What Forms, Maintains, and Changes the Boldness of Swimming Crabs (Portunus trituberculatus)?
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Collection and Maintenance
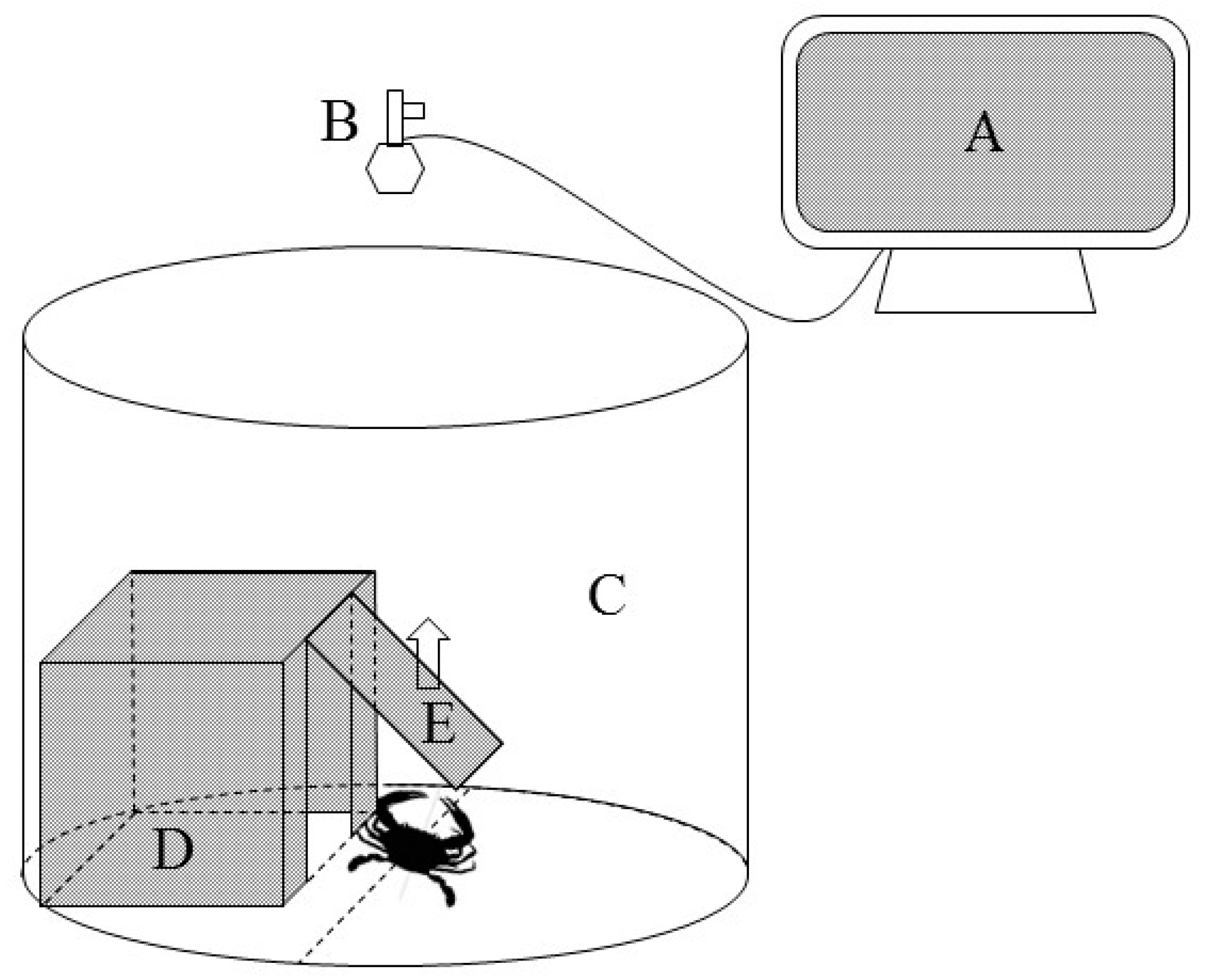
2.2. Experimental Process and Sample Collection
2.3. Data Collection and Measurement
2.3.1. Determination of Boldness
2.3.2. Energy Sources
2.3.3. mRNA Relative Expression of Energy-Metabolism-Related and 5-HT Genes
- RNA extraction and reverse transcription
- mRNA expression of related genes
2.4. Data Analysis
3. Results
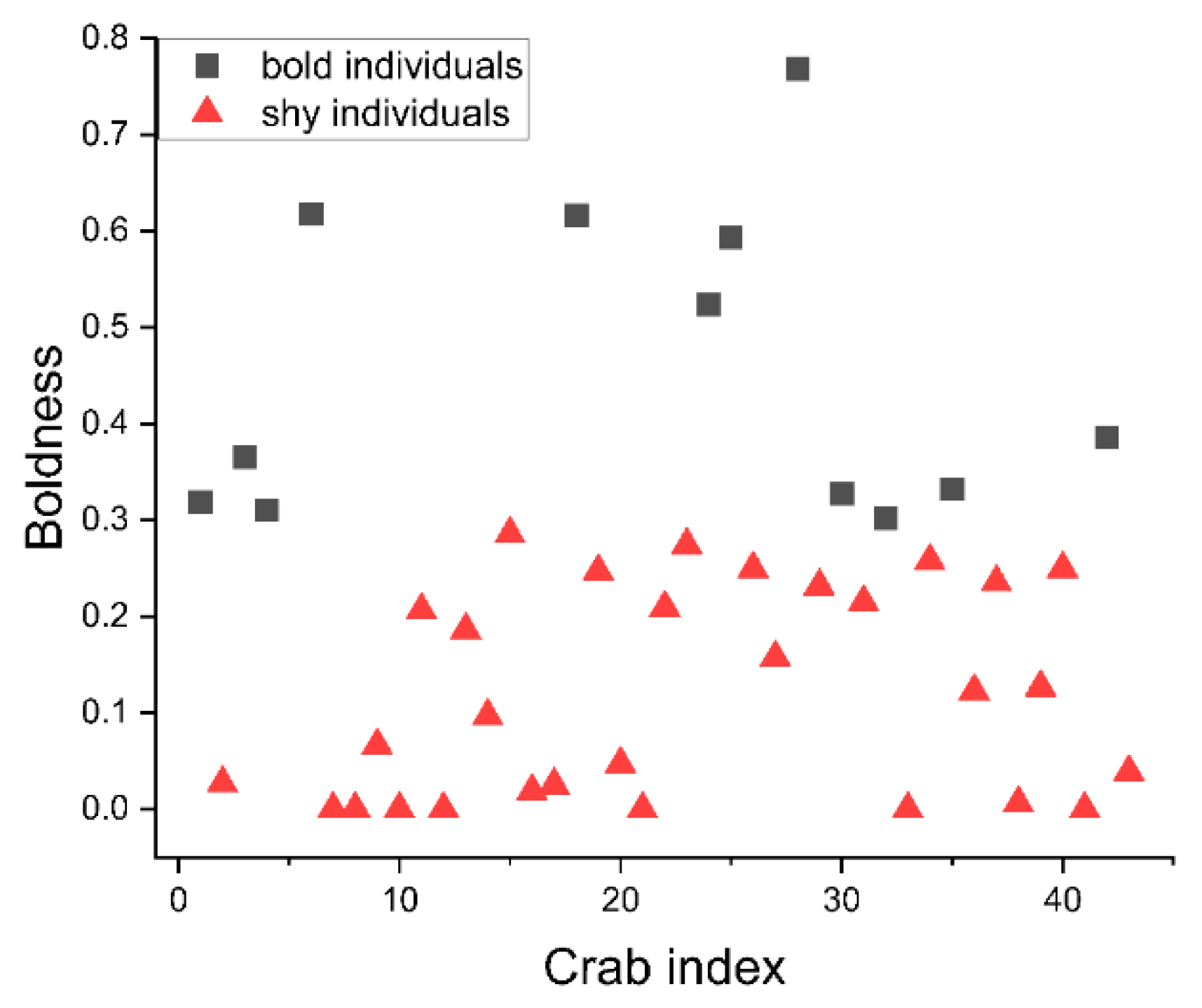
3.1. Cluster Analysis of Boldness
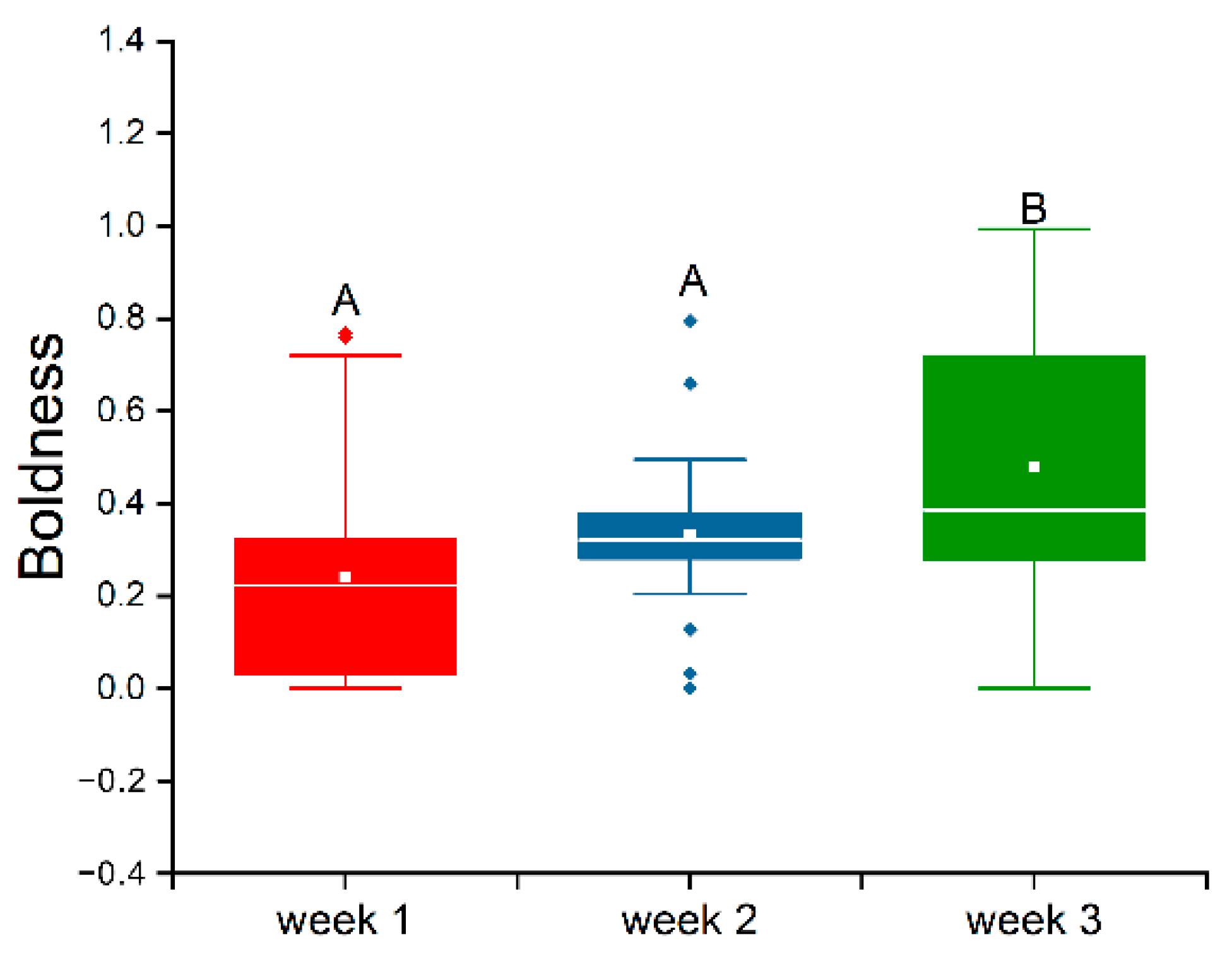
3.2. Stability of Boldness
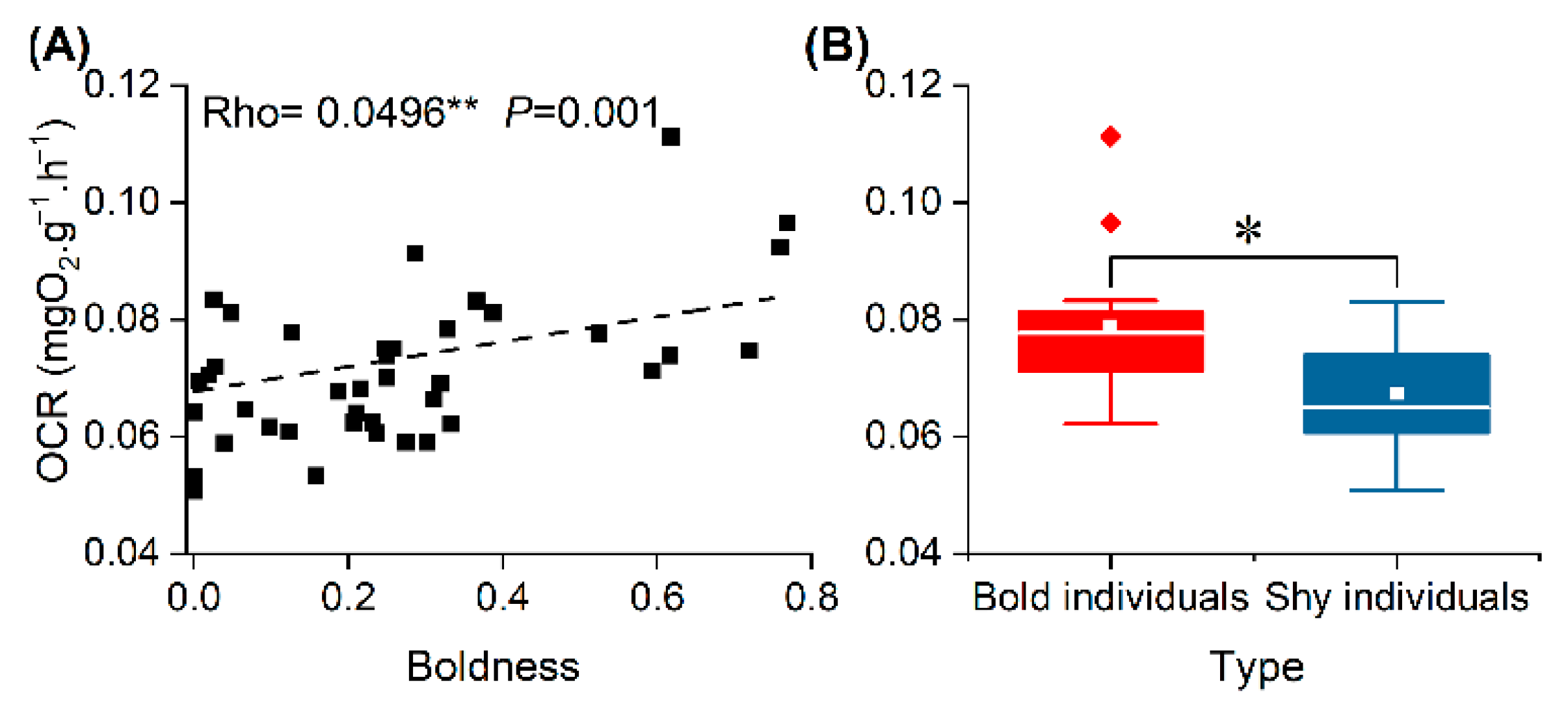
3.3. OCR
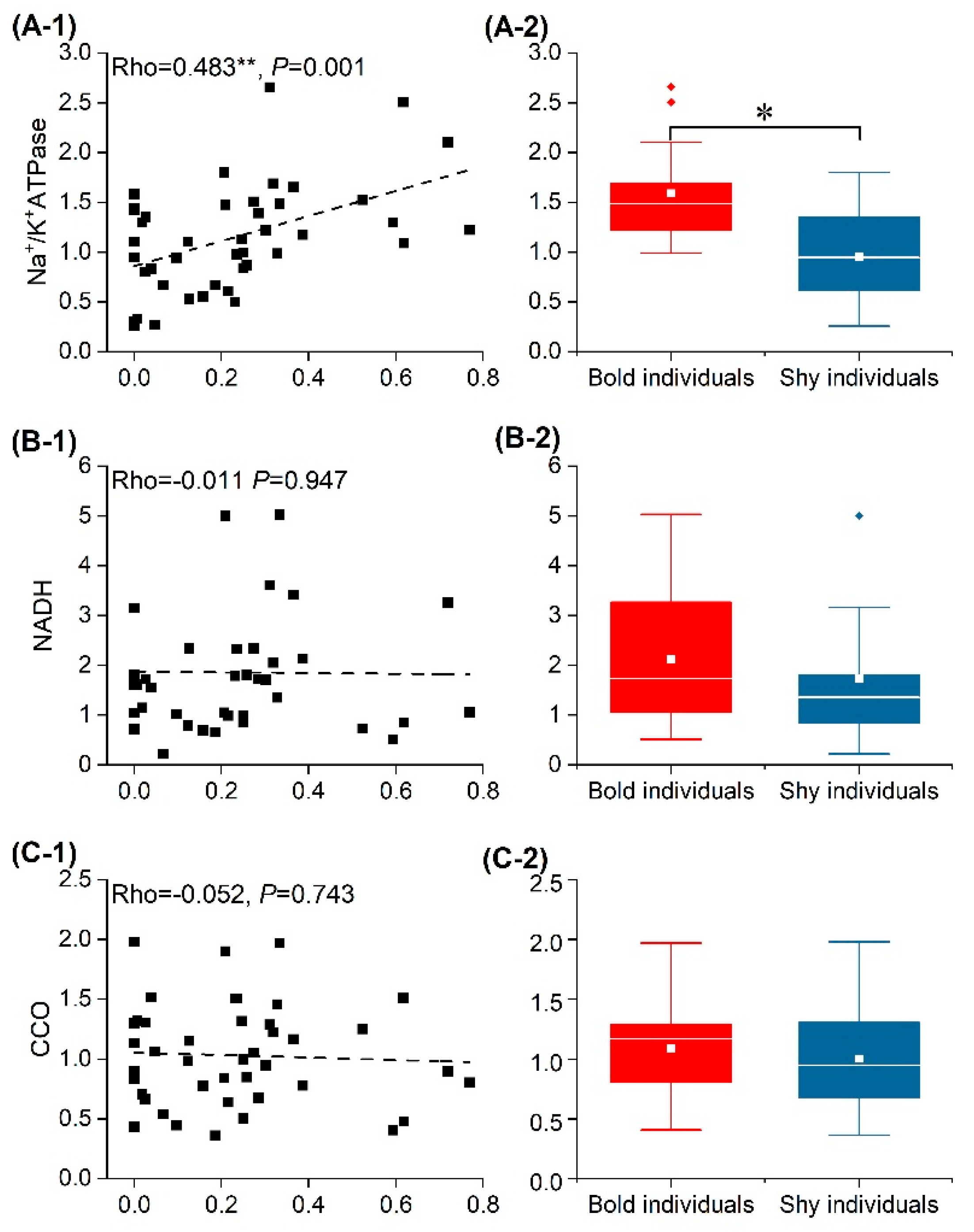
3.4. Energy Source
3.5. mRNA Relative Expression of Energy-Metabolism-Related and 5-HT Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cabrera, D.; Nilsson, J.R.; Griffen, B.D. The development of animal personality across ontogeny: A cross-species review. Anim. Behav. 2021, 173, 137–144. [Google Scholar] [CrossRef]
- Carere, C.; van Oers, K. Shy and bold great tits (Parus major): Body temperature and breath rate in response to handling stress. Physiol. Behav. 2004, 82, 905–912. [Google Scholar] [CrossRef]
- Rudin, F.S.; Briffa, M. Is boldness a resource-holding potential trait? Fighting prowess and changes in startle response in the sea anemone, Actinia equina. Proc. Biol. Sci. 2012, 279, 1904–1910. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Su, X.; Liu, D.; Guo, Z.; Wang, F.; Lu, Y. A New Method of Aquatic Animal Personality Analysis Based on Machine Learning (PAML): Taking Swimming Crab Portunus trituberculatus as an Example. Front. Mari. Sci. 2020, 7, 32. [Google Scholar] [CrossRef]
- Reaney, L.T.; Backwell, P.R.Y. Risk-taking behavior predicts aggression and mating success in a fiddler crab. Behav. Ecol. 2007, 18, 521–525. [Google Scholar] [CrossRef] [Green Version]
- Courtene-Jones, W.; Briffa, M. Boldness and asymmetric contests: Role- and outcome-dependent effects of fighting in hermit crabs. Behav. Ecol. 2014, 25, 1073–1082. [Google Scholar] [CrossRef] [Green Version]
- Briffa, M.; Rundle, S.D.; Fryer, A. Comparing the strength of behavioural plasticity and consistency across situations: Animal personalities in the hermit crab Pagurus bernhardus. Proc. Biol. Sci. 2008, 275, 1305–1311. [Google Scholar] [CrossRef] [Green Version]
- Vainikka, A.; Rantala, M.J.; Niemelä, P.; Hirvonen, H.; Kortet, R. Boldness as a consistent personality trait in the noble crayfish, Astacus astacus. Acta Ethol. 2010, 14, 17–25. [Google Scholar] [CrossRef]
- Favreau, F.-R.; Goldizen, A.W.; Fritz, H.; Blomberg, S.P.; Best, E.C.; Pays, O. Within-population differences in personality and plasticity in the trade-off between vigilance and foraging in kangaroos. Anim. Behav. 2014, 92, 175–184. [Google Scholar] [CrossRef]
- Smith, B.R.; Blumstein, D.T. Fitness consequences of personality: A meta-analysis. Behav. Ecol. 2008, 19, 448–455. [Google Scholar] [CrossRef] [Green Version]
- Le Galliard, J.-F.; Paquet, M.; Cisel, M.; Montes-Poloni, L.; Franklin, C. Personality and the pace-of-life syndrome: Variation and selection on exploration, metabolism and locomotor performances. Funct. Ecol. 2013, 27, 136–144. [Google Scholar] [CrossRef]
- Cutts, C.J.; Adams, C.E.; Campbell, A. Stability of physiological and behavioural determinants of performance in Arctic char (Salvelinus alpinus). Can. J. Fish. Aquat. Sci. 2001, 58, 961–968. [Google Scholar] [CrossRef]
- Kucharski, R.; Maleszka, J.; Foret, S.; Maleszka, R. Nutritional control of reproductive status in honeybees via DNA methylation. Science 2008, 319, 1827–1830. [Google Scholar] [CrossRef] [Green Version]
- Velando, A.; Costa, M.M.; Kim, S.-Y.; Holman, L. Sex-specific phenotypes and metabolism-related gene expression in juvenile sticklebacks. Behav. Ecol. 2017, 28, 1553–1563. [Google Scholar] [CrossRef]
- Briffa, M.; Sneddon, L.U.; Wilson, A.J. Animal personality as a cause and consequence of contest behaviour. Biol. Lett. 2015, 11, 20141007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Listerman, L.R.; Deskins, J.; Bradacs, H.; Cooper, R.L. Heart rate within male crayfish: Social interactions and effects of 5-HT. Comp. Biochem. Phys. A 2000, 125, 251–263. [Google Scholar] [CrossRef] [Green Version]
- Su, X.; Sun, Y.; Liu, D.; Wang, F.; Liu, J.; Zhu, B. Agonistic behaviour and energy metabolism of bold and shy swimming crabs Portunus trituberculatus. J. Exp. Biol. 2019, 222, jeb188706. [Google Scholar] [CrossRef] [Green Version]
- Wolf, M.; Weissing, F.J. Animal personalities: Consequences for ecology and evolution. Trends Ecol. Evol. 2012, 27, 452–461. [Google Scholar] [CrossRef]
- Coleman, K.; Wilson, D.S. Shyness and boldness in pumpkinseed sunfish: Individual differences are context-specific. Anim. Behav. 1998, 56, 927–936. [Google Scholar] [CrossRef] [Green Version]
- Hazlett, B.A. Behavioral plasticity in crustacea: Why not more? J. Exp. Mar. Biol. Ecol. 1995, 193, 57–66. [Google Scholar] [CrossRef]
- Belgrad, B.A.; Karan, J.; Griffen, B.D. Individual personality associated with interactions between physiological condition and the environment. Anim. Behav. 2017, 123, 277–284. [Google Scholar] [CrossRef] [Green Version]
- Ding, S.; Wang, F.; Dong, S.; Li, Y. Comparison of the respiratory metabolism of juvenile Litopenaeus vannamei cultured in seawater and freshwater. J. Ocean Univ. China 2013, 13, 331–337. [Google Scholar] [CrossRef]
- Ren, Q.; Pan, L. Digital gene expression analysis in the gills of the swimming crab (Portunus trituberculatus) exposed to elevated ambient ammonia-N. Aquaculture 2014, 434, 108–114. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, D.; Wang, F.; Dong, S. Hypothermal effects on survival, energy homeostasis and expression of energy-related genes of swimming crabs Portunus trituberculatus during air exposure. J. Therm. Biol. 2016, 60, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.Y.; Zhang, B.; Li, J.H.; Fan, Q.X.; Zhang, Y.; Mu, D.G.; Li, W.P.; Xu, M.; Zhao, P.T.; Jin, F.G. Sodium tanshinone iia sulfonate attenuates seawater aspiration-induced acute pulmonary edema by up-regulating Na(+),K(+)-ATPase activity. Exp. Lung. Res. 2011, 37, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Pan, L.; Ding, Y.; Ren, X. Effects of low salinity stress on immune response and evaluating indicators of the swimming crab Portunus trituberculatus. Aquac. Res. 2018, 49, 659–667. [Google Scholar] [CrossRef]
- Chapman, B.B.; Hegg, A.; Ljungberg, P. Sex and the syndrome: Individual and population consistency in behaviour in rock pool prawn Palaemon elegans. PLoS ONE 2013, 8, e59437. [Google Scholar] [CrossRef]
- Briffa, M.; Twyman, C. Do I stand out or blend in? Conspicuousness awareness and consistent behavioural differences in hermit crabs. Biol. Lett. 2011, 7, 330–332. [Google Scholar] [CrossRef] [Green Version]
- Briffa, M.; Bridger, D.; Biro, P.A. How does temperature affect behaviour? Multilevel analysis of plasticity, personality and predictability in hermit crabs. Anim. Behav. 2013, 86, 47–54. [Google Scholar] [CrossRef]
- Takahashi, M.; Suzuki, N.; Koga, T. Burrow defense behaviors in a sand-bubbler crab, Scopimera globosa, in relation to body size and prior residence. J. Ethol. 2001, 19, 93–96. [Google Scholar] [CrossRef]
- Liang, Q.; Su, X.; Wang, F.; Zhu, B.; He, M. The Developmental Plasticity of Boldness and Aggressiveness in Juvenile and Adult Swimming Crab (Portunus trituberculatus). Front. Mar. Sci. 2020, 7, 1008. [Google Scholar] [CrossRef]
- Mittelbach, G.G.; Ballew, N.G.; Kjelvik, M.K.; Fraser, D. Fish behavioral types and their ecological consequences. Can. J. Fish. Aquat. Sci. 2014, 71, 927–944. [Google Scholar] [CrossRef]
- Briffa, M.; Elwood, R.W. Use of energy reserves in fighting hermit crabs. Proc. Biol. Sci. 2004, 271, 373–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gribben, P.E.; O’Connor, J.; Pedini, L.; Biro, P.A. Personality and plasticity: Consistent responses within-, but not across-temperature situations in crabs. Behaviour 2013, 150, 799–811. [Google Scholar] [CrossRef]
- Decker, R.A.; Griffen, B.D. Correlating context-specific boldness and physiological condition of female sand fiddler crabs (Uca pugilator). J. Ethol. 2012, 30, 403–412. [Google Scholar] [CrossRef]
- Liu, S.; Fu, S.J. Effects of food availability on metabolism, behaviour, growth and their relationships in a triploid carp. J. Exp. Biol. 2018, 221, 4711–4719. [Google Scholar] [CrossRef] [Green Version]
- Huntingford, F.A.; Andrew, G.; Mackenzie, S.; Morera, D.; Coyle, S.M.; Pilarczyk, M.; Kadri, S. Coping strategies in a strongly schooling fish, the common carp Cyprinus carpio. J. Fish. Biol. 2010, 76, 1576–1591. [Google Scholar] [CrossRef]
- Zhu, B.; Wang, F.; Su, X.; Lu, Y.; Zhang, H. Effect of different amount of food and female resource on competitive strategy and agonistic behavior of swimming crab (Portunus trituberculatus). Aquaculture 2021, 536, 736471. [Google Scholar] [CrossRef]
- Careau, V.; Thomas, D.; Humphries, M.M.; Re´ale, D. Energy metabolism and animal personality. Oikos 2008, 117, 641–653. [Google Scholar] [CrossRef]
- Iftikar, F.I.; MacDonald, J.; Hickey, A.J.R. Thermal limits of portunid crab heart mitochondria: Could more thermo-stable mitochondria advantage invasive species? J. Exp. Mar. Biol. Ecol. 2010, 395, 232–239. [Google Scholar] [CrossRef]
- Frederich, M.; O′Rourke, M.R.; Furey, N.B.; Jost, J.A. AMP-activated protein kinase (AMPK) in the rock crab, Cancer irroratus: An early indicator of temperature stress. J. Exp. Biol. 2009, 212, 722–730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weihrauch, D.; Morris, S.; Towle, D.W. Ammonia excretion in aquatic and terrestrial crabs. J. Exp. Biol. 2004, 207, 4491–4504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coppens, C.M.; de Boer, S.F.; Koolhaas, J.M. Coping styles and behavioural flexibility: Towards underlying mechanisms. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2010, 365, 4021–4028. [Google Scholar] [CrossRef] [PubMed]
- Filby, A.L.; Paull, G.C.; Hickmore, T.F.; Tyler, C.R. Unravelling the neurophysiological basis of aggression in a fish model. BMC Genom. 2010, 11, 498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saudou, F.; Amara, D.A.; Dierich, A.; LeMeur, M.; Ramboz, S.; Segu, L.; Buhot, M.-C.; Hen, R. Enhanced aggressive behavior in mice lacking 5-HT1B receptor. Science 1994, 265, 1875–1878. [Google Scholar] [CrossRef] [PubMed]
- Broadhurst, H.E.; Morrell, L.J. Group size and individual ‘personality’ influence emergence times in hermit crabs. Biosci. Horiz. Int. J. Stud. Res. 2018, 11, hzy011. [Google Scholar] [CrossRef] [Green Version]


| Genes | Primer Sequence | |
|---|---|---|
| CCO | Forward | 5′-GGCTACCAGCAGTGACA-3′ |
| Reverse | 5′-ATGGCTGCCTCTCTCACCT-3′ | |
| NADH-QR | Forward | 5′-CAATCATCGCACAGGAGAG-3′ |
| Reverse | 5′-CCAGACGCTGTCGTTTCAA-3′ | |
| Na+/K+-ATPase | Forward | 5′-TACAAGAACGGAGACAACGA-3′ |
| Reverse | 5′-TCAAGCGACACACTTTCCTCT-3′ | |
| AMPKα | Forward | 5′-5GCAATAGCAGGCGTACAACA-3′ |
| Reverse | 5′-CTTCTGCCCTTTGATGATGAG-3′ | |
| 5-Hydroxytryptamine receptor 1 | Forward | 5’-TGTTAGCATTGCCCAGGT-3′ |
| Reverse | 5′-AGTCTCTATCCCGAGGTTCTAC-3′ | |
| β-actin | Forward | 5′-TGCTGTCCTTGTACGCCTCC-3′ |
| Reverse | 5′-CAGACGCAGGATAGCGTGA-3′ | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, B.; Su, X.; Yu, W.; Wang, F. What Forms, Maintains, and Changes the Boldness of Swimming Crabs (Portunus trituberculatus)? Animals 2022, 12, 1618. https://doi.org/10.3390/ani12131618
Zhu B, Su X, Yu W, Wang F. What Forms, Maintains, and Changes the Boldness of Swimming Crabs (Portunus trituberculatus)? Animals. 2022; 12(13):1618. https://doi.org/10.3390/ani12131618
Chicago/Turabian StyleZhu, Boshan, Xianpeng Su, Weiping Yu, and Fang Wang. 2022. "What Forms, Maintains, and Changes the Boldness of Swimming Crabs (Portunus trituberculatus)?" Animals 12, no. 13: 1618. https://doi.org/10.3390/ani12131618
APA StyleZhu, B., Su, X., Yu, W., & Wang, F. (2022). What Forms, Maintains, and Changes the Boldness of Swimming Crabs (Portunus trituberculatus)? Animals, 12(13), 1618. https://doi.org/10.3390/ani12131618







