in Lafora disease. Neurology 2000, 54, 488–490. [Google Scholar] [CrossRef]
- Minassian, B.A. Lafora’s disease: Towards a clinical, pathologic, and molecular synthesis. Pediatr. Neurol. 2001, 25, 21–29. [Google Scholar] [CrossRef]
- Pondrelli, F.; Muccioli, L.; Licchetta, L.; Mostacci, B.; Zenesini, C.; Tinuper, P.; Vignatelli, L.; Bisulli, F. Natural history of Lafora disease: A prognostic systematic review and individual participant data meta-analysis. Orphanet J. Rare Dis. 2021, 16, 362. [Google Scholar] [CrossRef]
- Franceschetti, S.; Gambardella, A.; Canafoglia, L.; Striano, P.; Lohi, H.; Gennaro, E.; Ianzano, L.; Veggiotti, P.; Sofia, V.; Biondi, R.; et al. Clinical and genetic findings in 26 Italian patients with Lafora disease. Epilepsia 2006, 47, 640–643. [Google Scholar] [CrossRef]
- Striano, P.; Zara, F.; Turnbull, J.; Girard, J.M.; Ackerley, C.A.; Cervasio, M.; De Rosa, G.; Del Basso-De Caro, M.L.; Striano, S.; Minassian, B.A. Typical progression of myoclonic epilepsy of the Lafora type: A case report. Nat. Clin. Pract. Neurol. 2008, 4, 106–111. [Google Scholar] [CrossRef]
- Janeway, R.; Ravens, J.R.; Pearce, L.A.; Odor, D.L.; Suzuki, K. Progressive myoclonus epilepsy with Lafora inclusion bodies. I. Clinical, genetic, histopathologic, and biochemical aspects. Arch. Neurol. 1967, 16, 565–582. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, F.; Santangelo, R.; Gasparo-Rippa, P.; Santoro, M. Biopsy findings (cerebral cortex, muscle, skin) in Lafora disease. Acta Neurol. 1987, 9, 81–94. [Google Scholar]
- Sakai, M.; Austin, J.; Witmer, F.; Trueb, L. Studies in myoclonus epilepsy (Lafora body form). II. Polyglucosans in the systemic deposits of myoclonus epilepsy and in corpora amylacea. Neurology 1970, 20, 160–176. [Google Scholar] [CrossRef] [PubMed]
- Shirozu, M.; Hashimoto, M.; Tomimatsu, M.; Nakazawa, Y.; Anraku, S.; Nagata, M. Lafora disease diagnosed by skin biopsy. Kurume Med. J. 1985, 32, 311–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gambetti, P.; Di Mauro, S.; Hirt, L.; Blume, R.P. Myoclonic epilepsy with lafora bodies. Some ultrastructural, histochemical, and biochemical aspects. Arch. Neurol. 1971, 25, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, S.; Karpati, G. Ultrastructural findings in Lafora disease. Ann. Neurol. 1981, 10, 63–64. [Google Scholar] [CrossRef]
- Brewer, M.K.; Putaux, J.-L.; Rondon, A.; Uittenbogaard, A.; Sullivan, M.A.; Gentry, M.S. Polyglucosan body structure in Lafora disease. Carbohydr. Polym. 2020, 240, 116260. [Google Scholar] [CrossRef]
- Mochel, F.; Schiffmann, R.; Steenweg, M.E.; Akman, H.O.; Wallace, M.; Sedel, F.; Laforêt, P.; Levy, R.; Powers, J.M.; Demeret, S.; et al. Adult polyglucosan body disease: Natural History and Key Magnetic Resonance Imaging Findings. Ann. Neurol. 2012, 72, 433–441. [Google Scholar] [CrossRef] [Green Version]
- Ward, T.L.; Valberg, S.J.; Adelson, D.L.; Abbey, C.A.; Binns, M.M.; Mickelson, J.R. Glycogen branching enzyme (GBE1) mutation causing equine glycogen storage disease IV. Mamm. Genome Off. J. Int. Mamm. Genome Soc. 2004, 15, 570–577. [Google Scholar] [CrossRef]
- Cavanagh, J.B. Corpora-amylacea and the family of polyglucosan diseases. Brain Res. Brain Res. Rev. 1999, 29, 265–295. [Google Scholar] [CrossRef]
- Suzuki, Y.; Kamiya, S.; Ohta, K.; Suu, S. Lafora-like bodies in a cat. Case report suggestive of glycogen metabolism disturbances. Acta Neuropathol. 1979, 48, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Simmons, M.M. Lafora disease in the cow? J. Comp. Pathol. 1994, 110, 389–401. [Google Scholar] [CrossRef]
- Gredal, H.; Berendt, M.; Leifsson, P.S. Progressive myoclonus epilepsy in a beagle. J. Small Anim. Pract. 2003, 44, 511–514. [Google Scholar] [CrossRef] [PubMed]
- Honnold, S.P.; Schulman, F.Y.; Bauman, K.; Nelson, K. Lafora’s-like disease in a fennec fox (Vulpes zerda). J. Zoo Wildl. Med. 2010, 41, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Loupal, G. A storage disease in a parakeet (Nymphicus hollandicus) morphologically similar to the systemic myoclonic disease (Lafora disease) in man and dog. Zent. Vet. A 1985, 32, 502–511. [Google Scholar] [CrossRef]
- von Klopmann, T.; Ahonen, S.; Espadas-Santiuste, I.; Matiasek, K.; Sanchez-Masian, D.; Rupp, S.; Vandenberghe, H.; Rose, J.; Wang, T.; Wang, P.; et al. Canine Lafora Disease: An Unstable Repeat Expansion Disorder. Life 2021, 11, 689. [Google Scholar] [CrossRef]
- Mari, L.; Comero, G.; Mueller, E.; Kuehnlein, P.; Kehl, A. NHLRC1 homozygous dodecamer expansion in a Newfoundland dog with Lafora disease. J. Small Anim. Pract. 2021, 62, 1030–1032. [Google Scholar] [CrossRef]
- Kehl, A.; Cizinauskas, S.; Langbein-Detsch, I.; Mueller, E. NHLRC1 dodecamer expansion in a Welsh Corgi (Pembroke) with Lafora disease. Anim. Genet. 2019, 50, 413–414. [Google Scholar] [CrossRef]
- Hajek, I.; Kettner, F.; Simerdova, V.; Rusbridge, C.; Wang, P.; Minassian, B.A.; Palus, V. NHLRC1 repeat expansion in two beagles with Lafora disease. J. Small Anim. Pract. 2016, 57, 650–652. [Google Scholar] [CrossRef] [Green Version]
- Sainsbury, R. DNA screening for Lafora’s disease in miniature wire-haired dachshunds. Vet. Rec. 2011, 169, 292. [Google Scholar] [CrossRef]
- Bradbury, J. Canine epilepsy gene mutation identified. Lancet Neurol. 2005, 4, 143. [Google Scholar] [CrossRef]
- Gabor, L.J.; Srivastava, M. Polyglucosan inclusions (Lafora bodies) in a gray-headed flying fox (Pteropus poliocephalus). J. Vet. Diagn. Investig. 2010, 22, 303–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamir, A.N. Spontaneous lesions in aged captive raccoons (Procyon lotor). J. Am. Assoc. Lab. Anim. Sci. 2011, 50, 322–325. [Google Scholar] [PubMed]
- Barrientos, L.; Maiolini, A.; Hani, A.; Jagannathan, V.; Leeb, T. NHLRC1 dodecamer repeat expansion demonstrated by whole genome sequencing in a Chihuahua with Lafora disease. Anim. Genet. 2019, 50, 118–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chambers, J.K.; Thongtharb, A.; Shiga, T.; Azakami, D.; Saito, M.; Sato, M.; Morozumi, M.; Nakayama, H.; Uchida, K. Accumulation of Laforin and Other Related Proteins in Canine Lafora Disease With EPM2B Repeat Expansion. Vet. Pathol. 2018, 55, 543–551. [Google Scholar] [CrossRef] [Green Version]
- Bras, I.C.; Xylaki, M.; Outeiro, T.F. Mechanisms of alpha-synuclein toxicity: An update and outlook. Prog. Brain Res. 2020, 252, 91–129. [Google Scholar] [CrossRef]
- Gómez-Benito, M.; Granado, N.; García-Sanz, P.; Michel, A.; Dumoulin, M.; Moratalla, R. Modeling Parkinson’s Disease With the Alpha-Synuclein Protein. Front. Pharmacol. 2020, 11, 356. [Google Scholar] [CrossRef]
- Sorrentino, Z.A.; Giasson, B.I. The emerging role of alpha-synuclein truncation in aggregation and disease. J. Biol. Chem. 2020, 295, 10224–10244. [Google Scholar] [CrossRef]
- Stefanis, L. α-Synuclein in Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2012, 2, a009399. [Google Scholar] [CrossRef] [Green Version]
- Sergi, C.M. Vitamin D and Primary Ciliary Dyskinesia: A Topic to Be Further Explored. Appl. Sci. 2021, 11, 3818. [Google Scholar] [CrossRef]
- Carson, F. Histotechnology: A Self-Instructional Text, 2nd ed; ASCP Pres: Chicago, IL, USA, 1997. [Google Scholar]
- Yokoi, S.; Austin, J.; Witmer, F.; Sakai, M. Studies in myoclonus epilepsy (Lafora body form). I. Isolation and preliminary characterization of Lafora bodies in two cases. Arch. Neurol. 1968, 19, 15–33. [Google Scholar] [CrossRef] [PubMed]
- Almaguer-Melian, W.; Vallejo, A.; Ramirez, M.; Capdevila, V.; Rosillo-Marti, J.C.; Bergado-Rosado, J.A. Comparative study of bilateral lesions in the entorhinal cortex and in the fimbria fornix. Rev. Neurol. 2003, 37, 619–622. [Google Scholar] [PubMed]
- Miller, V.M.; Best, P.J. Spatial correlates of hippocampal unit activity are altered by lesions of the fornix and entorhinal cortex. Brain Res. 1980, 194, 311–323. [Google Scholar] [CrossRef]
- Possin, K.L. Visual spatial cognition in neurodegenerative disease. Neurocase 2010, 16, 466–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maguire, E.A.; Gadian, D.G.; Johnsrude, I.S.; Good, C.D.; Ashburner, J.; Frackowiak, R.S.; Frith, C.D. Navigation-related structural change in the hippocampi of taxi drivers. Proc. Natl. Acad. Sci. USA 2000, 97, 4398–4403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alisauskaite, N.; Beckmann, K.; Dennler, M.; Zolch, N. Brain proton magnetic resonance spectroscopy findings in a Beagle dog with genetically confirmed Lafora disease. J. Vet. Intern. Med. 2020, 34, 1594–1598. [Google Scholar] [CrossRef]
- Webb, A.A.; McMillan, C.; Cullen, C.L.; Boston, S.E.; Turnbull, J.; Minassian, B.A. Lafora disease as a cause of visually exacerbated myoclonic attacks in a dog. Can. Vet. J. 2009, 50, 963–967. [Google Scholar]
- Stent, A.; Gosbell, M.; Tatarczuch, L.; Summers, B.A. Giant axonal neuropathy-like disease in an Alexandrine parrot (Psittacula eupatria). J. Vet. Diagn. Investig. 2015, 27, 611–615. [Google Scholar] [CrossRef] [Green Version]
- Kamiya, S.; Suzuki, Y. Polyglucosan bodies in the brain of the cat. J. Comp. Pathol. 1989, 101, 263–267. [Google Scholar] [CrossRef]
- Sullivan, M.A.; Nitschke, S.; Steup, M.; Minassian, B.A.; Nitschke, F. Pathogenesis of Lafora Disease: Transition of Soluble Glycogen to Insoluble Polyglucosan. Int. J. Mol. Sci. 2017, 18, 1743. [Google Scholar] [CrossRef] [Green Version]
- Turnbull, J.; Tiberia, E.; Striano, P.; Genton, P.; Carpenter, S.; Ackerley, C.A.; Minassian, B.A. Lafora disease. Epileptic Disord 2016, 18, 38–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turnbull, J.; DePaoli-Roach, A.A.; Zhao, X.; Cortez, M.A.; Pencea, N.; Tiberia, E.; Piliguian, M.; Roach, P.J.; Wang, P.; Ackerley, C.A.; et al. PTG depletion removes Lafora bodies and rescues the fatal epilepsy of Lafora disease. PLoS Genet. 2011, 7, e1002037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pederson, B.A.; Turnbull, J.; Epp, J.R.; Weaver, S.A.; Zhao, X.; Pencea, N.; Roach, P.J.; Frankland, P.W.; Ackerley, C.A.; Minassian, B.A. Inhibiting glycogen synthesis prevents Lafora disease in a mouse model. Ann. Neurol. 2013, 74, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, J.; Epp, J.R.; Goldsmith, D.; Zhao, X.; Pencea, N.; Wang, P.; Frankland, P.W.; Ackerley, C.A.; Minassian, B.A. PTG protein depletion rescues malin-deficient Lafora disease in mouse. Ann. Neurol. 2014, 75, 442–446. [Google Scholar] [CrossRef]
- Swain, L.; Key, G.; Tauro, A.; Ahonen, S.; Wang, P.; Ackerley, C.; Minassian, B.A.; Rusbridge, C. Lafora disease in miniature Wirehaired Dachshunds. PLoS ONE 2017, 12, e0182024. [Google Scholar] [CrossRef] [Green Version]
- Heitkotter, H.; Linderman, R.E.; Cava, J.A.; Woertz, E.N.; Mastey, R.R.; Summerfelt, P.; Chui, T.Y.; Rosen, R.B.; Patterson, E.J.; Vincent, A.; et al. Retinal alterations in patients with Lafora disease. Am. J. Ophthalmol. Case Rep. 2021, 23, 101146. [Google Scholar] [CrossRef]
- Vincent, A.; Macrì, A.; Tumber, A.; Koukas, N.; Ahonen, S.; Striano, P.; Minassian, B. Ocular phenotype and electroretinogram abnormalities in Lafora disease: A “window to the brain”. Neurology 2018, 91, 137–139. [Google Scholar] [CrossRef]
- Sánchez Romano, J.; Leijon, M.; Hagström, Å.; Jinnerot, T.; Rockström, U.K.; Tryland, M. Chlamydia pecorum Associated With an Outbreak of Infectious Keratoconjunctivitis in Semi-domesticated Reindeer in Sweden. Front. Vet. Sci. 2019, 6, 14. [Google Scholar] [CrossRef]
- Dias-Alves, A.; Cabezón, O.; Borel, N.; López-Olvera, J.R.; Mentaberre, G.; Lavín, S.; Fernández Aguilar, X. Molecular Detection and Identification of Chlamydiaceae in the Eyes of Wild and Domestic Ruminant Hosts from Northern Spain. Pathogens 2021, 10, 383. [Google Scholar] [CrossRef]
- Hedberg-Oldfors, C.; Oldfors, A. Polyglucosan storage myopathies. Mol. Asp. Med. 2015, 46, 85–100. [Google Scholar] [CrossRef]
- Hansen, D.; Ling, H.; Lashley, T.; Holton, J.L.; Warner, T.T. Review: Clinical, neuropathological and genetic features of Lewy body dementias. Neuropathol. Appl. Neurobiol. 2019, 45, 635–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kövari, E.; Horvath, J.; Bouras, C. Neuropathology of Lewy body disorders. Brain Res. Bull. 2009, 80, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Shahmoradian, S.H.; Lewis, A.J.; Genoud, C.; Hench, J.; Moors, T.E.; Navarro, P.P.; Castaño-Díez, D.; Schweighauser, G.; Graff-Meyer, A.; Goldie, K.N.; et al. Lewy pathology in Parkinson’s disease consists of crowded organelles and lipid membranes. Nature Neurosci. 2019, 22, 1099–1109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chia, S.J.; Tan, E.K.; Chao, Y.X. Historical Perspective: Models of Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 2464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dickson, D.W.; Crystal, H.; Mattiace, L.A.; Kress, Y.; Schwagerl, A.; Ksiezak-Reding, H.; Davies, P.; Yen, S.H. Diffuse Lewy body disease: Light and electron microscopic immunocytochemistry of senile plaques. Acta Neuropathol. 1989, 78, 572–584. [Google Scholar] [CrossRef]
- Zucca, F.A.; Vanna, R.; Cupaioli, F.A.; Bellei, C.; De Palma, A.; Di Silvestre, D.; Mauri, P.; Grassi, S.; Prinetti, A.; Casella, L.; et al. Neuromelanin organelles are specialized autolysosomes that accumulate undegraded proteins and lipids in aging human brain and are likely involved in Parkinson’s disease. Npj Parkinson’s Dis. 2018, 4, 17. [Google Scholar] [CrossRef]
- Xu, S.; Chan, P. Interaction between Neuromelanin and Alpha-Synuclein in Parkinson’s Disease. Biomolecules 2015, 5, 1122–1142. [Google Scholar] [CrossRef]
- Xuan, Q.; Xu, S.L.; Lu, D.H.; Yu, S.; Zhou, M.; Uéda, K.; Cui, Y.Q.; Zhang, B.Y.; Chan, P. Increased expression of α-synuclein in aged human brain associated with neuromelanin accumulation. J. Neural Transm. 2011, 118, 1575–1583. [Google Scholar] [CrossRef]
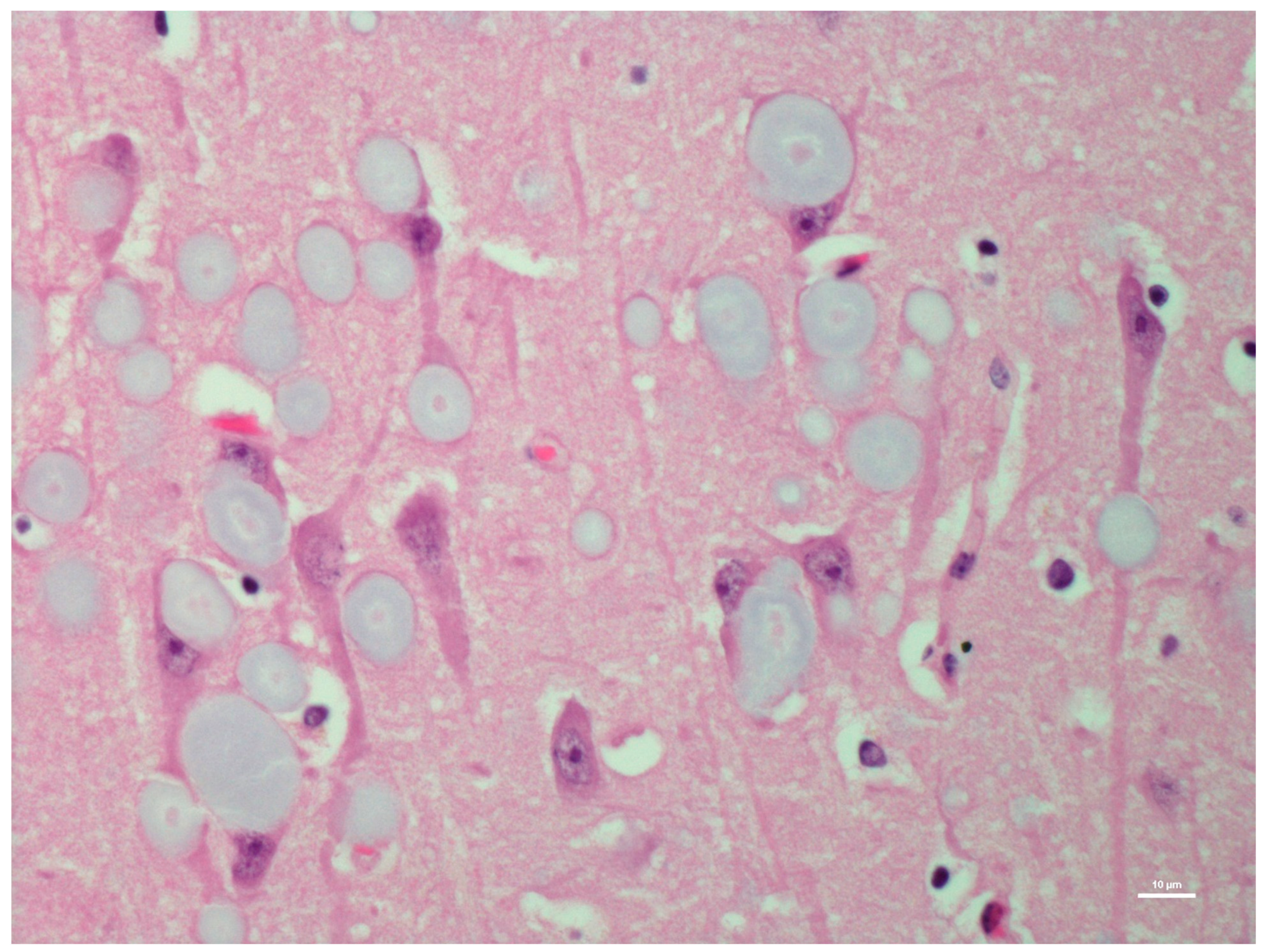
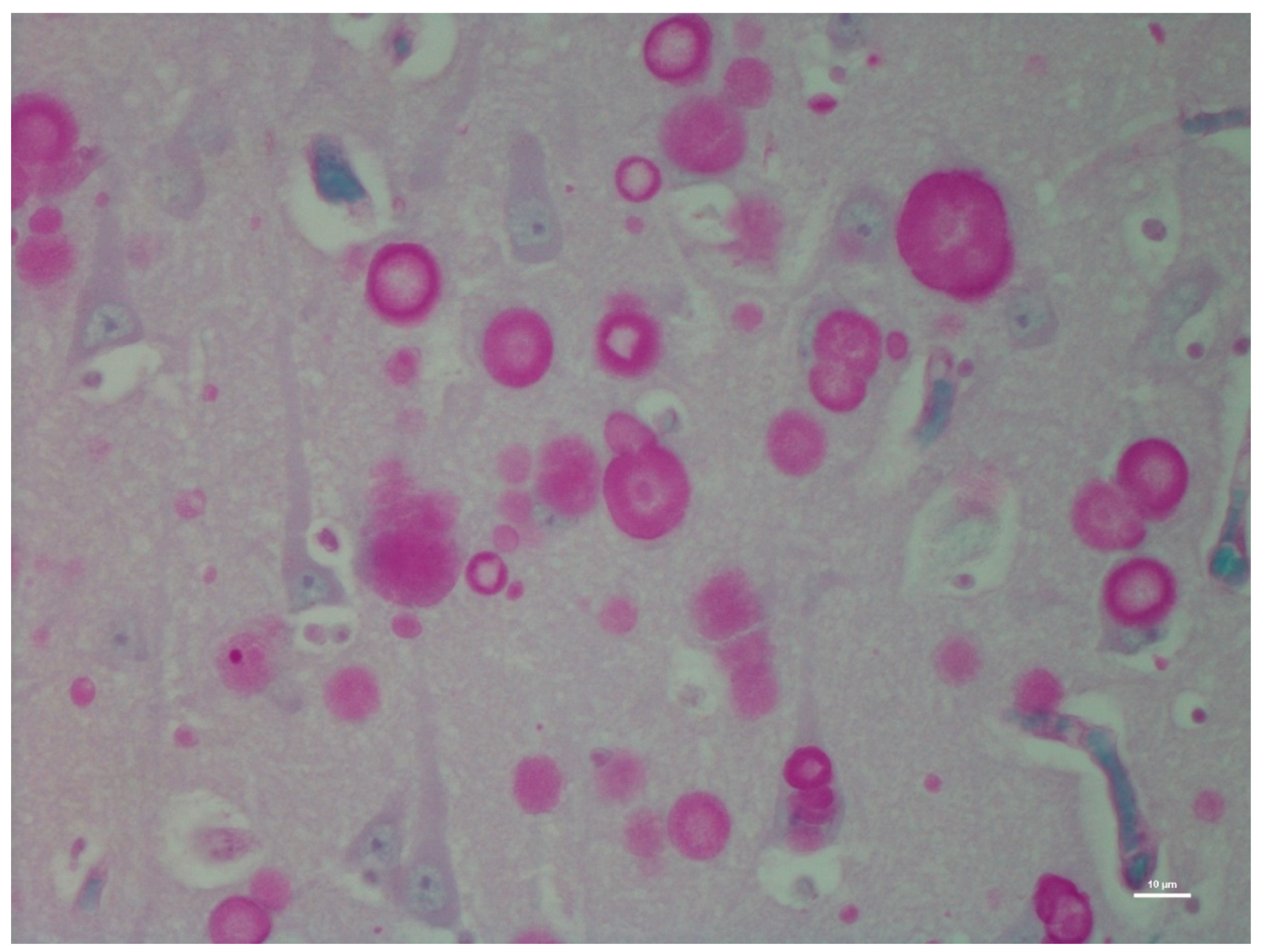
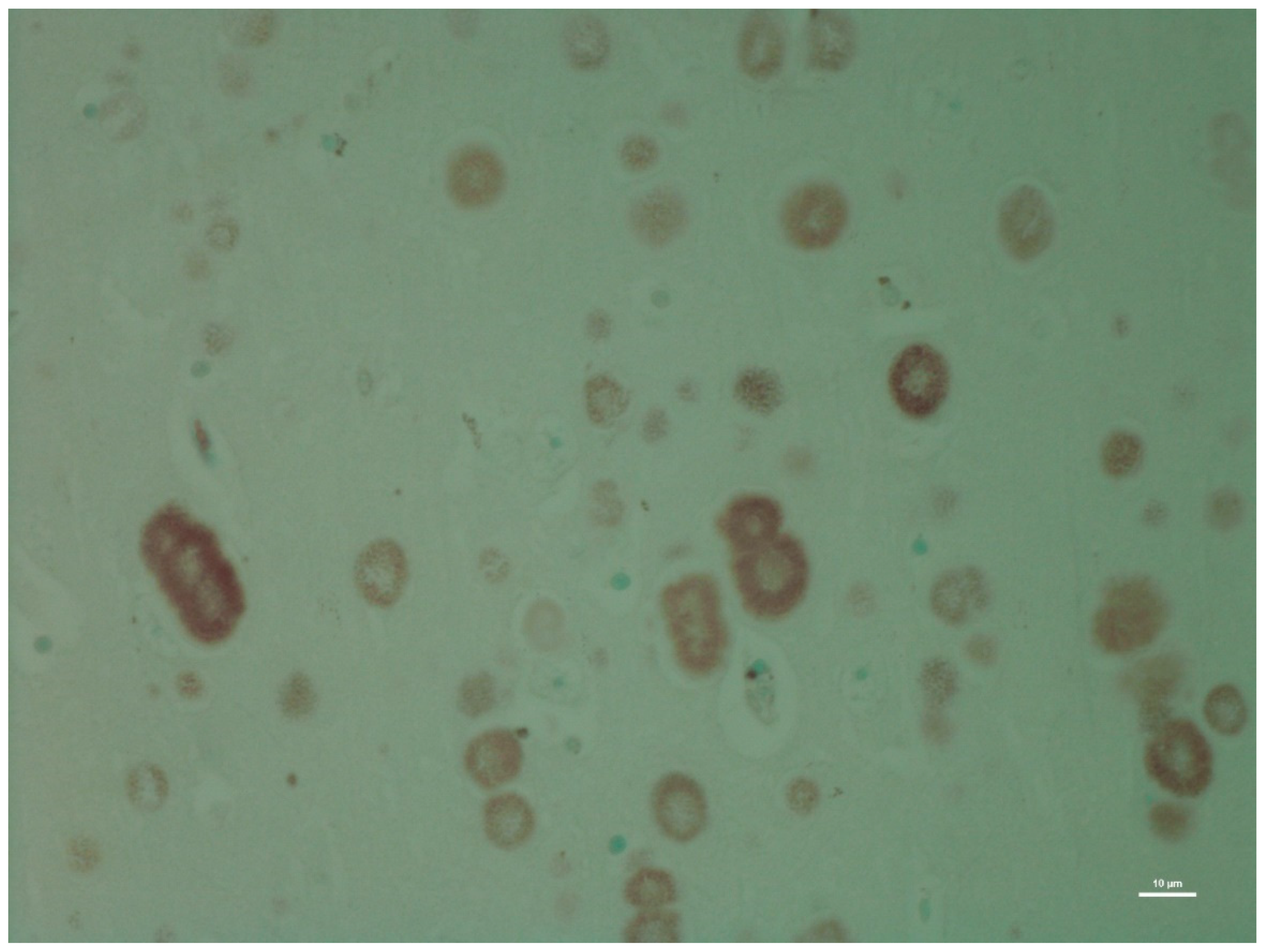
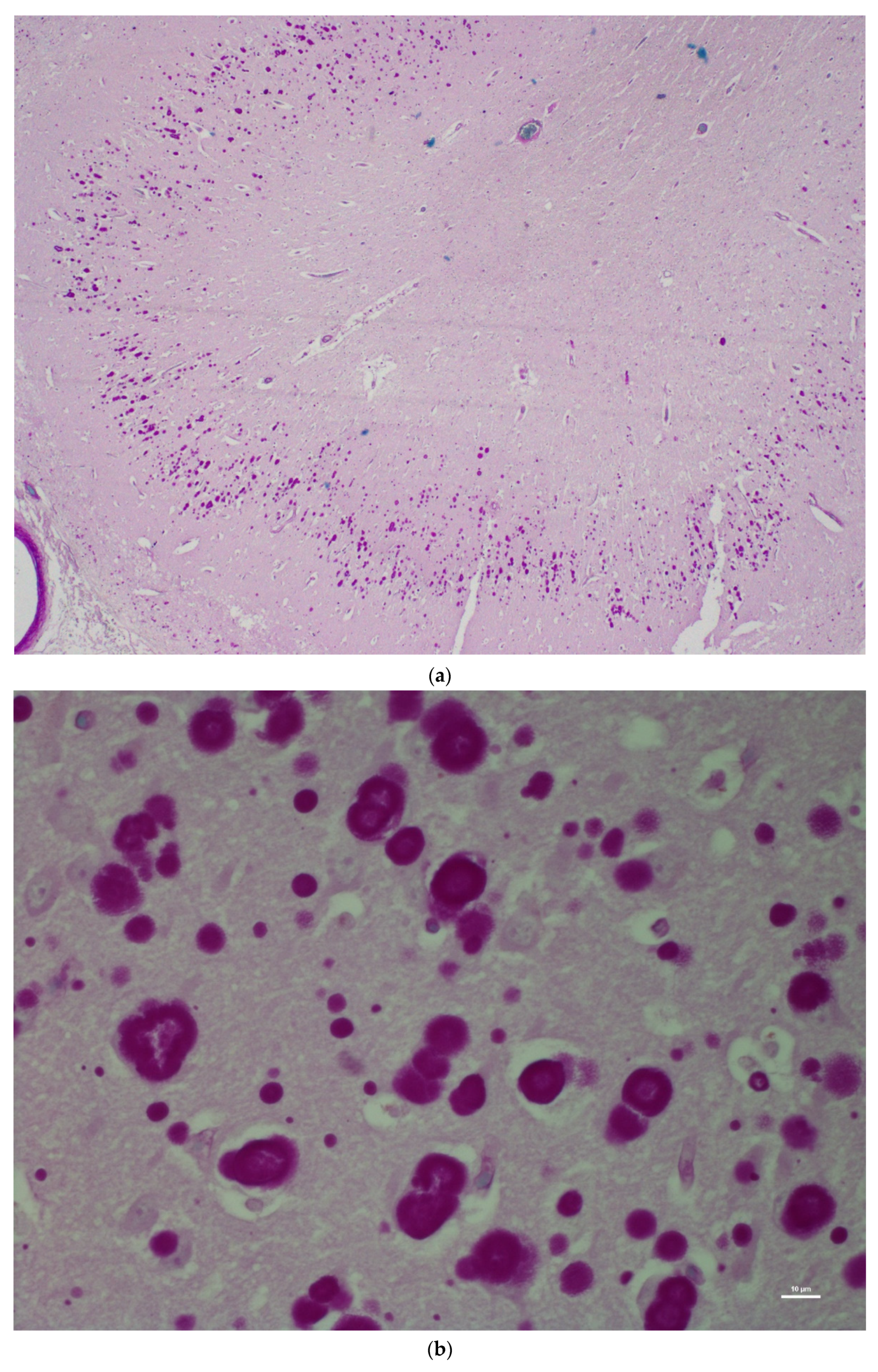
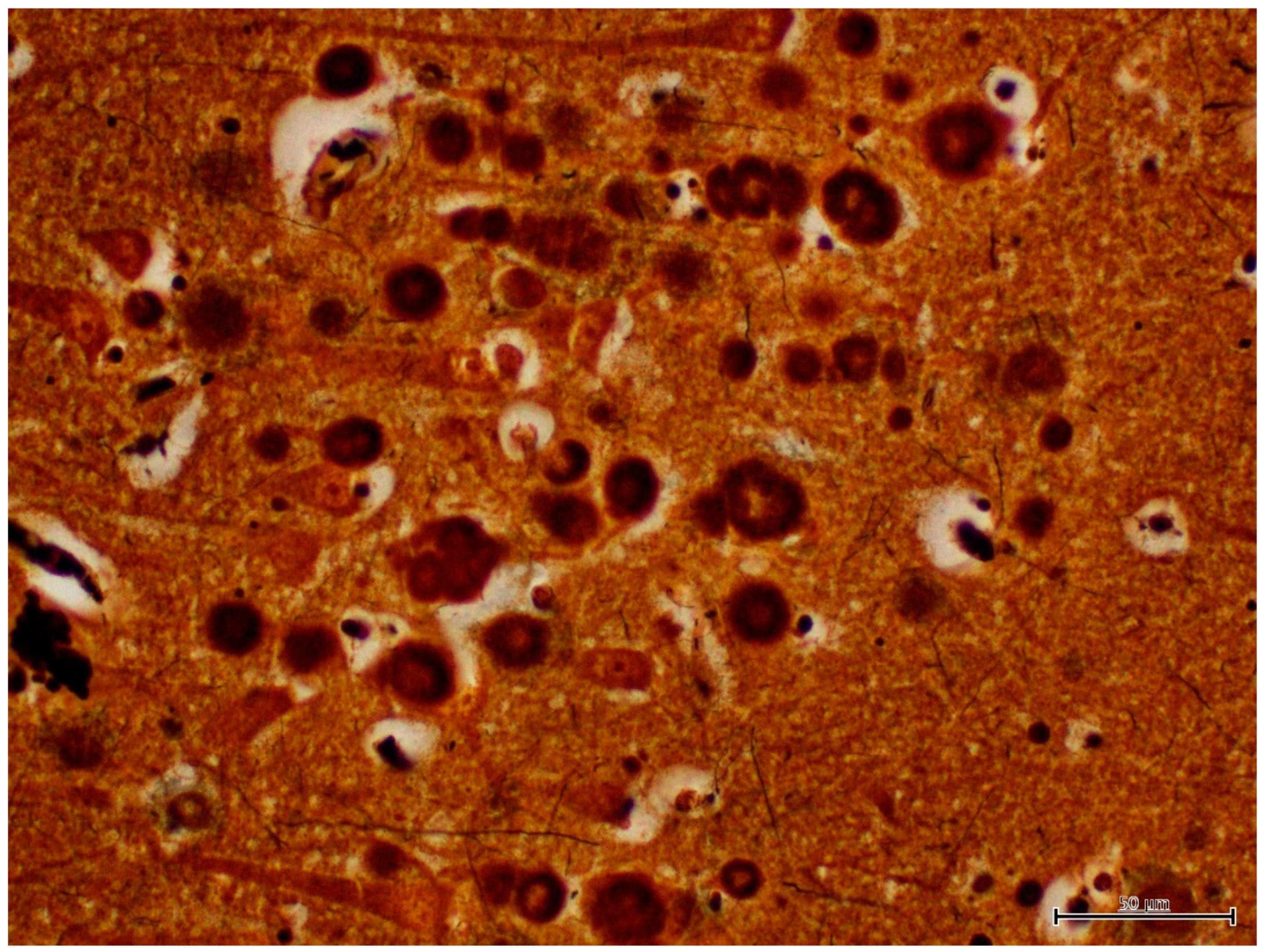
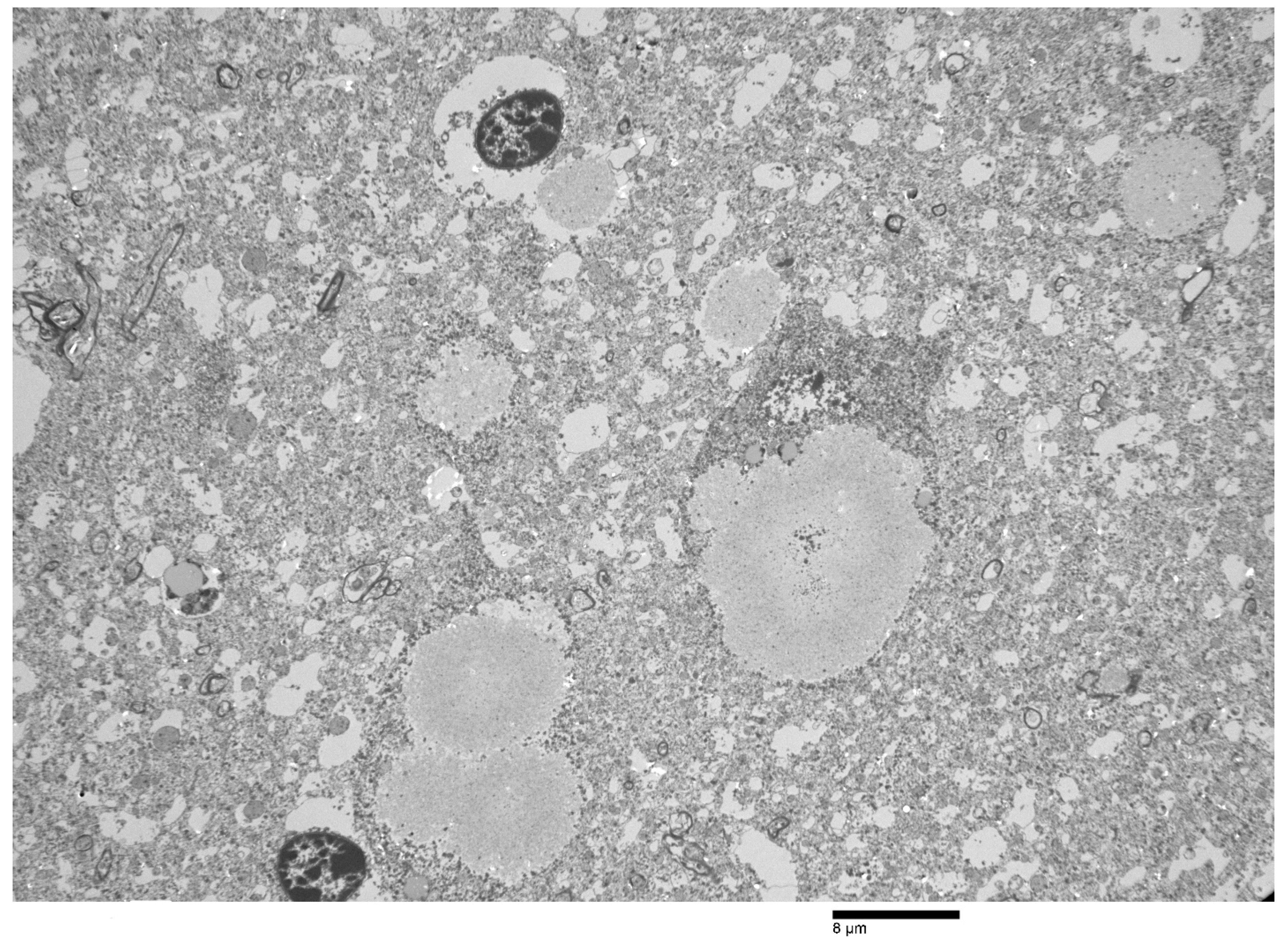
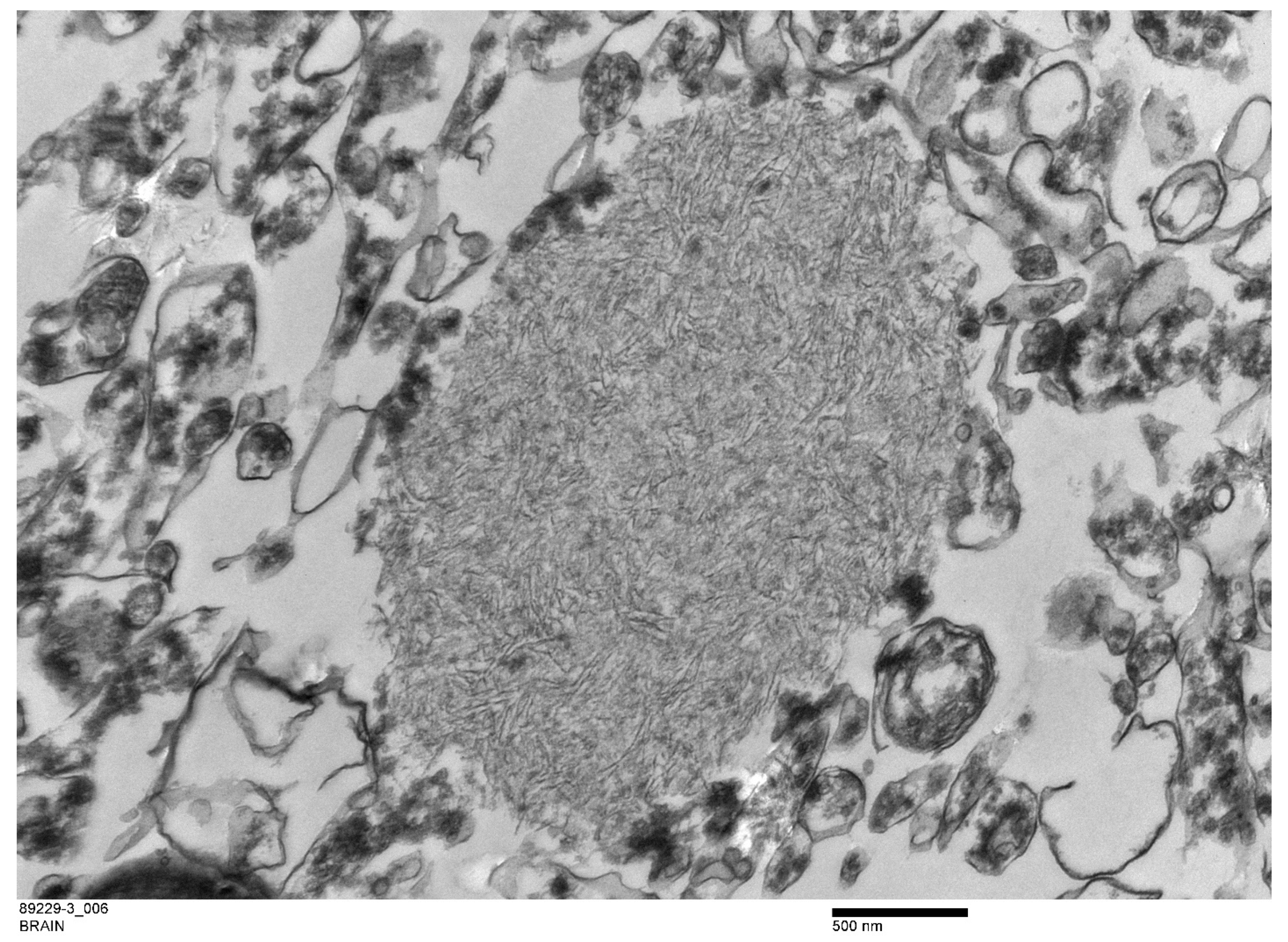
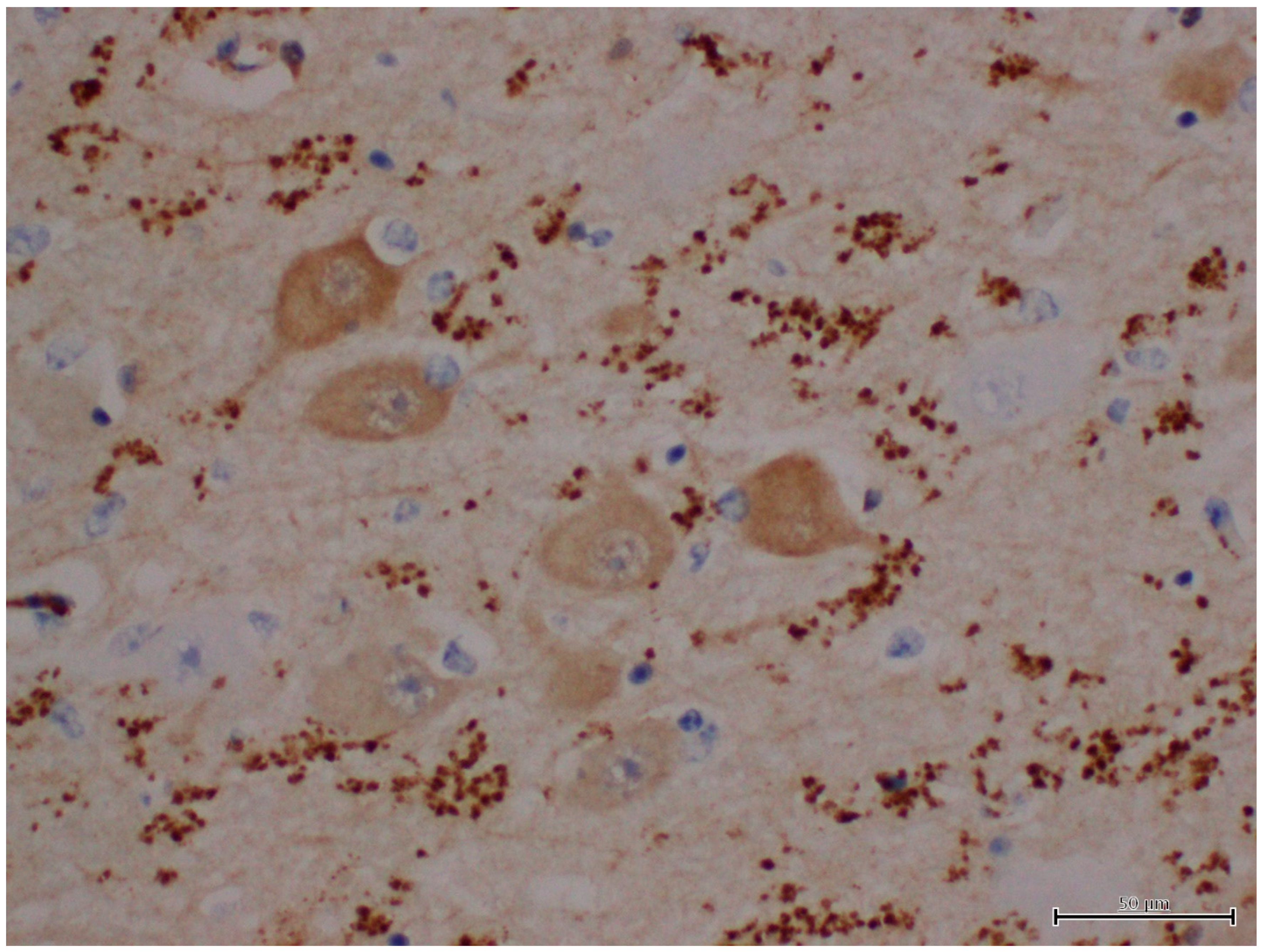
| H&E | PAS | PASD | Bielschowsky | α-Synuclein | Ultrastructure | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study Subject | Hippocampus Region | PGB | LB | PGB | LB | PGB | LB | PGB | LB | PGB | LB | PGB | LB |
| Moose 1 | Hippocampus | ||||||||||||
| CA1 | +++ | − | +++ | − | +++ | − | +++ | − | − | ± | +++ | + | |
| CA2 | +++ | − | +++ | − | +++ | − | +++ | - | − | + | +++ | + | |
| CA3 | +++ | − | +++ | − | +++ | − | +++ | + | − | +++ | +++ | + | |
| CA4 | − | − | − | − | − | − | − | + | − | +++ | − | − | |
| Dentate neurons | +++ | − | +++ | − | +++ | − | +++ | − | − | − | +++ | − | |
| Subdentate layer | +++ | − | +++ | − | +++ | − | +++ | − | − | − | +++ | ND | |
| Subiculum | +++ | − | +++ | − | +++ | − | +++ | − | − | ++ | +++ | ND | |
| White matter | − | − | − | − | − | − | − | − | − | ++ | − | ND | |
| Moose 2 | Hippocampus | ||||||||||||
| CA1 | + | − | +++ | − | +++ | − | ++ | - | − | − | ++ | − | |
| CA2 | + | − | +++ | − | +++ | − | ++ | - | − | − | ++ | − | |
| CA3 | − | − | − | − | − | − | − | − | − | − | − | − | |
| CA4 | − | − | − | − | − | − | − | − | − | − | − | − | |
| Dentate neurons | − | − | − | − | − | − | − | − | − | − | − | ++ | |
| Subdentate layer | − | − | − | − | − | − | − | − | − | − | − | ++ | |
| Subiculum | − | − | − | − | − | − | − | − | − | + | − | ++ | |
| White matter | − | − | − | − | − | − | − | − | − | + | − | ++ | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ravi, M.; Lacson, A.; Pybus, M.; Ball, M.C. Lafora Disease and Alpha-Synucleinopathy in Two Adult Free-Ranging Moose (Alces alces) Presenting with Signs of Blindness and Circling. Animals 2022, 12, 1633. https://doi.org/10.3390/ani12131633
Ravi M, Lacson A, Pybus M, Ball MC. Lafora Disease and Alpha-Synucleinopathy in Two Adult Free-Ranging Moose (Alces alces) Presenting with Signs of Blindness and Circling. Animals. 2022; 12(13):1633. https://doi.org/10.3390/ani12131633
Chicago/Turabian StyleRavi, Madhu, Atilano Lacson, Margo Pybus, and Mark C. Ball. 2022. "Lafora Disease and Alpha-Synucleinopathy in Two Adult Free-Ranging Moose (Alces alces) Presenting with Signs of Blindness and Circling" Animals 12, no. 13: 1633. https://doi.org/10.3390/ani12131633
APA StyleRavi, M., Lacson, A., Pybus, M., & Ball, M. C. (2022). Lafora Disease and Alpha-Synucleinopathy in Two Adult Free-Ranging Moose (Alces alces) Presenting with Signs of Blindness and Circling. Animals, 12(13), 1633. https://doi.org/10.3390/ani12131633






