Application of Felid Hair for Non-Invasive Tracking of Animal Reproductive Status and Adrenal Activity
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Objects
2.3. Husbandry Conditions
2.4. Samples Collection
2.5. Steroid Extraction and Analysis
2.6. Statistics
3. Results
3.1. Sex Differences in Testosterone and Cortisol Levels
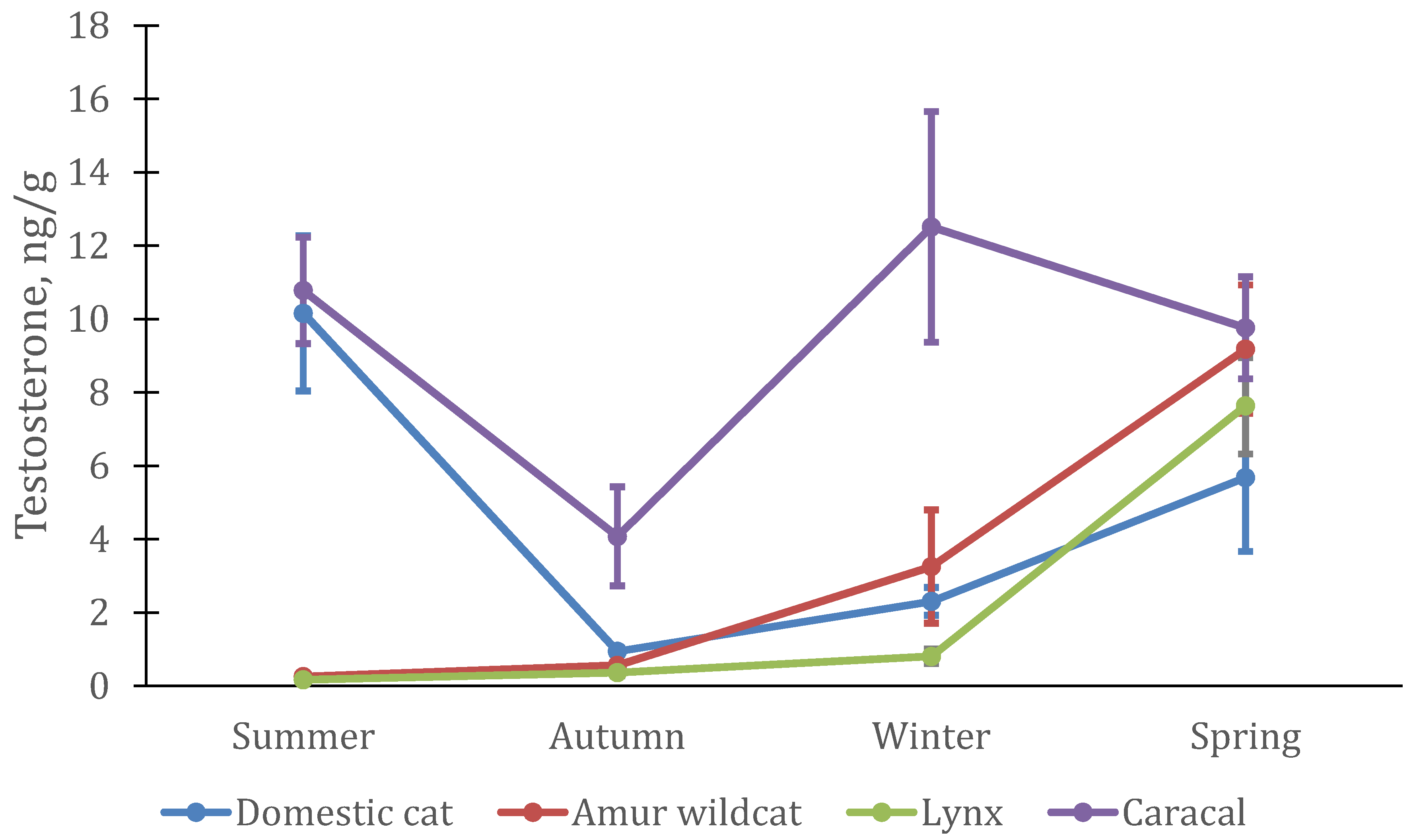
3.2. Seasonal Features of the Dynamics of Testosterone and Cortisol in Felines
3.3. Interspecific Features of Testosterone and Cortisol Dynamics in Felines
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alekseeva, G.S.; Loshchagina, J.A.; Erofeeva, M.N.; Naidenko, S.V. Stressed by Maternity: Changes of Cortisol Level in Lactating Domestic Cats. Animals 2020, 10, 903. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.C.S.; Berndtson, W.E.; Cardoso, F.M. Plasma and testicular testosterone levels, volume density and number of Leydig cells and spermatogenic efficiency of rabbits. Physiology and biophysics. Braz. J. Med. Biol. Res. 2002, 35, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Naidenko, S.V.; Berezhnoi, M.A.; Kumar, V.; Umapathy, G. Comparison of tigers’ fecal glucocorticoids level in two extreme habitats. PLoS ONE 2019, 14, e0214447. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.L.; Wemmer, C.M.; Lehnhardt, J. Urinary cortisol analysis for monitoring adrenal activity in elephants. Zoo Biol. 1995, 14, 533–542. [Google Scholar] [CrossRef]
- Strzelec, K.; Kankofer, M.; Pietrzak, S. Cortisol concentration in the saliva of horses subjected to different kinds of exercise. Acta Vet. Brno 2011, 80, 101–105. [Google Scholar] [CrossRef]
- Diaz, J.R.; Alejandro, M.; Romero, G.; Moya, F.; Peris, C. Variation in milk cortisol during lactation in Murciano-Granadina goats. J. Dairy Sci. 2013, 96, 897–905. [Google Scholar] [CrossRef]
- Bechshøft, T.O.; Sonne, C.; Riget, F.F.; Letcher, R.J.; Novak, M.A.; Henchey, E.; Meyer, J.S.; Eulaers, I.; Jaspers, V.L.B.; Covaci, A.; et al. Polar bear stress hormone cortisol fluctuates with the North Atlantic Oscillation climate index. Polar Biol. 2013, 36, 1525–1529. [Google Scholar] [CrossRef]
- Deng, X.-S.; Kurosu, A.; Pounder, D.J. Detection of anabolic steroids in head hair. J. Forensic Sci. 1999, 44, 343–346. [Google Scholar] [CrossRef]
- Deshmukha, N.; Hussaina, I.; Barker, J.; Petroczi, A.; Naughton, D.P. Analysis of anabolic steroids in human hair using LC–MS/MS. Steroids 2010, 75, 710–714. [Google Scholar] [CrossRef]
- Terwissen, C.V.; Mastromonaco, G.F.; Murray, D.L. Influence of adrenocorticotrophin hormone challenge and external factors (age, sex, and body region) on hair cortisol concentration in Canada lynx (Lynx canadensis). Gen. Comp. Endocrinol. 2013, 194, 162–167. [Google Scholar] [CrossRef]
- Heimburge, S.; Kanitz, E.; Otten, W. The use of hair cortisol for the assessment of stress in animals. Gen. Comp. Endocrinol. 2019, 270, 10–17. [Google Scholar] [CrossRef]
- Kapoor, A.; Schultz-Darken, N.; Ziegler, T.E. Radiolabel validation of cortisol in the hair of rhesus monkeys. Psychoneuroendocrinology 2018, 97, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.H.; Doan, S.N. Innovations in biological assessments of chronic stress through hair and nail cortisol: Conceptual, developmental, and methodological issues. Dev. Psychobiol. 2019, 61, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Pokharel, S.S.; Yoneda, H.; Yanagi, M.; Sukumar, R.; Kinoshita, K. The tail-tale of stress: An exploratory analysis of cortisol levels in the tail-hair of captive Asian elephants. PeerJ 2021, 9, e10445. [Google Scholar] [CrossRef]
- Carlitz, E.H.D.; Kirschbaum, C.; Stalder, T.; van Schaik, C. Hair as a long-term retrospective cortisol calendar in orangutans (Pongo spp.): New perspectives for stress monitoring in captive management and conservation. Gen. Comp. Endocrinol. 2014, 195, 151–156. [Google Scholar] [CrossRef]
- Keogh, M.J.; Charapata, P.; Fadely, B.S.; Zeppelin, T.; Rea, L.; Waite, J.N.; Burkanov, V.; Marshall, C.; Jones, A.; Sprowls, C.; et al. Whiskers as a novel tissue for tracking reproductive and stress-related hormones in North Pacific otariid pinnipeds. Conserv. Physiol. 2021, 9, coaa134. [Google Scholar] [CrossRef] [PubMed]
- Hunt, K.E.; Lysiak, N.S.; Moore, M.J.; Rolland, R.M. Longitudinal progesterone profiles in baleen from female North Atlantic right whales (Eubalaena glacialis) match known calving history. Conserv. Physiol. 2016, 4, cow014. [Google Scholar] [CrossRef] [PubMed]
- Colding-Jørgensen, P.; Hestehave, S.; Abelson, K.S.P.; Kalliokoski, O. Hairs do not contain a historical record of stress—elucidating hair glucocorticoid kinetics in laboratory rats. In Proceedings of the 7th Conference of the International Society of Wildlife Endocrinology, Skukuza, Kruger National Park, South Africa, 13–16 October 2019. [Google Scholar]
- Accorsi, P.A.; Carloni, E.; Valsecchi, P.; Viggiani, R.; Garnberoni, M.; Tarnanini, C.; Seren, E. Cortisol determination in hair and faeces from domestic cats and dogs. Gen. Comp. Endocr. 2008, 155, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Davenport, M.D.; Tiefenbacher, S.; Lutz, C.K.; Novak, M.A.; Meyer, J.S. Analysis of endogenous cortisol concentrations in the hair of rhesus macaques. Gen. Comp. Endocr. 2006, 147, 255–261. [Google Scholar] [CrossRef]
- Franchini, M.; Prandi, A.; Filacorda, S.; Pezzin, E.N.; Fanin, Y.; Comin, A. Cortisol in hair: A comparison between wild and feral cats in the north-eastern Alps. Eur. J. Wildl. Res. 2019, 65, 90. [Google Scholar] [CrossRef]
- Azevedo, A.; Wauters, J.; Kirschbaum, C.; Serra, R.; Rivas, A.; Jewgenow, K. Sex steroids and glucocorticoid ratios in Iberian lynx hair. Conserv. Physiol. 2020, 8, coaa075. [Google Scholar] [CrossRef] [PubMed]
- Lafferty, D.J.R.; Laudenslager, M.L.; Mowat, G.; Heard, D.; Belant, J.L. Sex, diet, and the social environment: Factors influencing hair cortisol concentration in free-ranging black bears (Ursus americanus). PLoS ONE 2015, 10, e0141489. [Google Scholar] [CrossRef] [PubMed]
- Macbeth, B.J.; Cattet, M.R.L.; Stenhouse, G.B.; Gibeau, M.L.; Janz, D.M. Hair cortisol concentration as a noninvasive measure of long-term stress in free-ranging grizzly bears (Ursus arctos): Considerations with implications for other wildlife. Can. J. Zool. 2010, 88, 935–949. [Google Scholar] [CrossRef]
- Jewgenow, K.; Azevedo, A.; Albrecht, M.; Kirschbaum, C.; Dehnhard, M. Hair cortisol analyses in different mammal species: Choosing the wrong assay may lead to erroneous results. Conserv. Physiol. 2020, 8, coaa009. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, V.E.; Naidenko, S.V.; Serbenyuk, M.A. Specific fights in lynx early ontogenesis (Felis lynx, Felidae, Carnivora). Zool. Zh. 1994, 73, 132–138. [Google Scholar]
- Pavlova, E.V.; Alekseeva, G.S.; Erofeeva, M.N.; Vasilieva, N.A.; Tchabovsky, A.V.; Naidenko, S.V. The method matters: The effect of handling time on cortisol level and blood parameters in wild cats. J. Exp. Zool. 2018, 329, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Goeritz, F.; Neubauer, K.; Naidenko, S.; Fickel, J.; Jewgenow, K. Experimental investigations on reproductive physiology in male Eurasian Lynx (Lynx lynx). Theriogenology 2006, 66, 1751–1754. [Google Scholar] [CrossRef]
- Mastromonaco, G.F.; Gunn, K.; McCurdy-Adams, H.; Edwards, D.B.; Schulte-Hostedde, A.I. Validation and use of hair cortisol as a measure of chronic stress in eastern chipmunks (Tamias striatus). Conserv. Physiol. 2014, 2, cou055. [Google Scholar] [CrossRef] [PubMed]
- Naidenko, S.V. Biology of reproduction of felids. The mechanism of changes of reproductive success. Diss. Dr. Habilit. Degree 2016, 1, 1–276. [Google Scholar]
- Glickman, S.E.; Frank, L.G.; Davidson, J.M.; Smith, E.R.; Sieteri, P.K. Androstenedione may organize or activate sex-reversed traits in female spotted hyenas. Proc. Natl. Acad. Sci. USA 1987, 84, 3444–3447. [Google Scholar] [CrossRef] [PubMed]
- von Engelhardt, N.; Kappeler, P.M.; Heistermann, M. Androgen levels and female social dominance in Lemur catta. Proc. R. Soc. London 2000, 267, 1533–1539. [Google Scholar] [CrossRef] [PubMed]
- Glukhov, D.V.; Naidenko, S.V. Seasonal changes of reproductive characteristics in teratospermic and normospermic males of domestic cats (Felis silvestris catus). Zool. Zh. 2013, 92, 1269–1274. [Google Scholar]
- Brown, J.L. Comparative endocrinology of domestic and nondomestic felids. Theriogenology 2006, 66, 25–36. [Google Scholar] [CrossRef]
- Blottner, S.; Jewgenow, K. Moderate seasonality in testis function of domestic cat. Reprod. Domest. Anim. 2007, 42, 536–540. [Google Scholar] [CrossRef]
- Erofeeva, M.N.; Pavlova, E.V.; Antonevich, A.L.; Naidenko, S.V. Seasonal changes in activity of males’ reproductive system in Eurasian lynx. Russ. J. Theriol. 2014, 13, 9–16. [Google Scholar] [CrossRef]
- Pavlova, E.V.; Naidenko, S.V. Characteristics of the dynamic of basal adrenal activity and its relation to the behavior of Far-Eastern cat (Prionailurus bengalensis euptilura). Zool. Zh. 2012, 91, 1261–1272. [Google Scholar]
- Bernar, R.T.F.; Stuart, C.T. Reproduction of the caracal Felis caracal from the Cape Province of South Africa. S. Afr. J. Zool. 1987, 22, 177–182. [Google Scholar] [CrossRef]
- Graham, L.H.; Goodrowe, K.L.; Raeside, J.I.; Liptrap, R.M. Non-invasive monitoring of ovarian function in several felid species by measurement of fecal estradiol-17β and progestins. Zoo Biol. 1995, 14, 223–237. [Google Scholar]
- Goodrowe, K.L.; GJCrawshaw Mehren, K.G. 1991. Stimulation of ovarian activity and oocyte recovery in the caracal (Felis caracal) and cheetah (Acinonyx jubatus). J. Zoo Wildl. Med. 1991, 22, 42–48. [Google Scholar]
- Kraus, C.; Pfannkuche, K.; Trillmich, F.; Groothuis, T.G.G. High maternal androstenedione levels during pregnancy in a small precocial mammal with female genital masculinization. MPIDR Work. Pap. 2008, 1–23. [Google Scholar] [CrossRef]
- Makieva, S.; Saunders, P.T.K.; Norman, J.E. Androgens in pregnancy: Roles in parturition. Hum. Reprod. Update 2014, 20, 542–559. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.S.; Birnie, A.K.; French, J.A. Prenatal Androgens Affect Development and Behavior in Primates. In Building Babies: Primate Development in Proximate and Ultimate Perspective, Developments in Primatology: Progress and Prospects; Clancy, K.B.H., Ed.; Springer: New York, NY, USA, 2013; Volume 37, pp. 103–131. [Google Scholar]
- Bélanger, A.; Cusan, A.L.; Caron, S.; Barden, N.; Dupont, A. Ovarian progestins, androgens and estrogen throughout the 4-day estrous cycle in the rat. Biol. Reprod. 1981, 24, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Fochi, R.A.; Perez, A.P.S.; Bianchi, C.V.; Rochel, S.S.; Goes, R.M.; Vilamaior, P.S.L.; Taboga, S.R.; Santos, F.C.A. Hormonal oscillations during the estrous cycle influence the morphophysiology of the gerbil (Meriones unguiculatus) female prostate (skene paraurethral glands). Biol. Reprod. 2008, 79, 1084–1091. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Wang, W.X.; Li, L.H.; Liu, B.Q.; Liu, G.; Liu, S.Q.; Qi, L.; Hu, D.F. Effects of crowding and sex on fecal cortisol levels of captive forest musk deer. Biol. Res. 2014, 47, 48. [Google Scholar] [CrossRef] [PubMed]
- Garber, P.A.; McKenney, A.; Bartling-John, E.; Bicca-Marques, J.C.; De la Fuente, M.F.; Abreu, F.; Schiel, N.; Souto, A.; Phillips, K.A. Life in a harsh environment: The effects of age, sex, reproductive condition, and season on hair cortisol concentration in a wild non-human primate. PeerJ 2020, 8, e9365. [Google Scholar] [CrossRef] [PubMed]
- Bechshøft, T.Ø.; Sonne, C.; Dietz, R.; Born, E.W.; Novak, M.A.; Henchey, E.; Meyer, J.S. Cortisol levels in hair of East Greenland polar bears. Sci. Total Environ. 2011, 409, 831–834. [Google Scholar] [CrossRef] [PubMed]
- Cattet, M.; Macbeth, B.J.; Janz, D.M.; Zedrosser, A.; Swenson, J.E.; Dumond, M.; Stenhouse, G.B. Quantifying long-term stress in brown bears with the hair cortisol concentration: A biomarker that may be confounded by rapid changes in response to capture and handling. Conserv. Physiol. 2014, 2, cou026. [Google Scholar] [CrossRef] [PubMed]
- Wielebnowski, N.C.; Ziegler, K.; Wildt, D.E.; Lukas, J.; Brown, J.L. Impact of social management on reproductive, adrenal and behavioral activity in the cheetah (Acinonyx jubatus). Anim. Conserv. 2002, 5, 291–301. [Google Scholar] [CrossRef]
- Pavlova, E.V.; Naidenko, S.V. Noninvasive monitoring of glucocorticoids in feces of the Bengal cat (Prionailurus bengalensis euptilura). Zool. Zh. 2008, 87, 1375–1381. [Google Scholar]
- Ramos, D.; Reche-Junior, A.; Fragoso, P.L.; Palme, R.; Yanasse, N.K.; Gouvêa, V.R.; Beck, A.; Mills, D.S. Are cats (Felis catus) from multi-cat households more stressed? Evidence from assessment of fecal glucocorticoid metabolite analysis. Physiol. Behav. 2013, 122, 72–75. [Google Scholar] [CrossRef]
- Sokolov, V.E.; Naidenko, S.V.; Serbenyuk, M.A. Marking bahaviour of European lynx (Felis lynx, Felidae, Carnivora). Izvestiya RAN 1995, 3, 345–355. [Google Scholar]
- Johnson, L.; Thompson, D.L.J.R. Age-related and seasonal variation in the Sertoli cell population, daily sperm production and serum concentrations of follicle-stimulating hormone, luteinizing hormone and testosterone in stallions. Biol. Reprod. 1983, 29, 777–789. [Google Scholar] [CrossRef]
- Hochereau-de Reviers, M.T.; Monet-Kuntz CCourot, M. Spermatogenesis and Sertoli cell markers and function in rams and bulls. J. Reprod. Fert. 1987, 34, 101–114. [Google Scholar]
- Jewgenow, K.; Goritz, F.; Neubauer, K.; Fickel, J.; Naidenko, S. Characterization of reproductive activity in captive male Eurasian lynx (Lynx lynx). J. Eur. Wildl. Res. 2006, 52, 34–38. [Google Scholar] [CrossRef]
- Swanson, W.F.; Brown, J.L.; Wildt, D.E. Influence of seasonality on reproductive traits in the male Pallas cat (Felis manul) and implications for captive management. J. Zoo Wildl. Med. 1996, 27, 234–240. [Google Scholar]
- Naidenko, S.V.; Hupe, K. Seasonal changes in home range use in feral tomcats in Solling, Central Germany. Zool. Zh. 2002, 81, 1371–1381. [Google Scholar]
- Breitenmoser, U.; Kaczensky, P.; Ditherer, M.; Breitenmoser-Wursten, C.; Capt, S.; Bernhart, F.; Liberek, M. Spatial organization and recruitment of lynx (Lynx lynx) in a re-introduced population in the Swiss Jura Mountains. J. Zool. 1993, 231, 449–464. [Google Scholar] [CrossRef]
- Naidenko, S.V.; Ivanov, E.A.; Lukarevskii, V.S.; Hernandes-Blanco, J.A.; Sorokin, P.A.; Litvinov, M.N.; Kotlyar, A.K.; Rozhnov, V.V. Activity of the hypothalamo-pituitary-adrenals axis in the Siberian tiger (Panthera tigris altaica) in captivity and in the wild, and its dynamics throughout the year. Biol. Bull. 2011, 38, 301–305. [Google Scholar] [CrossRef]
- Ivanov, E.A.; Rozhnov, V.V.; Naidenko, S.V. The effect of ambient temperature on glucocorticoid level in the Amur tiger (Panthera tigris altaica). Russ. J. Ecol. 2017, 48, 294–297. [Google Scholar] [CrossRef]
- Wielebnowski, N.C.; Fletchall, N.; Carlstead, K.; Busso, J.M.; Brown, J.L. Non-invasive assessment of adrenal activity associated with husbandry and behavioral factors in the North American clouded leopard population. Zoo Biol. 2002, 21, 77–98. [Google Scholar] [CrossRef]
- Carter, C.S.; Perkeybile, A.M. The Monogamy Paradox: What Do Love and Sex Have to Do with It? Front. Ecol. Evol. 2018, 6, 202. [Google Scholar] [CrossRef]
- Wasser, S.K.; Papageorge, S.; Foley, C.; Brown, J.L. Excretory fate of estradiol and progesterone in the African elephant (Loxodonta africana) and patterns of fecal steroid concentrations throughout the estrous cycle. Gen. Comp. Endocrin. 1996, 102, 255–262. [Google Scholar] [CrossRef]
- Heistermann, M.; Agil, M.; Buthe, A.; Hodges, J.K. Metabolism and excretion of oestradiol-17b and progesterone in the Sumatran rhinoceros Dicerorhinus sumatrensis. Anim. Reprod. Sci. 1998, 53, 157–172. [Google Scholar] [CrossRef]
- Dehnhard, M.; Fanson, K.; Frank, A.; Naidenko, S.V.; Vargas, A.; Jewgenow, K. Comparative metabolism of gestagens and estrogens in the four lynx species, the Eurasian (Lynx lynx), the Iberian (L. pardinus), the Canada lynx (L. canadensis) and the bobcat (L. rufus). Gen. Comp. Endocrin. 2010, 167, 287–296. [Google Scholar] [CrossRef] [PubMed]





| Species | Number of Males | Number of Females | Age Limits (Years) |
|---|---|---|---|
| Domestic cat | 5 | 12 | 1–6 |
| Eurasian lynx | 4 | 4 | 3–12 |
| Caracal | 4 | 2 | 2–9 |
| Amur wildcat | 4 | 4 | 2–7 |
| Species | Type of Enclosure | Size, m2 |
|---|---|---|
| Domestic cat | Outdoor | 4–6 |
| Eurasian lynx | Outdoor | 74 |
| Caracal (summer–autumn) | Outdoor | 12–74 |
| Caracal (winter–spring) | Indoor (+15 °C) with regular walking hours in outdoor enclosure | 4 (indoor) + 12 (outdoor) |
| Amur wildcat | Outdoor | 8–16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naidenko, S.V.; Alekseeva, G.S.; Klyuchnikova, P.S.; Erofeeva, M.N. Application of Felid Hair for Non-Invasive Tracking of Animal Reproductive Status and Adrenal Activity. Animals 2022, 12, 2792. https://doi.org/10.3390/ani12202792
Naidenko SV, Alekseeva GS, Klyuchnikova PS, Erofeeva MN. Application of Felid Hair for Non-Invasive Tracking of Animal Reproductive Status and Adrenal Activity. Animals. 2022; 12(20):2792. https://doi.org/10.3390/ani12202792
Chicago/Turabian StyleNaidenko, Sergey V., Galina S. Alekseeva, Polina S. Klyuchnikova, and Mariya N. Erofeeva. 2022. "Application of Felid Hair for Non-Invasive Tracking of Animal Reproductive Status and Adrenal Activity" Animals 12, no. 20: 2792. https://doi.org/10.3390/ani12202792
APA StyleNaidenko, S. V., Alekseeva, G. S., Klyuchnikova, P. S., & Erofeeva, M. N. (2022). Application of Felid Hair for Non-Invasive Tracking of Animal Reproductive Status and Adrenal Activity. Animals, 12(20), 2792. https://doi.org/10.3390/ani12202792





