Validation of an Enzyme Immunoassay to Measure Faecal Glucocorticoid Metabolites in Common Brushtail Possums (Trichosurus vulpecula) to Evaluate Responses to Rehabilitation
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Validation of Enzyme Immunoassays
2.1.1. Animal Capture, Handling, and Husbandry
2.1.2. Adrenocorticotrophic Hormone (ACTH) Challenge
2.1.3. Steroid Extraction and Analysis
2.2. Assessment of FGM Levels of Possums in Care
2.2.1. Sample Collection
2.2.2. Steroid Extraction and Analysis
2.3. Data Analysis
3. Results
3.1. Validation of Enzyme Immunoassays
3.2. Assessment of FGM Levels of Possums in Rehabilitation
3.2.1. Sample Collection and Volunteer Response
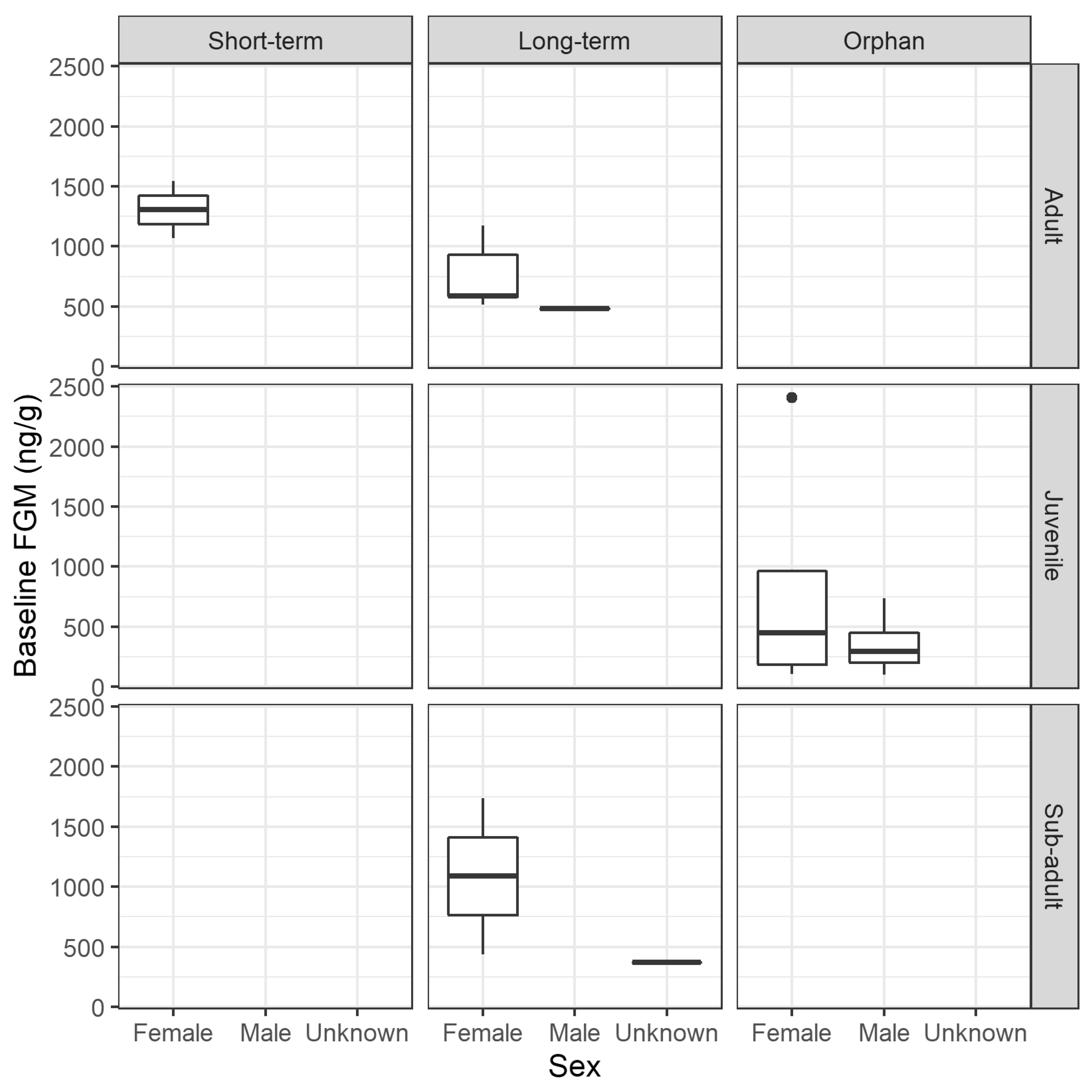
3.2.2. FGM Concentration
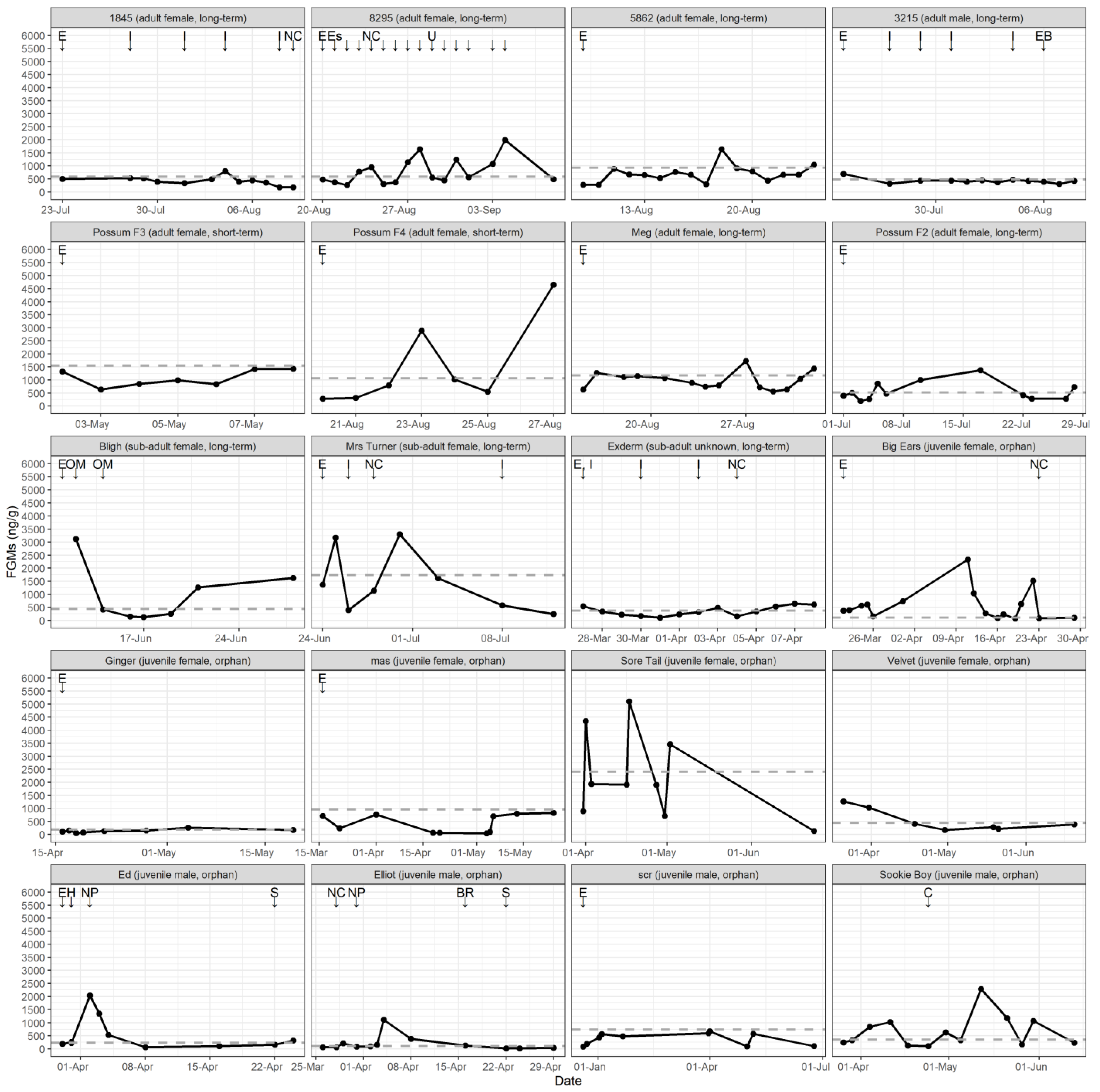
3.2.3. Physiological Response to Potential Stressors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kwok, A.B.C.; Haering, R.; Travers, S.K.; Stathis, P. Trends in wildlife rehabilitation rescues and animal fate across a six-year period in New South Wales, Australia. PLoS ONE 2021, 16, e0257209. [Google Scholar] [CrossRef] [PubMed]
- Tribe, A.; Brown, P.R. The role of wildlife rescue groups in the care and rehabilitation of Australian fauna. Hum. Dimens Wildl. 2000, 5, 69–85. [Google Scholar] [CrossRef]
- Booth, C.; Curtis, L.K. A measure of greatness: Wildlife care in Australia. Wildl. Aust. 2014, 51, 43–47. [Google Scholar]
- Pyke, G.H.; Szabo, J.K. What can we learn from untapped wildlife rescue databases? the masked lapwing as a case study. Pac. Conserv. Biol. 2018, 24, 148–156. [Google Scholar] [CrossRef]
- Mo, M.; Roache, M.; Haering, R.; Kwok, A. Using wildlife carer records to identify patterns in flying-fox rescues: A case study in New South Wales, Australia. Pac. Conserv. Biol. 2020, 27, 61–69. [Google Scholar] [CrossRef]
- DPIE. NSW Wildlife Rehabilitation: 2019-20 Annual Report; Department of Planning, Industry and Environment: Parramatta, NSW, Australia, 2021; p. 44.
- Haering, R.; Wilson, V.; Zhuo, A.; Stathis, P. A survey of veterinary professionals about their interactions with free-living native animals and the volunteer wildlife rehabilitation sector in New South Wales, Australia. Aust. Zool. 2021, 41, 254–282. [Google Scholar] [CrossRef]
- Englefield, B.; Candy, S.; Starling, M.; McGreevy, P. The demography and practice of Australians caring for native wildlife and the psychological, physical and financial effects of rescue, rehabilitation and release of wildlife on the welfare of carers. Animals 2019, 9, 1127. [Google Scholar] [CrossRef]
- Haering, R.; Wilson, V.; Zhuo, A.; Stathis, P. Towards a more effective model of wildlife care and rehabilitation: A survey of volunteers in New South Wales, Australia. Aust. Zool. 2020, 40, 605–627. [Google Scholar] [CrossRef]
- Guy, A.J.; Curnoe, D.; Banks, P.B. A survey of current mammal rehabilitation and release practices. Biodivers. Conserv. 2013, 22, 825–837. [Google Scholar] [CrossRef]
- OEH. Code of Practice for Injured, Sick and Orphaned Protected Fauna; Office of Environment and Heritage: Parramatta, NSW, Australia, 2011.
- Narayan, E.; Vanderneut, T. Physiological stress in rescued wild koalas are influenced by habitat demographics, environmental stressors, and clinical intervention. Front. Endocrinol. 2019, 10, 18. [Google Scholar] [CrossRef]
- Dickens, M.J.; Earle, K.A.; Romero, L.M. Initial transference of wild birds to captivity alters stress physiology. Gen. Comp. Endocrinol. 2009, 160, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Dickens, M.J.; Delehanty, D.J.; Michael Romero, L. Stress: An inevitable component of animal translocation. Biol. Conserv. 2010, 143, 1329–1341. [Google Scholar] [CrossRef]
- Diehl, S.; Diehl, C. Temporary care of wildlife in the animal shelter. In Shelter Medicine for Veterinarians and Staff, 2nd ed.; Miller, L., Zawistowski, S., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 247–262. [Google Scholar]
- Moberg, G.P.; Mench, J.A. The Biology of Animal Stress: Basic Principles and Implications for Animal Welfare; CABI Publishing: Oxon, UK, 2000. [Google Scholar]
- Möstl, E.; Palme, R. Hormones as indicators of stress. Domest. Anim. Endocrinol. 2002, 23, 67–74. [Google Scholar] [CrossRef]
- Matteri, R.; Carroll, J.; Dyer, C. Neuroendocrine responses to stress. In The Biology of Animal Stress: Basic Principles and Implications for Animal Welfare; Moberg, G.P., Mench, J.A., Eds.; CABI Publishing: Oxon, UK, 2000; pp. 43–76. [Google Scholar]
- McCarty, R. Chapter 4—The fight-or-flight response: A cornerstone of stress research. In Stress: Concepts, Cognition, Emotion, and Behavior; Fink, G., Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 33–37. [Google Scholar]
- Cannon, W.B. The Wisdom of the Body; W. W. Norton and Company: New York, NY, USA, 1939. [Google Scholar]
- Wingfield, J.C.; Romero, L.M. Adrenocortical responses to stress and their modulation in free-living vertebrates. In Comprehensive Physiology; Terjung, R., Ed.; Wiley: Hoboken, NJ, USA, 2011; pp. 211–234. [Google Scholar]
- Sapolsky, R.M.; Romero, L.M.; Munck, A.U. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000, 21, 55–89. [Google Scholar] [CrossRef] [PubMed]
- Packard, A.E.B.; Egan, A.E.; Ulrich-Lai, Y.M. HPA axis interactions with behavioral systems. Compr. Physiol. 2016, 6, 1897–1934. [Google Scholar] [CrossRef]
- Baxter, J.D. Glucocorticoid hormone action. Pharmacol. Ther. Part B Gen. Syst. Pharmacol. 1976, 2, 605–659. [Google Scholar] [CrossRef]
- Adamo, S.; Roberts, J.; Easy, R.; Ross, N. Competition between immune function and lipid transport for the protein apolipophorin III leads to stress-induced immunosuppression in crickets. J. Exp. Biol. 2008, 211, 531–538. [Google Scholar] [CrossRef]
- Downs, C.J.; Boan, B.V.; Lohuis, T.D.; Stewart, K.M. Investigating relationships between reproduction, immune defenses, and cortisol in dall sheep. Front. Immunol. 2018, 9, 105. [Google Scholar] [CrossRef]
- Munck, A.; Guyre, P.M.; Holbrook, N.J. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocr. Rev. 1984, 5, 25–44. [Google Scholar] [CrossRef]
- Cabezas, S.; Blas, J.; Marchant, T.A.; Moreno, S. Physiological stress levels predict survival probabilities in wild rabbits. Horm Behav 2007, 51, 313–320. [Google Scholar] [CrossRef]
- Yan, D.; Hu, D.; Li, K.; Li, B.; Zeng, X.; Chen, J.; Li, Y.; Wronski, T. Effects of chronic stress on the fecal microbiome of Malayan pangolins (Manis javanica) rescued from the illegal wildlife trade. Curr. Microbiol. 2021, 78, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Wey, T.W.; Lin, L.; Patton, M.L.; Blumstein, D.T. Stress hormone metabolites predict overwinter survival in yellow-bellied marmots. Acta Ethol. 2015, 18, 181–185. [Google Scholar] [CrossRef]
- Ethan Pride, R. High faecal glucocorticoid levels predict mortality in ring-tailed lemurs (Lemur catta). Biol. Lett. 2005, 1, 60–63. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Boonstra, R. Reality as the leading cause of stress: Rethinking the impact of chronic stress in nature. Funct. Ecol. 2013, 27, 11–23. [Google Scholar] [CrossRef]
- Touma, C.; Palme, R. Measuring fecal glucocorticoid metabolites in mammals and birds: The importance of validation. Ann. N. Y. Acad. Sci. 2005, 1046, 54–74. [Google Scholar] [CrossRef]
- Palme, R. Non-invasive measurement of glucocorticoids: Advances and problems. Physiol. Behav. 2019, 199, 229–243. [Google Scholar] [CrossRef]
- Sheriff, M.J.; Dantzer, B.; Delehanty, B.; Palme, R.; Boonstra, R. Measuring stress in wildlife: Techniques for quantifying glucocorticoids. Oecologia 2011, 166, 869–887. [Google Scholar] [CrossRef]
- Narayan, E.J. Evaluation of physiological stress in Australian wildlife: Embracing pioneering and current knowledge as a guide to future research directions. Gen. Comp. Endocrinol. 2017, 244, 30–39. [Google Scholar] [CrossRef]
- Fanson, K.V.; Best, E.C.; Bunce, A.; Fanson, B.G.; Hogan, L.A.; Keeley, T.; Narayan, E.J.; Palme, R.; Parrott, M.L.; Sharp, T.M. One size does not fit all: Monitoring faecal glucocorticoid metabolites in marsupials. Gen. Comp. Endocrinol. 2017, 244, 146–156. [Google Scholar] [CrossRef]
- Palme, R. Measuring fecal steroids: Guidelines for practical application. Ann. N. Y. Acad. Sci. 2005, 1046, 75–80. [Google Scholar] [CrossRef]
- Davies, N.; Gillett, A.; McAlpine, C.; Seabrook, L.; Baxter, G.; Lunney, D.; Bradley, A. The effect of ACTH upon faecal glucocorticoid excretion in the koala. J. Endocrinol. 2013, 219, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Narayan, E.J.; Webster, K.; Nicolson, V.; Mucci, A.; Hero, J.M. Non-invasive evaluation of physiological stress in an iconic Australian marsupial: The koala (Phascolarctos cinereus). Gen. Comp. Endocrinol. 2013, 187, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, F.; Barlow, C.K.; Schlagloth, R.; Schittenhelm, R.B.; Palme, R.; Henning, J. Identification of Koala (Phascolarctos cinereus) Faecal Cortisol Metabolites Using Liquid Chromatography-Mass Spectrometry and Enzyme Immunoassays. Metabolites 2021, 11, 393. [Google Scholar] [CrossRef] [PubMed]
- Morris, K.; Woinarski, J.; Friend, T.; Foulkes, J.; Kerle, A.; Ellis, M. Trichosurus Vulpecula. Available online: https://www.iucnredlist.org/species/40585/21952080 (accessed on 30 July 2021).
- How, R.; Hillcox, S. Brushtail possum, Trichosurus vulpecula, populations in south-western Australia: Demography, diet and conservation status. Wildl. Res. 2000, 27, 81–89. [Google Scholar] [CrossRef]
- Morgan, K.N.; Tromborg, C.T. Sources of stress in captivity. Appl. Anim. Behav. Sci. 2007, 102, 262–302. [Google Scholar] [CrossRef]
- Charalambous, R.; Simonato, T.; Peel, M.; Narayan, E. Physiological stress in rescued wild koalas (Phascolarctos cinereus) being held in a rehabilitation sanctuary: A pilot study. Animals 2021, 11, 2864. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, J.A.; Sherwood, A.; Smith, P.J.; Watkins, L.; Mabe, S.; Kraus, W.E.; Ingle, K.; Miller, P.; Hinderliter, A. Enhancing Cardiac Rehabilitation With Stress Management Training. Circulation 2016, 133, 1341–1350. [Google Scholar] [CrossRef]
- Frasure-Smith, N. In-hospital symptoms of psychological stress as predictors of long-term outcome after acute myocardial infarction in men. Am. J. Cardiol. 1991, 67, 121–127. [Google Scholar] [CrossRef]
- Hannibal, K.E.; Bishop, M.D. Chronic stress, cortisol dysfunction, and pain: A psychoneuroendocrine rationale for stress management in pain rehabilitation. Phys. Ther. 2014, 94, 1816–1825. [Google Scholar] [CrossRef]
- Hing, S.; Narayan, E.J.; Thompson, R.A.; Godfrey, S.S. The relationship between physiological stress and wildlife disease: Consequences for health and conservation. Wildl. Res. 2016, 43, 51–60. [Google Scholar] [CrossRef]
- Fanson, K.V.; Wielebnowski, N.C.; Shenk, T.M.; Lucas, J.R. Comparative patterns of adrenal activity in captive and wild Canada lynx (Lynx canadensis). J. Comp. Physiol. B 2012, 182, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Narayan, E.; Hero, J.M.; Evans, N.; Nicolson, V.; Mucci, A. Non-invasive evaluation of physiological stress hormone responses in a captive population of the greater bilby Macrotis lagotis. Endanger Spec. Res. 2012, 18, 279–289. [Google Scholar] [CrossRef]
- Van der Weyde, L.K.; Martin, G.B.; Paris, M.C.J. Monitoring stress in captive and free-ranging African wild dogs (Lycaon pictus) using faecal glucocorticoid metabolites. Gen. Comp. Endocrinol. 2016, 226, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.L.; Kalliokoski, O.; Dabelsteen, T.; Abelson, K. An exploratory investigation of glucocorticoids, personality and survival rates in wild and rehabilitated hedgehogs (Erinaceus europaeus) in Denmark. BMC Ecol. Evol. 2021, 21, 96. [Google Scholar] [CrossRef] [PubMed]
- Hing, S.; Narayan, E.; Thompson, R.C.A.; Godfrey, S. A review of factors influencing the stress response in Australian marsupials. Conserv. Physiol. 2014, 2, cou027. [Google Scholar] [CrossRef]
- Wyman, T.E.; Kelly, D. Quantifying seed dispersal by birds and possums in a lowland New Zealand forest. N. Z. J. Ecol. 2017, 41, 47–55. [Google Scholar] [CrossRef]
- Arbor Assays. Corticosterone ISWE Mini-Kit. Available online: https://www.arborassays.com/product/corticosterone-iswe-mini-kit/ (accessed on 4 April 2022).
- Watson, R.; Munro, C.; Edwards, K.L.; Norton, V.; Brown, J.L.; Walker, S.L. Development of a versatile enzyme immunoassay for non-invasive assessment of glucocorticoid metabolites in a diversity of taxonomic species. Gen. Comp. Endocrinol. 2013, 186, 16–24. [Google Scholar] [CrossRef]
- Möstl, E.; Rettenbacher, S.; Palme, R. Measurement of corticosterone metabolites in birds’ droppings: An analytical approach. Ann. N Y Acad. Sci. 2005, 1046, 17–34. [Google Scholar] [CrossRef]
- Palme, R.; Mostl, E. Measurement of cortisol metabolites in faeces of sheep as a parameter of cortisol concentration in blood. Int. J. Mammal. Biol. 1997, 62, 192–197. [Google Scholar]
- Fanson, K.V.; Lynch, M.; Vogelnest, L.; Miller, G.; Keeley, T. Response to long-distance relocation in Asian elephants (Elephas maximus): Monitoring adrenocortical activity via serum, urine, and feces. Eur. J. Wildl. Res. 2013, 59, 655–664. [Google Scholar] [CrossRef]
- Young, K.M.; Walker, S.L.; Lanthier, C.; Waddell, W.T.; Monfort, S.L.; Brown, J.L. Noninvasive monitoring of adrenocortical activity in carnivores by fecal glucocorticoid analyses. Gen. Comp. Endocrinol. 2004, 137, 148–165. [Google Scholar] [CrossRef] [PubMed]
- Whitten, P.; Brockman, D.; Stavisky, R. Recent advances in noninvasive techniques to monitor hormone-behavior interactions. Am. J. Phys. Anthropol. 1998, 107, 1–23. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Fanson, B.; Fanson, K.V. hormLong: An R package for longitudinal data analysis in wildlife endocrinology studies. PeerJ PrePrints 2015, 3, e1546v1541. [Google Scholar] [CrossRef][Green Version]
- Raña, A. The Influences of Physiological Stress, Personality, and Behavioural Mechanisms on Problem Solving by Common Brushtail Possums. Honours Thesis, University of Sydney, Sydney, NSW, Australia, 2017. [Google Scholar]
- Palme, R.; Rettenbacher, S.; Touma, C.; El-Bahr, S.M.; Möstl, E. Stress hormones in mammals and birds: Comparative aspects regarding metabolism, excretion, and noninvasive measurement in fecal samples. Ann. N. Y. Acad. Sci. 2005, 1040, 162–171. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, S.; Deane, E.M. Faecal corticosteroid levels as an indicator of well-being in the tammar wallaby, Macropus eugenii. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2005, 140, 81–87. [Google Scholar] [CrossRef]
- Shutt, K.; Setchell, J.M.; Heistermann, M. Non-invasive monitoring of physiological stress in the Western lowland gorilla (Gorilla gorilla gorilla): Validation of a fecal glucocorticoid assay and methods for practical application in the field. Gen. Comp. Endocrinol. 2012, 179, 167–177. [Google Scholar] [CrossRef]
- Gardiner, R.; Cristescu, R.; Keeley, T. Hormones in the wild: The influence of environmental conditions on steroid hormone concentrations in koala faecal pellets. In Proceedings of the 67th Annual Scientific Meeting of the Australian Mammal Society Virtual 2021, Perth, WA, Australia, 28 September–October 2021. [Google Scholar]
- Bosson, C.O.; Palme, R.; Boonstra, R. Assessment of the stress response in Columbian ground squirrels: Laboratory and field validation of an enzyme immunoassay for fecal cortisol metabolites. Physiol. Biochem. Zool. 2009, 82, 291–301. [Google Scholar] [CrossRef]
- McDowell, A.; Nicoll, J.J.; McLeod, B.J.; Tucker, I.G.; Davies, N.M. Gastrointestinal transit in the common brushtail possum measured by gamma scintigraphy. Int. J. Pharm. 2005, 302, 125–132. [Google Scholar] [CrossRef]
- Eymann, J.; Herbert, C.A.; Cooper, D.W. Management issues of urban common brushtail possums Trichosurus vulpecula: A loved or hated neighbour. Aust. Mammal 2006, 28, 153–171. [Google Scholar] [CrossRef]
- Hufschmid, J.; Handasyde, K.; Beveridge, I. The role of host and environmental factors in the epidemiology of rumpwear in brushtail possums. Aust. J. Zool. 2010, 58, 250–262. [Google Scholar] [CrossRef]
- Ringwaldt, E.M.; Brook, B.W.; Carver, S.; Buettel, J.C. The patterns and causes of dermatitis in terrestrial and semi-aquatic mammalian wildlife. Animals 2021, 11, 1691. [Google Scholar] [CrossRef] [PubMed]
- Chilvers, B.L.; Finlayson, G.; Candy, E.J.; Sriram, A.; Morgan, K.J.; Cockrem, J.F. Corticosterone stress hormone responses in oil rehabilitated and non-rehabilitated little penguins. Mar. Pollut. Bull. 2016, 113, 312–315. [Google Scholar] [CrossRef] [PubMed]
- Viljoen, J.J.; Ganswindt, A.; Toit, J.T.D., Jr. Translocation stress and faecal glucocorticoid metabolite levels in free-ranging African savanna elephants: Research article. S. Afr. J. Wildl. Res. 2008, 38, 146–152. [Google Scholar] [CrossRef]
- MacLeod, K.; Sheriff, M.; Ensminger, D.; Owen, D.; Langkilde, T. Survival and reproductive costs of repeated acute glucocorticoid elevations in a captive, wild animal. Gen. Comp. Endocrinol. 2018, 268, 1–6. [Google Scholar] [CrossRef]
- Fey, S.B.; Siepielski, A.M.; Nusslé, S.; Cervantes-Yoshida, K.; Hwan, J.L.; Huber, E.R.; Fey, M.J.; Catenazzi, A.; Carlson, S.M. Recent shifts in the occurrence, cause, and magnitude of animal mass mortality events. Proc. Natl. Acad. Sci. USA 2015, 112, 1083–1088. [Google Scholar] [CrossRef]
- Davies, N.; Gramotnev, G.; Seabrook, L.; McAlpine, C.; Baxter, G.; Lunney, D.; Bradley, A. Climate-driven changes in diet composition and physiological stress in an arboreal folivore at the semi-arid edge of its distribution. Biol. Conserv. 2014, 172, 80–88. [Google Scholar] [CrossRef]
- Hogan, L.A.; Lisle, A.T.; Johnston, S.D.; Robertson, H. Non-invasive assessment of stress in captive numbats, Myrmecobius fasciatus (Mammalia: Marsupialia), using faecal cortisol measurement. Gen. Comp. Endocrinol. 2012, 179, 376–383. [Google Scholar] [CrossRef]
- Rothschild, D.M.; Serfass, T.L.; Seddon, W.L.; Hegde, L.; Fritz, R.S. Using fecal glucocorticoids to assess stress levels in captive river otters. J. Wildl. Manag. 2008, 72, 138–142. [Google Scholar] [CrossRef]
- Mella, V.S.; Ward, A.J.; Banks, P.B.; McArthur, C. Personality affects the foraging response of a mammalian herbivore to the dual costs of food and fear. Oecologia 2015, 177, 293–303. [Google Scholar] [CrossRef]
- Baker, M.R.; Gobush, K.S.; Vynne, C.H. Review of factors influencing stress hormones in fish and wildlife. J. Nat. Conserv. 2013, 21, 309–318. [Google Scholar] [CrossRef]
- Fardell, L.L.; Bedoya-Pérez, M.A.; Dickman, C.R.; Crowther, M.S.; Pavey, C.R.; Narayan, E.J. Are physiological and behavioural responses to stressors displayed concordantly by wild urban rodents? Sci. Nat. 2021, 108, 5. [Google Scholar] [CrossRef] [PubMed]
- McArthur, C.; Banks, P.B.; Boonstra, R.; Forbey, J.S. The dilemma of foraging herbivores: Dealing with food and fear. Oecologia 2014, 176, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Terio, K.A.; Marker, L.; Munson, L. Evidence for chronic stress in captive but not free-ranging cheetahs (Acinonyx jubatus) based on adrenal morphology and function. J. Wildl. Dis. 2004, 40, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Young, A.J.; Carlson, A.A.; Monfort, S.L.; Russell, A.F.; Bennett, N.C.; Clutton-Brock, T. Stress and the suppression of subordinate reproduction in cooperatively breeding meerkats. Proc. Natl. Acad. Sci. USA 2006, 103, 12005–12010. [Google Scholar] [CrossRef] [PubMed]
- Fries, E.; Hesse, J.; Hellhammer, J.; Hellhammer, D.H. A new view on hypocortisolism. Psychoneuroendocrinology 2005, 30, 1010–1016. [Google Scholar] [CrossRef] [PubMed]
- Heim, C.M.; Nater, U.M. Hypocortisolism and Stress. In Encyclopedia of Stress, 2nd ed.; Fink, G., Ed.; Academic Press: New York, NY, USA, 2007; pp. 400–407. [Google Scholar]
- Dickens, M.J.; Romero, L.M. A consensus endocrine profile for chronically stressed wild animals does not exist. Gen. Comp. Endocrinol. 2013, 191, 177–189. [Google Scholar] [CrossRef]
- Bradley, A.; Stoddart, D. Seasonal changes in plasma androgens, glucocorticoids and glucocorticoid-binding proteins in the marsupial sugar glider Petaurus breviceps. J. Endocrinol. 1992, 132, 21–31. [Google Scholar] [CrossRef]
- Santamaria, F.; Palme, R.; Schlagloth, R.; Klobetz-Rassam, E.; Henning, J. Seasonal Variations of Faecal Cortisol Metabolites in Koalas in South East Queensland. Animals 2021, 11, 1622. [Google Scholar] [CrossRef]
- Reeder, D.M.; Kramer, K.M. Stress in free-ranging mammals: Integrating physiology, ecology, and natural history. J. Mammal 2005, 86, 225–235. [Google Scholar] [CrossRef]
- Perry, D.J.; Averka, J.P. Caring for the circle of life: Wildlife rehabilitation and sanctuary care. Hum. Wildl. Interact. 2020, 14, 18. [Google Scholar]
- DPIE. Initial Treatment and Care Guidelines for Rescued Possums and Gliders; Department of Planning, Industry and Environment: Parramatta, NSW, Australia, 2021; pp. 1–43.
- Goody, A.; Ferris, R.; Gelatos, M.; Yim, C. Background music to reduce startle response in wild avian species during rehabilitation. J. Wildl. Rehabil. 2013, 33, 13–18. [Google Scholar]
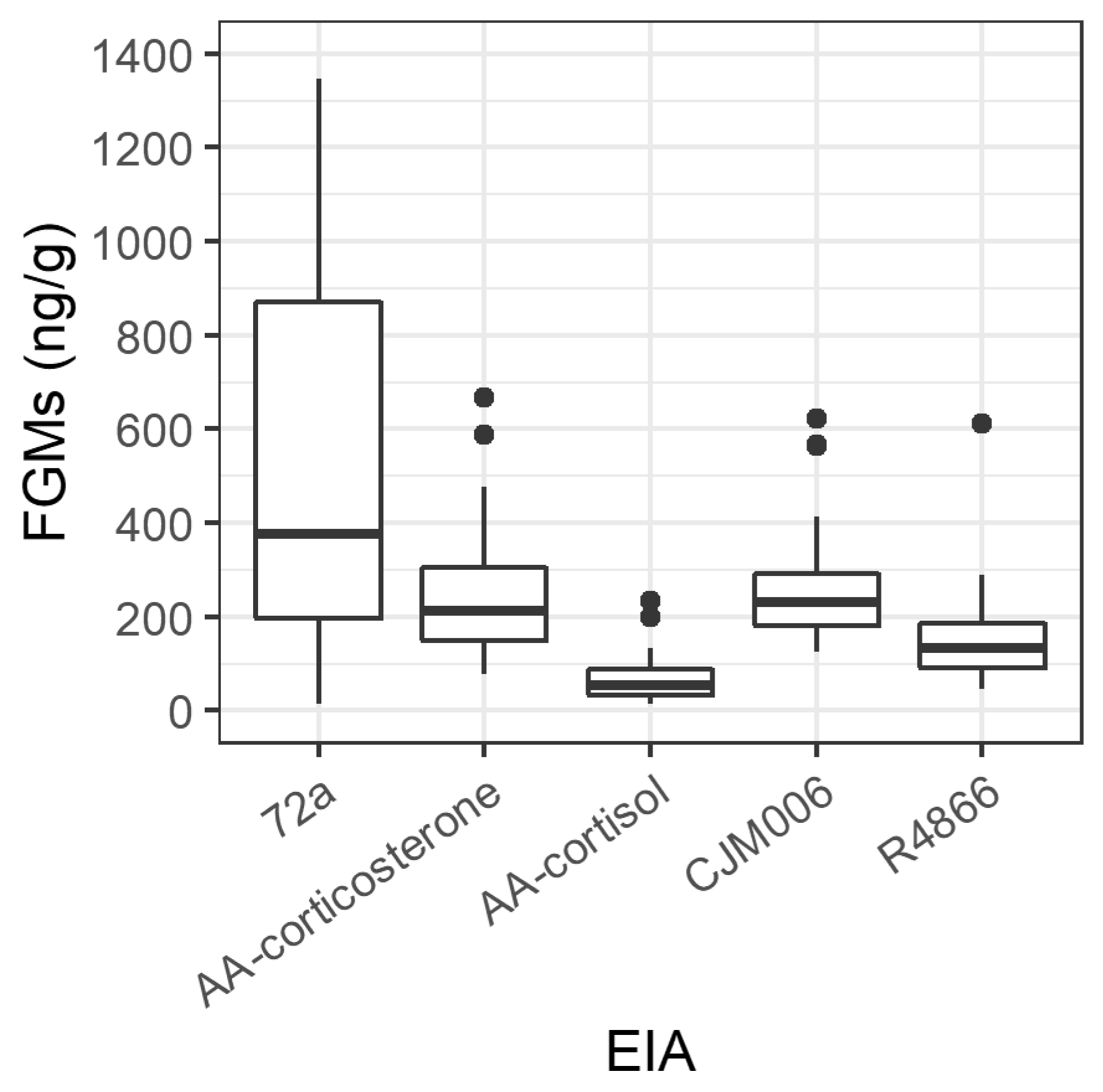
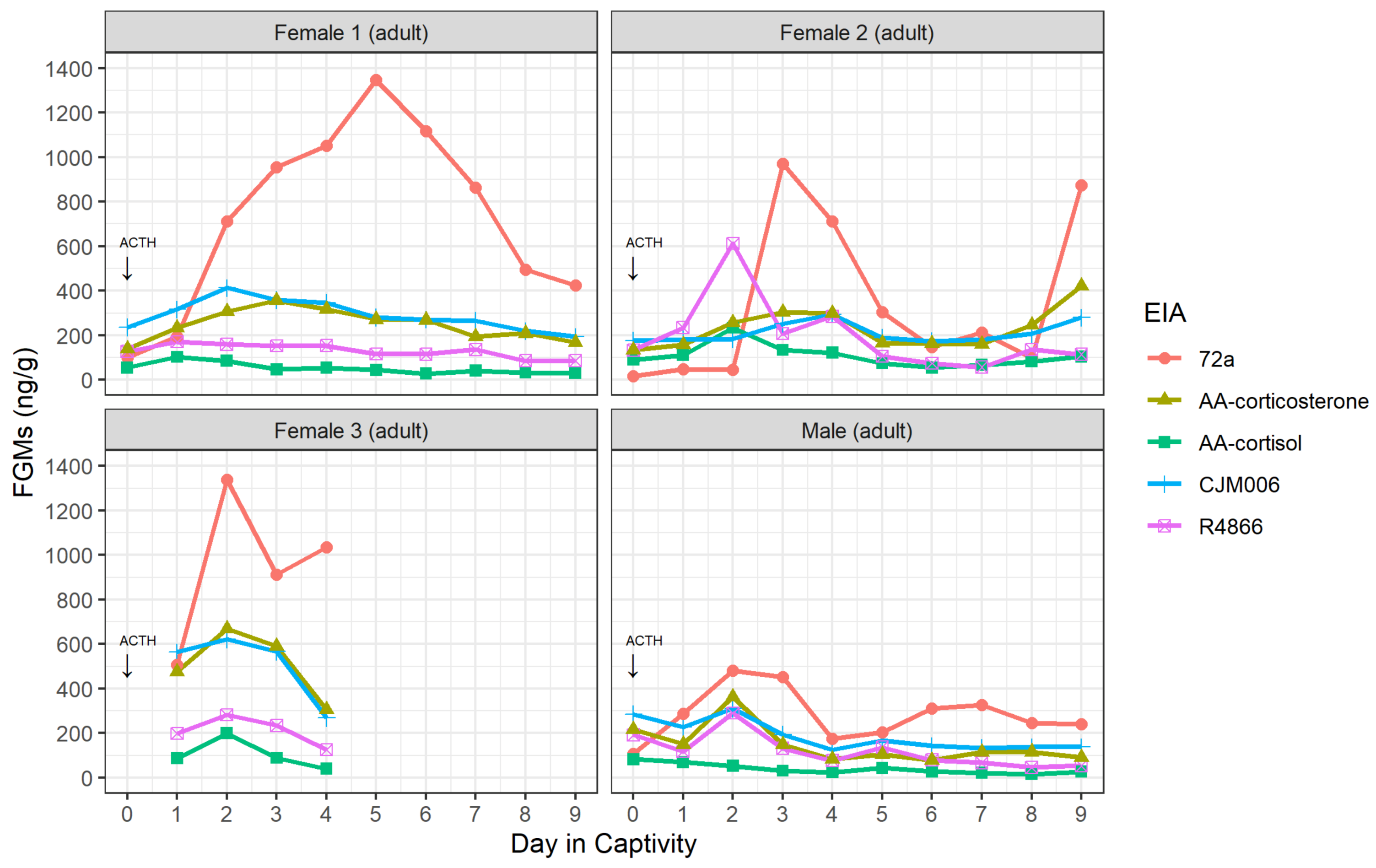
| Adult Possum | 72a | AA-Corticosterone | AA-Cortisol | CJM006 | R4866 | |
|---|---|---|---|---|---|---|
| Time to first FGM rise above baseline threshold (and time to maximum FGM if different from first rise) | Female 1 | 55 h 0 min (105 h 30 min) | 31 h 30 min | 7 h 0 min | 31 h 30 min | - (7 h 0 min) |
| Female 2 | 55 h 27 min | 55 h 27 min (200 h 40 min) | 32 h 6 min | 55 h 27 min (82 h 31 min) | 32 h 6 min | |
| Female 3 | 31 h 25 min | - (31 h 25 min) | 31 h 25 min | - (31 h 25 min) | - (31 h 25 min) | |
| Male | 8 h 40 min (31 h 40 min) | 31 h 40 min | - (8 h 40 min) | - (31 h 40 min) | - (31 h 40 min) | |
| Mean baseline FGM concentration (ng/g) | Female 1 | 385 | 189 | 46 | 254 | 130 |
| Female 2 | 124 | 184 | 93 | 185 | 150 | |
| Female 3 | 709 | 510 | 72 | 505 | 210 | |
| Male | 194 | 122 | 40 | 186 | 119 | |
| Mean peak FGM concentration (ng/g) | Female 1 | 1066 | 304 | 103 | 373 | - |
| Female 2 | 852 | 342 | 234 | 275 | 612 | |
| Female 3 | 1187 | - | 199 | - | - | |
| Male | 372 | 363 | - | - | - | |
| Magnitude of change from first sample at peak FGM (%) | Female 1 | 1330 | 254 | 186 | 175 | 135 |
| Female 2 | 6277 | 314 | 262 | 167 | 461 | |
| Female 3 | 264 | 140 | 229 | 110 | 142 | |
| Male | 454 | 168 | 100 | 109 | 150 |
| Event | FGM Response | No FGM Response | Total Events |
|---|---|---|---|
| Enter rehabilitation | 6 | 9 | 15 |
| Cover removed | 1 | 0 | 1 |
| New cage | 4 | 1 | 5 |
| New possum | 1 | 1 | 2 |
| Back rider | 0 | 1 | 1 |
| Handling | 2 | 0 | 2 |
| Separated | 1 | 1 | 2 |
| Injection | 4 | 8 | 12 |
| Oral medication | 0 | 2 | 2 |
| Escaped | 0 | 1 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cope, H.R.; Keeley, T.; Keong, J.; Smith, D.; Silva, F.R.O.; McArthur, C.; Webster, K.N.; Mella, V.S.A.; Herbert, C.A. Validation of an Enzyme Immunoassay to Measure Faecal Glucocorticoid Metabolites in Common Brushtail Possums (Trichosurus vulpecula) to Evaluate Responses to Rehabilitation. Animals 2022, 12, 1627. https://doi.org/10.3390/ani12131627
Cope HR, Keeley T, Keong J, Smith D, Silva FRO, McArthur C, Webster KN, Mella VSA, Herbert CA. Validation of an Enzyme Immunoassay to Measure Faecal Glucocorticoid Metabolites in Common Brushtail Possums (Trichosurus vulpecula) to Evaluate Responses to Rehabilitation. Animals. 2022; 12(13):1627. https://doi.org/10.3390/ani12131627
Chicago/Turabian StyleCope, Holly R., Tamara Keeley, Joy Keong, Daniel Smith, Fabiola R. O. Silva, Clare McArthur, Koa N. Webster, Valentina S. A. Mella, and Catherine A. Herbert. 2022. "Validation of an Enzyme Immunoassay to Measure Faecal Glucocorticoid Metabolites in Common Brushtail Possums (Trichosurus vulpecula) to Evaluate Responses to Rehabilitation" Animals 12, no. 13: 1627. https://doi.org/10.3390/ani12131627
APA StyleCope, H. R., Keeley, T., Keong, J., Smith, D., Silva, F. R. O., McArthur, C., Webster, K. N., Mella, V. S. A., & Herbert, C. A. (2022). Validation of an Enzyme Immunoassay to Measure Faecal Glucocorticoid Metabolites in Common Brushtail Possums (Trichosurus vulpecula) to Evaluate Responses to Rehabilitation. Animals, 12(13), 1627. https://doi.org/10.3390/ani12131627






