Corynebacterium pseudotuberculosis Infections in Alpacas (Vicugna pacos)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Alpaca Herds and Samples
2.2. Serological Examinations
2.3. Bacteriological Examinations
2.4. Post-Mortem Examinations
2.5. Statistical Analyses
3. Results
3.1. Herd Study
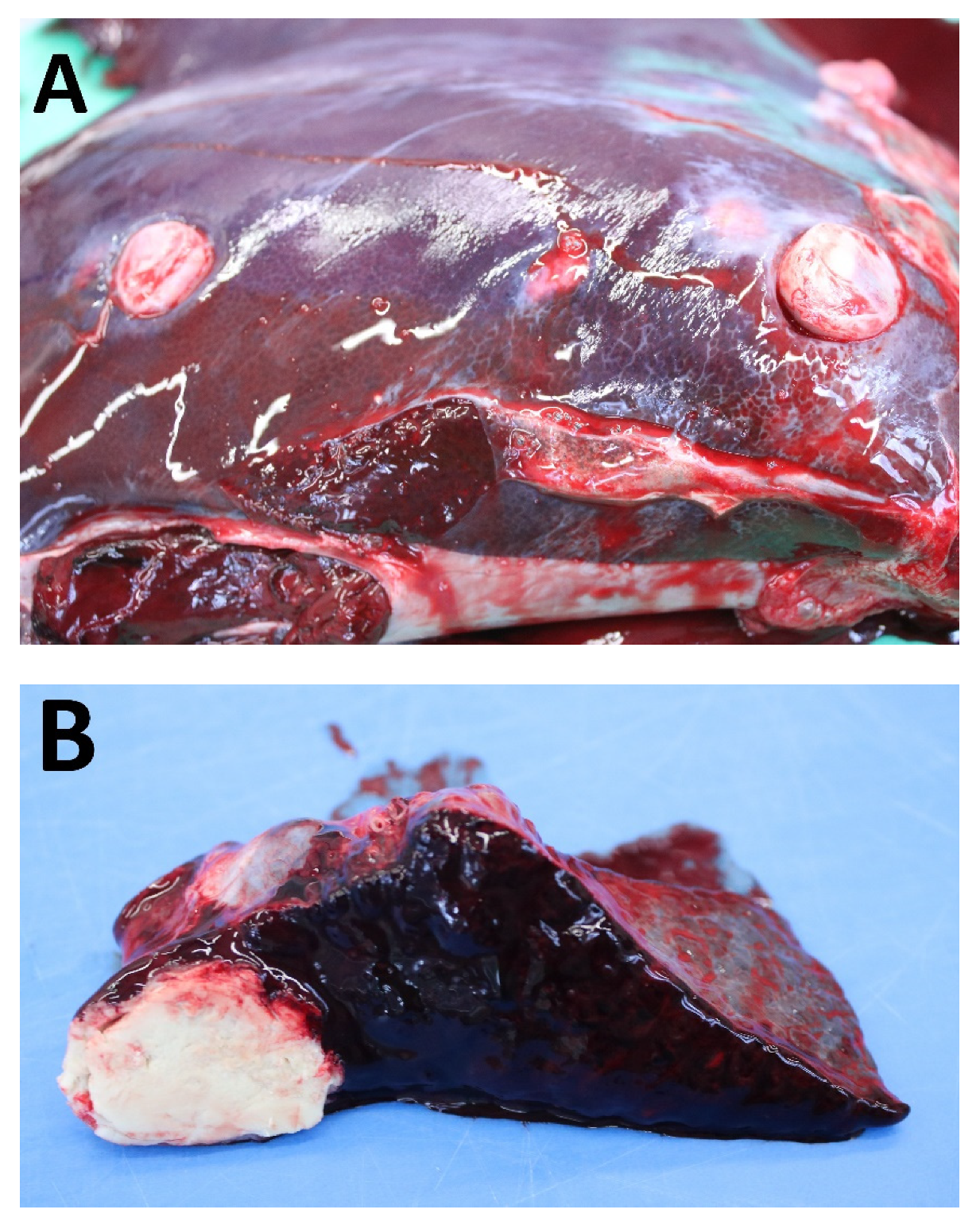
3.2. Post-Mortem Examinations
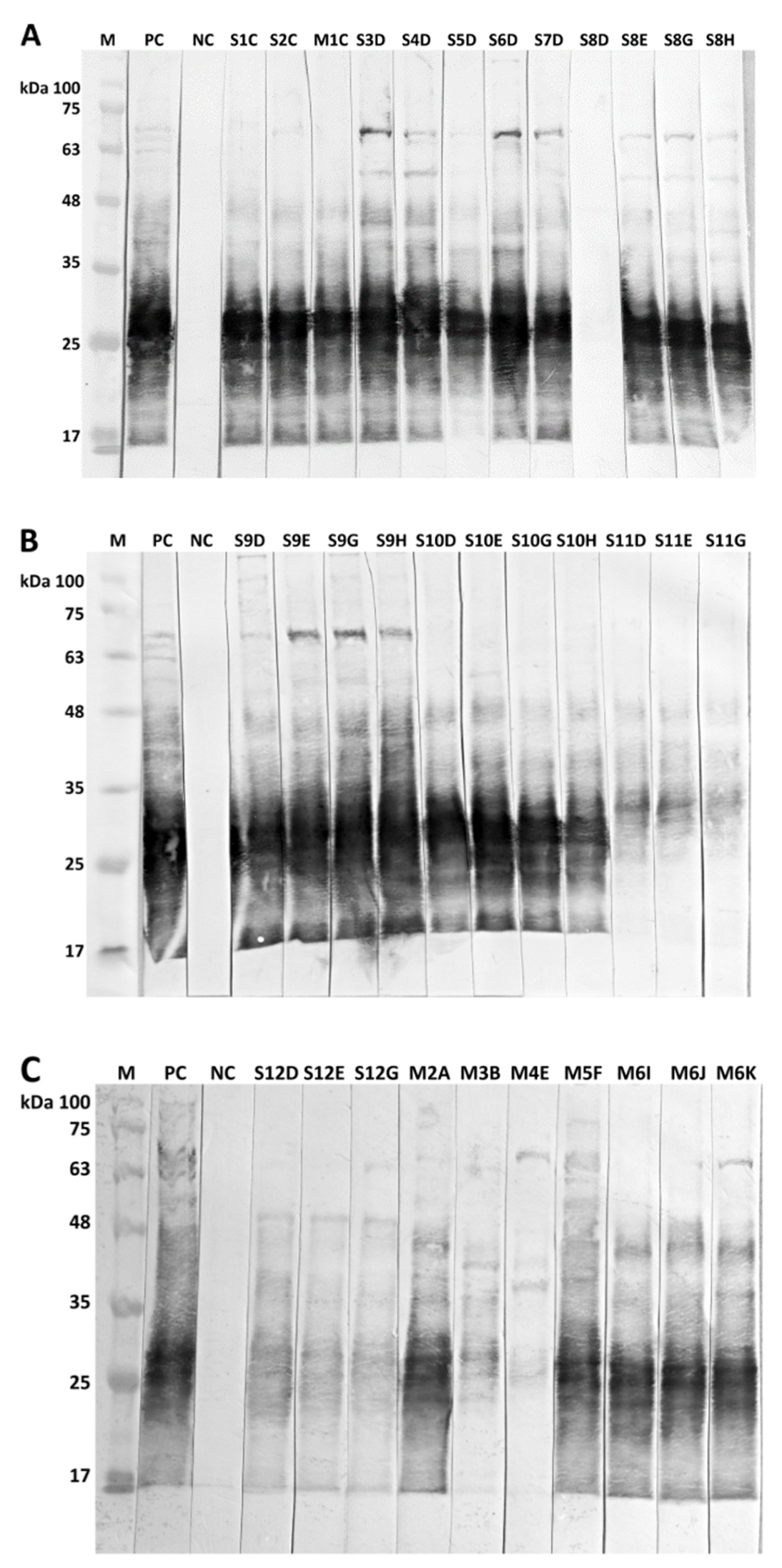
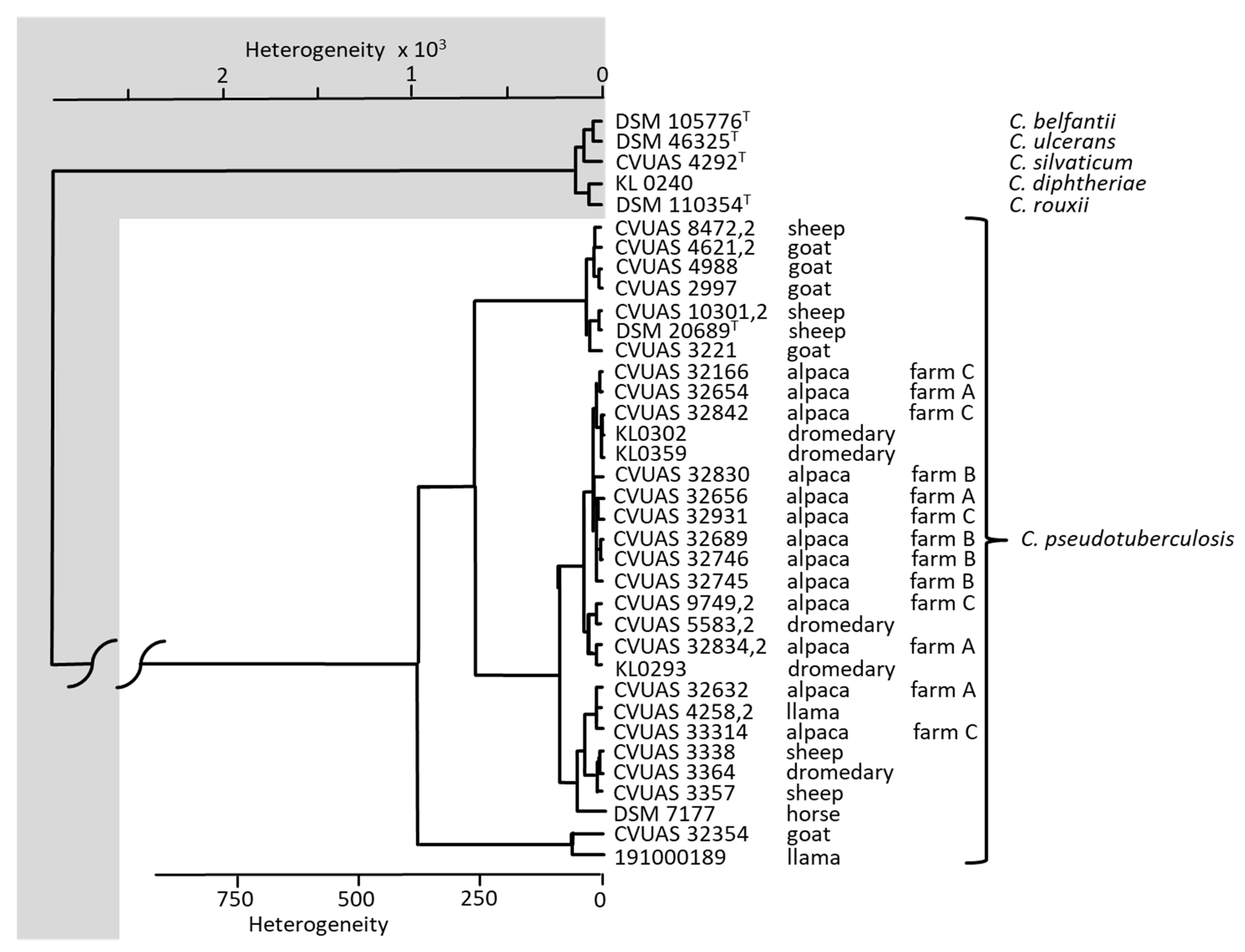
3.3. Bacteriological Examinations
3.4. Comparative Serological Examinations
4. Discussion
4.1. Post-Mortem Examinations
4.2. Bacteriological Examinations
4.3. Serological Examinations
4.4. Source of Infection, Incubation Period
4.5. Treatment and Control of CLA
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- D’Alterio, G.L.; Knowles, T.G.; Eknaes, E.I.; Loevland, I.E.; Foster, A.P. Postal survey of the population of South American camelids in the United Kingdom in 2000/01. Vet. Rec. 2006, 158, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Neubert, S.; von Altrock, A.; Wendt, M.; Wagener, M.G. Llama and alpaca management in Germany—Results of an online survey among owners on farm structure, health problems and self-reflection. Animals 2021, 11, 102. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.E.; Rings, D.M.; Kowalski, J. Infection with Corynebacterium pseudotuberculosis in five alpacas. J. Am. Vet. Med. Assoc. 2004, 225, 1743–1747. [Google Scholar] [CrossRef] [PubMed]
- Wernery, U.; Kinne, J. Caseous Lymphadenitis (Pseudotuberculosis) in Camelids: A Review. Austin J. Vet. Sci. Animal Husb. 2016, 3, 1022. [Google Scholar]
- Braga, W.U.; Chavera, A.E.; Gonzalez, A.E. Corynebacterium pseudotuberculosis infection in highland alpacas (Lama pacos) in Peru. Vet. Rec. 2006, 159, 23–24. [Google Scholar] [CrossRef]
- Sting, R.; Rietschel, W.; Polley, B.; Süß-Dombrowski, C.; Rau, J. Corynebacterium pseudotuberculosis infection in a dromedary (Camelus dromedaries) in Germany. Berl. Munch. Tierarztl. Wochenschr. 2017, 130, 511–516. [Google Scholar] [CrossRef]
- Sprake, P.; Gold, J.R. Corynebacterium pseudotuberculosis liver abscess in a mature alpaca (Lama pacos). Can. Vet. J. 2012, 53, 387–390. [Google Scholar]
- Braga, W.U.; Chavera, A.E.; Gonzalez, A.E. Clinical, humoral, and pathologic findings in adult alpacas with experimentally induced Corynebacterium pseudotuberculosis infection. Am. J. Vet. Res. 2006, 67, 1570–1574. [Google Scholar] [CrossRef]
- Braga, W.U. Protection in alpacas against Corynebacterium pseudotuberculosis using different bacterial components. Vet. Microbiol. 2007, 119, 297–303. [Google Scholar] [CrossRef]
- Braga, W.U.; Schul, S.; Nunez, A.; Pezo, D.; Franco, E. A primary Corynebacterium pseudotuberculosis low dose infection in alpacas (Lama pacos) protects against a lethal challenge exposure. Small Rumin. Res. 2007, 72, 81–86. [Google Scholar] [CrossRef]
- Spier, S.; Leutenegger, C.; Carroll, S.P.; Loye, J.E.; Berger Pusterla, J.; Carpenter, T.E.; Mihalyi, J.E.; Madigan, J.E. Use of a real-time polymerase chain reaction-based fluorogenic 5’ nuclease assay to evaluate insect vectors of Corynebacterium pseudotuberculosis infections in horses. Am. J. Vet. Res. 2004, 65, 829–834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoelzle, L.E.; Scherrer, T.; Muntwyler, J.; Wittenbrink, M.M.; Philipp, W.; Hoelzle, K. Differences in the antigen structures of Corynebacterium pseudotuberculosis and the induced humoral immune response in sheep and goats. Vet. Microbiol. 2013, 164, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Sting, R.; Schneider-Bühl, L.; Wagner, H.; Maget, J.; Polley, B.; Bürstel, D.; Axt, H. Clinical and serological investigations on caseous lymphadenitis in goat breeding herds in Baden-Wuerttemberg. Berl. Munch. Tierarztl. Wochenschr. 2017, 130, 136–143. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Rau, J.; Eisenberg, T.; Männig, A.; Wind, C.; Lasch, P.; Sting, R. MALDI-UP—An Internet Platform for the Exchange of MALDI-TOF Mass Spectra—User guide. Asp. Food Control. Anim. Health 2016, 1, 1–17. Available online: https://ejournal.cvuas.de/issue201603 (accessed on 28 March 2022).
- Dangel, A.; Berger, A.; Rau, J.; Eisenberg, T.; Kämpfer, P.; Margos, G.; Contzen, M.; Busse, H.J.; Konrad, R.; Peters, M.; et al. Corynebacterium silvaticum sp. nov., a unique group of NTTB corynebacteria in wild boar and roe deer. Int. J. Syst. Evol. Microbiol. 2020, 70, 3614–3624. [Google Scholar] [CrossRef]
- Barksdale, L.; Linder, R.; Sulea, I.T.; Pollice, M. Phospholipase D activity of Corynebacterium pseudotuberculosis (Corynebacterium ovis) and Corynebacterium ulcerans, a distinctive marker within the genus Corynebacterium. J. Clin. Microbiol. 1981, 13, 335–343. [Google Scholar] [CrossRef] [Green Version]
- MacFaddin, J.F. Biochemical Tests for Identification of Medical Bacteria, 3rd ed.; Williams and Wilkins: Baltimore, MD, USA, 2000; pp. 348–362. [Google Scholar]
- Thrusfield, M.; Ortega, C.; de Blas, I.; Noordhuizen, J.P.; Frankena, K. Win Episcope 2.0: Improved epidemiological software for veterinary medicine. Vet. Rec. 2001, 148, 567–572. [Google Scholar] [CrossRef] [Green Version]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [Green Version]
- Villena, S.C. Abscesos en Alpacas: Aspectos Microbiológicos. Ph.D. Thesis, Facultad de Medicina Veterinaria, Universidad Nacional Mayor de San Marcos, Lima, Peru, 1985. [Google Scholar]
- Kobera, R.; Poehle, D. Case report in South American camelid. In Proceedings of the 4th European Symposium on South American Camelid and DECAMA European Seminar, Göttingen, Germany, 7–9 October 2004. [Google Scholar]
- Sting, R.; Schwalm, A.K.; Contzen, M.; Roller, M.; Rau, J. Actinomycetes associated with abscess formation in a goat, a llama and two alpacas. Berl. Munch. Tierarztl. Wochenschr. 2020, 2020, 133. [Google Scholar] [CrossRef]
- Rau, J.; Eisenberg, T.; Peters, M.; Berger, A.; Kutzer, P.; Lassnig, H.; Hotzel, H.; Sing, A.; Sting, R.; Contzen, M. Reliable differentiation of a non-toxigenic tox gene-bearing Corynebacterium ulcerans variant frequently isolated from game animals using MALDI-TOF MS. Vet. Microbiol. 2019, 237, 108399. [Google Scholar] [CrossRef] [PubMed]
- Schulthess, S. Wirksamkeit Antimikrobieller Wirkstoffe bei Corynebacterium pseudotuberculosis: Etablierung und Anwendung einer Empfindlichkeitstestung Mittels Bouillonmikrodilution und Agardiffusion. Ph.D. Thesis, University of Zurich, Zurich, Switzerland, 2013. [Google Scholar]
- Costa, L.R.R.; Spier, S.J.; Hirssh, D.C. Comparative molecular characterization of Corynebacterium pseudotuberculosis of different origin. Vet. Microbiol. 1998, 62, 135–143. [Google Scholar] [CrossRef]
- Fernández, E.P.; Vela, A.I.; Heras, A.L.; Dominguez, L.; Fernández-Garayzábal, J.F.; Morena, M.A. Antimicrobial susceptibility of corynebacteria isolated from ewe’s mastitis. Int. J. Antimicrob. Agents 2001, 18, 571–574. [Google Scholar] [CrossRef]
- Muckle, C.A.; Gyles, C.L. Characterization of strains of Corynebacterium pseudotuberculosis. Can. J. Comp. Med. 1982, 46, 206–208. [Google Scholar]
- Pépin, M.; Boisramé, A.; Marly, J. Corynebacterium pseudotuberculosis: Biochemical properties, production of toxin and virulence of ovine and caprine strains. Ann. Rech. Vet. 1989, 20, 111–115. [Google Scholar]
- Olson, M.E.; Ceri, H.; Morck, D.W.; Buret, A.G.; Read, R.R. Biofilm bacteria: Formation and comparative susceptibility to antibiotics. Can. J. Vet. Res. 2002, 66, 86–92. [Google Scholar]
- Senturk, S.; Temizel, M. Clinical efficacy of rifampicin SV combined with oxytetracycline in the treatment of caseous lymphadenitis in sheep. Vet. Rec. 2006, 159, 216–217. [Google Scholar] [CrossRef]
- Borham, M.A.; Oreiby, A.F.; El-Gedawy, A.A.; Al-Gaabary, M.H. Serological surveillance of caseous lymphadenitis in Sudanese and Somali camels slaughtered at Al-warraq abattoir, Giza, Egypt. World Vet. J. 2016, 6, 89–94. [Google Scholar] [CrossRef]
- Paule, B.J.A.; Azevedo, V.; Regis, L.F.; Carminati, R.; Bahia, C.R.; Vale, V.L.C.; Moura-Costa, L.F.; Freire, S.M.; Nascimento, I.; Schaer, R.; et al. Experimental Corynebacterium pseudotuberculosis primary infection in goats: Kinetics of IgG and interferon-gamma production, IgG avidity and antigen recognition by Western blotting. Vet. Immunol. Immunopathol. 2003, 96, 129–130. [Google Scholar] [CrossRef] [Green Version]
- Sá, M.C.A.; Oliveira, S.A.S.; Dantas, E.M., Jr.; Gisele, V.; Gouveia, G.V.; Gouveia, J.J.S.; Veschi, J.L.A.; Costa, M.M. Resistance of Corynebacterium pseudotuberculosis in the Brazilian semiarid environment. Pesq. Vet. Bras. 2018, 38, 1091–1096. [Google Scholar] [CrossRef]

| Animal | Herd | Date of Sampling for Serological Testing | Result PLD-ELISA | Result WCA-ELISA | Result Immunoblot |
|---|---|---|---|---|---|
| Stallion S1 1,2 euthanized on 14 December 2020 (CVUAS 32834.2) | A | 21 September 2020 | – | + | + |
| Stallion S2 1,3 | A | 21 September2020 | – | + | + |
| Mare M1 2 (CVUAS 32654, 22 September 2020) | A | 22 September 2020 | + | + | + |
| Stallion S3 1,2,4 (CVUAS 32746, 20 October 2020) | B | 20 October2020 | + | + | + |
| Stallion S4 4,5 | B | 20 October 2020 | + | + | + |
| Stallion S5 4,6 | B | 20 October 2020 | + | + | + |
| Stallion S6 4,6 | B | 20 October 2020 | + | + | + |
| Stallion S7 4,6 | B | 20 October 2020 | + | + | + |
| Stallion S8 2 (CVUAS 32830, 9 December 2020) | B | 20 October2020 | – | – | – |
| 9 December 2020 | + | + | + | ||
| 15 April 2021 | + | + | + | ||
| 21 September 2021 | + | + | + | ||
| Stallion S9 2,7 (CVUAS S32745, 20 October 2020) | B | 20 October 2020 | – | + | + |
| 9 December 2020 | + | + | + | ||
| 15 April 2021 | + | + | + | ||
| 21 September 2021 | + | + | + | ||
| Stallion S10 7 | B | 20 October 2020 | – | + | + |
| 9 December 2020 | + | + | + | ||
| 15 April 2021 | + | + | + | ||
| 21 September 2021 | + | + | + | ||
| Stallion S11 7 | B | 20 October 2020 | – | – | +(weak) |
| 9 December 2020 | – | – | +(weak) | ||
| 15 April 2021 | – | – | +(weak) | ||
| Stallion S12 7 | B | 20 October 2020 | – | – | +(weak) |
| 9 December 2020 | – | – | +(weak) | ||
| 15 April 2021 | – | – | +(weak) | ||
| Mare M2 2 died on 16 January 2020 (CVUAS 32166) | C | 15 January 2020 | + | + | + |
| Mare M3 2 (CVUAS 9749.2, 6 March 2020) | C | 6 March 2020 | – | – | +(weak) |
| Mare M4 2 (CVUAS 32842, 11 December 2020) | C | 11 December 2020 | + | – | +(weak) |
| Mare M5 2 (CVUAS 32931, 2 February 2021) | C | 8 February 2021 | + | + | + |
| Mare M6 2 (CVUAS 33314, 8 October 2021) | C | 8 October 2021 | – | + | + |
| 3 November 2021 | – | + | + | ||
| 1 December 2021 | + | + | + | ||
| Mare M7 2 died on 10 October 2020 (CVUAS 32689) | B | / | n.t. | n.t. | n.t. |
| MareM8 2 euthanized on 24 September 2020 (CVUAS 32656) | A | / | n.t. | n.t. | n.t. |
| Mare M9 2 (CVUAS 32632, 12 September 2020) | A | / | n.t. | n.t. | n.t. |
| Animal, Herd | Herd | Date | Visible Abscesses in | Absecces in | |||
|---|---|---|---|---|---|---|---|
| Lungs | Liver | Spleen | Kidney | ||||
| Stallion S1 1 (CVUAS 32834.2) | A | Euthanized on 14 December 2020 | Ln. cervicalis superficialis | − | + | + | − |
| Mare M2 2,3 (CVUAS 32166) | C | Died on 16 January 2020 | Ln. mandibularis, Ln. subiliacus | + | + | − | − |
| Mare M7 1,4,5 (CVUAS 32689) | B | Died on 10 October 2020 | Subcutis of the neck | + | + | + | + |
| Mare M8 (CVUAS 32656) | A | Euthanized on 24 September 2020 | Subcutis of the scapula and abdominal wall | + | − | − | − |
| Antibiotic | MIC | Result | Number of Strains | MIC | Result | Number of Strains |
|---|---|---|---|---|---|---|
| Amoxycillin/Clavulanic acid | ≤2/1 | S | 13 | |||
| Ampicillin | ≤0.25 | S | 13 | |||
| Cefazolin | ≤1 | S | 13 | |||
| Cefoxitin | =4 | S | 11 | >4 | R | 2 |
| Cefquinom | ≤2 | S | 13 | |||
| Ceftiofur | =2 | S | 13 | |||
| Cephalothin | ≤1 | S | 13 | |||
| Clindamycin | ≤0.125 | S | 13 | |||
| Colistin | >2 | R | 13 | |||
| Doxycyclin | ≤0.125 | S | 13 | |||
| Enrofloxacin | =0.625 | S | 13 | |||
| Erythromycin | ≤0.125 | S | 13 | |||
| Florfenicol | =2 | S | 13 | |||
| Gentamicin | ≤1 | S | 13 | |||
| Neomycin | ≤8 | S | 12 | >16 | R | 1 |
| Nitrofurantoin | >64 | R | 13 | |||
| Oxacillin | ≥4 | R | 13 | |||
| Penicillin G-potassium | =0.25 | I | 13 | |||
| Rifampin | ≤0.0625 | S | 13 | |||
| Spectinomycin | =32 | S | 9 | =64 | I | 4 |
| Sulfamethoxazol/trimethoprim | ≤4.75/0.25 | S | 13 | |||
| Tetracycline | ≤0.25 | S | 13 | |||
| Tiamulin | ≤0.25 | S | 13 | |||
| Tilmicosin | =2 | S | 13 | |||
| Tulathromycin | ≤1 | S | 13 | |||
| Vancomycin | ≤0.5 | S | 13 |
| PLD ELISA | WCA ELISA | ||||
|---|---|---|---|---|---|
| Herd | Positive | Negative | Positive | Negative | Total |
| A | 110 (62.9%) | 65 (37.1%) | 124 (70.9%) | 51 (29.1%) | 175 (75.4%) |
| B | 8 (20.5%) | 31 (79.5%) | 8 (20.5%) | 31 (79.5%) | 39 (16.8%) |
| C | 12 (66.7%) | 6 (33.3%) | 10 (55.6%) | 8 (44.4%) | 18 (7.8%) |
| Total | 130 (56%) | 102 (44%) | 142 (61.2%) | 90 (38.8%) | 232 (100%) |
| PLD ELISA | WCA ELISA | Total | |
|---|---|---|---|
| Positive | Negative | ||
| Positive | 131 (93.3%) | 5 (3.7%) | 136 (55.1%) |
| Negative | 21 (18.9%) | 90 (81.1) | 111 (44.9%) |
| Total | 152 (61.5%) | 95 (38.5%) | 247 (100%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sting, R.; Geiger, C.; Rietschel, W.; Blazey, B.; Schwabe, I.; Rau, J.; Schneider-Bühl, L. Corynebacterium pseudotuberculosis Infections in Alpacas (Vicugna pacos). Animals 2022, 12, 1612. https://doi.org/10.3390/ani12131612
Sting R, Geiger C, Rietschel W, Blazey B, Schwabe I, Rau J, Schneider-Bühl L. Corynebacterium pseudotuberculosis Infections in Alpacas (Vicugna pacos). Animals. 2022; 12(13):1612. https://doi.org/10.3390/ani12131612
Chicago/Turabian StyleSting, Reinhard, Claudia Geiger, Wolfram Rietschel, Birgit Blazey, Ingo Schwabe, Jörg Rau, and Lisa Schneider-Bühl. 2022. "Corynebacterium pseudotuberculosis Infections in Alpacas (Vicugna pacos)" Animals 12, no. 13: 1612. https://doi.org/10.3390/ani12131612
APA StyleSting, R., Geiger, C., Rietschel, W., Blazey, B., Schwabe, I., Rau, J., & Schneider-Bühl, L. (2022). Corynebacterium pseudotuberculosis Infections in Alpacas (Vicugna pacos). Animals, 12(13), 1612. https://doi.org/10.3390/ani12131612






