Gut Microbiota Composition and Diversity in Different Commercial Swine Breeds in Early and Finishing Growth Stages
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals and Sample Collection
2.3. Growth Curve Models
2.4. Pearson Correlation and Path Analyses
2.5. DNA Isolation and 16S rRNA Gene Sequencing
2.6. Data Analysis
3. Results
3.1. Comparison of Growth Curve Models
3.2. Correlation and Path Analyses of Body Weight and Body Size Indexes
3.3. Taxonomy and Diversity of Gut Microbiota of Gilts
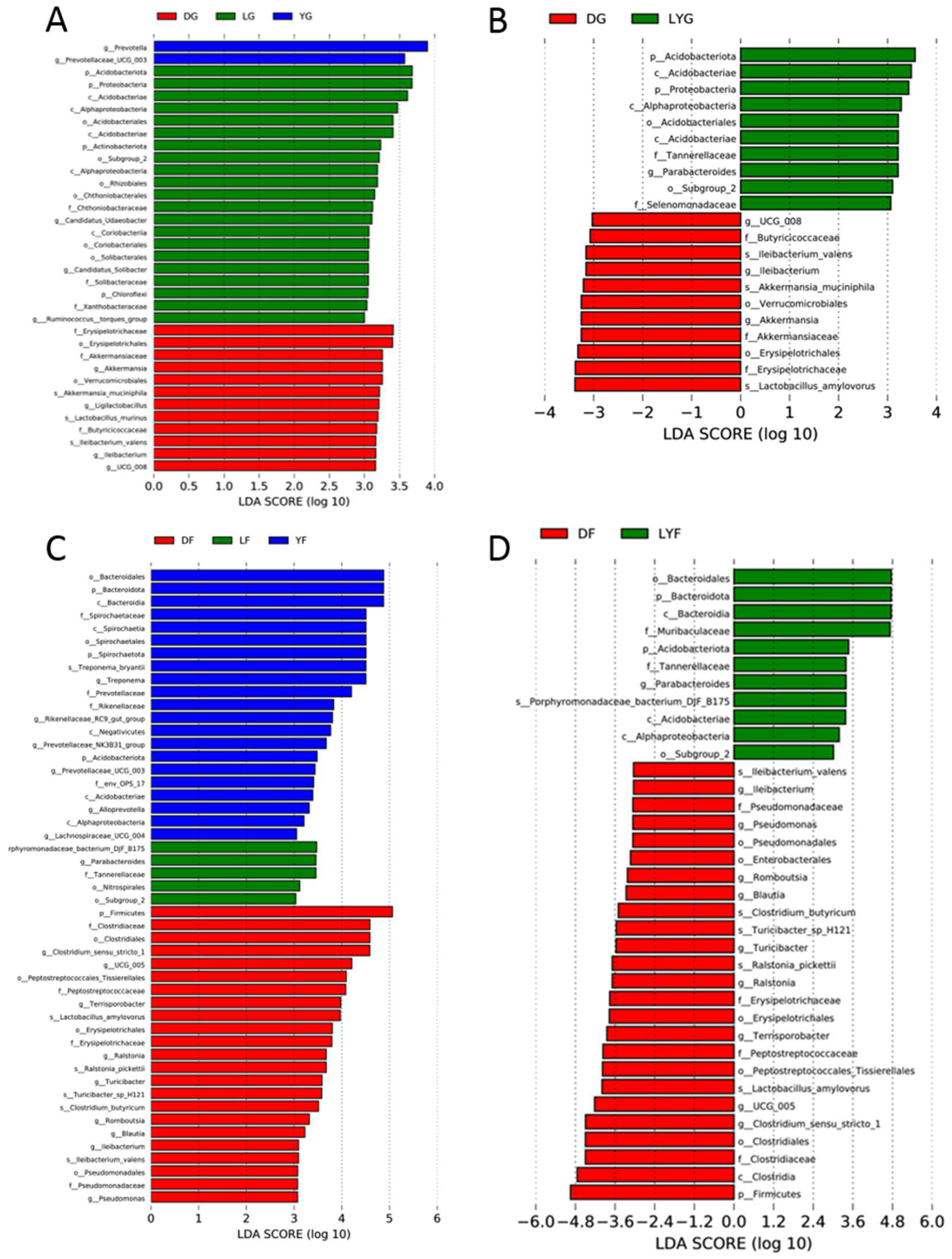
3.4. Differences in Gut Microbiota Composition among the Three Pig Breeds
3.5. Comparison of the Functions of the Gut Microbiota of Pigs at Early and Finishing Growth Stages
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Teleken, J.T.; Galvão, A.C.; Robazza, W.D.S. Comparing Non-Linear Mathematical Models to Describe Growth of Different Animals. Acta Sci. Anim. Sci. 2017, 39, 73. [Google Scholar] [CrossRef]
- Shen, L.; Luo, J.; Du, J.; Liu, C.; Wu, X.; Pu, Q.; Fu, Y.; Tang, Q.; Liu, Y.; Li, Q.; et al. Transcriptome Analysis of Liangshan Pig Muscle Development at the Growth Curve Inflection Point and Asymptotic Stages Using Digital Gene Expression Profiling. PLoS ONE 2015, 10, e0135978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vincek, D.; Sabo, K.; Kušec, G.; Kralik, G.; Đurkin, I.; Scitovski, R. Modeling of Pig Growth by S-Function—Least Absolute Deviation Approach for Parameter Estimation. Arch. Anim. Breed. 2012, 55, 364–374. [Google Scholar] [CrossRef] [Green Version]
- de Lange, C.F.M.; Marty, B.J.; Birkett, S.; Morel, P.; Szkotnicki, B. Application of Pig Growth Models in Commercial Pork Production. Can. J. Anim. Sci. 2001, 81, 1–8. [Google Scholar] [CrossRef]
- Dominguez-Bello, M.G.; Godoy-Vitorino, F.; Knight, R.; Blaser, M.J. Role of the Microbiome in Human Development. Gut 2019, 68, 1108–1114. [Google Scholar] [CrossRef]
- Heintz-Buschart, A.; Wilmes, P. Human Gut Microbiome: Function Matters. Trends Microbiol. 2018, 26, 563–574. [Google Scholar] [CrossRef]
- Tan, Z.; Wang, Y.; Yang, T.; Ao, H.; Chen, S.; Xing, K.; Zhang, F.; Zhao, X.; Liu, J.; Wang, C. Differences in Gut Microbiota Composition in Finishing Landrace Pigs with Low and High Feed Conversion Ratios. Antonie Van Leeuwenhoek 2018, 111, 1673–1685. [Google Scholar] [CrossRef] [Green Version]
- Bergamaschi, M.; Tiezzi, F.; Howard, J.; Huang, Y.J.; Gray, K.A.; Schillebeeckx, C.; McNulty, N.P.; Maltecca, C. Gut Microbiome Composition Differences among Breeds Impact Feed Efficiency in Swine. Microbiome 2020, 8, 110. [Google Scholar] [CrossRef]
- Donaldson, G.P.; Lee, S.M.; Mazmanian, S.K. Gut Biogeography of the Bacterial Microbiota. Nat. Rev. Microbiol. 2016, 14, 20–32. [Google Scholar] [CrossRef] [Green Version]
- Pluske, J.R. Invited Review: Aspects of Gastrointestinal Tract Growth and Maturation in the Pre- and Postweaning Period of Pigs. J. Anim. Sci. 2016, 94, 399–411. [Google Scholar] [CrossRef] [Green Version]
- Shao, M.; Wang, Z.; He, Y.; Tan, Z.; Zhang, J. Fecal Microbial Composition and Functional Diversity of Wuzhishan Pigs at Different Growth Stages. AMB Expr. 2021, 11, 88. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, W.; Blaine, A.; Kane, S.T.; Zijlstra, R.T.; Gänzle, M.G. Impact of Probiotic Lactobacillus Spp. on Autochthonous Lactobacilli in Weaned Piglets. J. Appl. Microbiol. 2019, 126, 242–254. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xu, J.; Ren, E.; Su, Y.; Zhu, W. Co-Occurrence of Early Gut Colonization in Neonatal Piglets with Microbiota in the Maternal and Surrounding Delivery Environments. Anaerobe 2018, 49, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liu, H.; Wang, S.; Zhang, W.; Wang, J.; Tian, H.; Wang, Y.; Ji, H. Fecal Microbiota and Its Correlation with Fatty Acids and Free Amino Acids Metabolism in Piglets after a Lactobacillus Strain Oral Administration. Front. Microbiol. 2019, 10, 785. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xu, Y.; Chen, X.; Fang, C.; Zhao, L.; Chen, F. The Maturing Development of Gut Microbiota in Commercial Piglets during the Weaning Transition. Front. Microbiol. 2017, 8, 1688. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Wu, J.; Huang, X.; Zhou, Y.; Zhang, Y.; Liu, M.; Liu, Q.; Ke, S.; He, M.; Fu, H.; et al. ABO Genotype Alters the Gut Microbiota by Regulating GalNAc Levels in Pigs. Nature 2022, 606, 358–367. [Google Scholar] [CrossRef]
- Winsor, C.P. The Gompertz Curve as a Growth Curve. Proc. Natl. Acad. Sci. USA 1932, 18, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Shen, L.; Gan, M.; Tang, Q.; Tang, G.; Jiang, Y.; Li, M.; Chen, L.; Bai, L.; Shuai, S.; Wang, J.; et al. Comprehensive Analysis of LncRNAs and CircRNAs Reveals the Metabolic Specialization in Oxidative and Glycolytic Skeletal Muscles. Int. J. Mol. Sci. 2019, 20, 2855. [Google Scholar] [CrossRef] [Green Version]
- Malhado, C.H.M.; Carneiro, P.L.S.; Affonso, P.R.A.M.; Souza, A.A.O.; Sarmento, J.L.R. Growth Curves in Dorper Sheep Crossed with the Local Brazilian Breeds, Morada Nova, Rabo Largo, and Santa Inês. Small Rumin. Res. 2009, 84, 16–21. [Google Scholar] [CrossRef]
- Knížetová, H.; Hyánek, J.; Kníže, B.; Roubíček, J. Analysis of Growth Curves of Fowl. I. Chickens. Br. Poult. Sci. 1991, 32, 1027–1038. [Google Scholar] [CrossRef]
- Sambrook, J.; Maniatis, T.E.; Fritsch, E.F. (Eds.) Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratories: Cold Spring Harbor, NY, USA, 1989. [Google Scholar]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global Patterns of 16S RRNA Diversity at a Depth of Millions of Sequences per Sample. Proc. Natl. Acad. Sci. USA 2011, 108, 4516–4522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, K.; Men, X.; Wu, J.; Deng, B.; Xu, Z. Effects of Growth Stage and Rearing Pattern on Pig Gut Microbiota. Curr. Microbiol. 2022, 79, 136. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME Improves Sensitivity and Speed of Chimera Detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naïve Bayesian Classifier for Rapid Assignment of RRNA Sequences into the New Bacterial Taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [Green Version]
- Khardori, N.M. In-Feed Antibiotic Effects on the Swine Intestinal Microbiome. Yearb. Med. 2012, 2012, 61–63. [Google Scholar] [CrossRef]
- Langille, M.G.I.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Vega Thurber, R.L.; Knight, R.; et al. Predictive Functional Profiling of Microbial Communities Using 16S RRNA Marker Gene Sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef]
- Parks, D.H.; Tyson, G.W.; Hugenholtz, P.; Beiko, R.G. STAMP: Statistical Analysis of Taxonomic and Functional Profiles. Bioinformatics 2014, 30, 3123–3124. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.H.; Nguyen, C.X.; Luu, M.Q.; Nguyen, A.T.; Bui, D.H.; Pham, D.K.; Do, D.N. Mathematical Models to Describe the Growth Curves of Vietnamese Ri Chicken. Braz. J. Biol. 2021, 83, e249756. [Google Scholar] [CrossRef]
- Koivula, M.; Sevón-Aimonen, M.-L.; Strandén, I.; Matilainen, K.; Serenius, T.; Stalder, K.J.; Mäntysaari, E.A. Genetic (Co)Variances and Breeding Value Estimation of Gompertz Growth Curve Parameters in Finnish Yorkshire Boars, Gilts and Barrows. J. Anim. Breed. Genet. 2008, 125, 168–175. [Google Scholar] [CrossRef]
- Sévon-Aimonen, M.-L.; Sternberg, K.; Ojala, M. Genetic Parameters for Growth Traits in Pigs Estimated Using Third Degree Polynomial Functions. Agric. Food Sci. 1997, 6, 1–10. [Google Scholar] [CrossRef]
- Strathe, A.B.; Danfær, A.; Sørensen, H.; Kebreab, E. A Multilevel Nonlinear Mixed-Effects Approach to Model Growth in Pigs. J. Anim. Sci. 2010, 88, 638–649. [Google Scholar] [CrossRef] [PubMed]
- Kundu, P.; Blacher, E.; Elinav, E.; Pettersson, S. Our Gut Microbiome: The Evolving Inner Self. Cell 2017, 171, 1481–1493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, Y.; Kong, F.; Xiang, Y.; Zhou, W.; Wang, J.; Yang, H.; Zhang, G.; Zhao, J. Comparative Biogeography of the Gut Microbiome between Jinhua and Landrace Pigs. Sci. Rep. 2018, 8, 5985. [Google Scholar] [CrossRef] [PubMed]
- Han, G.G.; Lee, J.-Y.; Jin, G.-D.; Park, J.; Choi, Y.H.; Kang, S.-K.; Chae, B.J.; Kim, E.B.; Choi, Y.-J. Tracing of the Fecal Microbiota of Commercial Pigs at Five Growth Stages from Birth to Shipment. Sci. Rep. 2018, 8, 6012. [Google Scholar] [CrossRef] [Green Version]
- Yao, G.; Wu, S.; Zeng, X.; Zhao, H.; Wang, G.; Chen, M.; Qian, N. Different Gut Microbiome Composition in Obese Guizhou Minipigs between Female and Castrated Male. Folia Microbiol. 2019, 64, 889–898. [Google Scholar] [CrossRef]
- Pajarillo, E.A.B.; Chae, J.P.; Balolong, M.P.; Kim, H.B.; Seo, K.-S.; Kang, D.-K. Pyrosequencing-Based Analysis of Fecal Microbial Communities in Three Purebred Pig Lines. J. Microbiol. 2014, 52, 646–651. [Google Scholar] [CrossRef]
- Niu, Q.; Li, P.; Hao, S.; Zhang, Y.; Kim, S.W.; Li, H.; Ma, X.; Gao, S.; He, L.; Wu, W.; et al. Dynamic Distribution of the Gut Microbiota and the Relationship with Apparent Crude Fiber Digestibility and Growth Stages in Pigs. Sci. Rep. 2015, 5, 9938. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Nguyen, S.G.; Guevarra, R.B.; Lee, I.; Unno, T. Analysis of Swine Fecal Microbiota at Various Growth Stages. Arch. Microbiol. 2015, 197, 753–759. [Google Scholar] [CrossRef]
- Kumar, H.; Park, W.; Srikanth, K.; Choi, B.-H.; Cho, E.-S.; Lee, K.-T.; Kim, J.-M.; Kim, K.; Park, J.; Lim, D.; et al. Comparison of Bacterial Populations in the Ceca of Swine at Two Different Stages and Their Functional Annotations. Genes 2019, 10, 382. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.H.; Kim, K.S.; Choi, B.H.; Yoon, D.H.; Jang, G.W.; Lee, K.T.; Chung, H.Y.; Lee, H.Y.; Park, H.S.; Lee, J.W. Genetic Structure of Pig Breeds from Korea and China Using Microsatellite Loci Analysis1. J. Anim. Sci. 2005, 83, 2255–2263. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, R.; Ingerslev, H.-C.; Sturek, M.; Alloosh, M.; Cirera, S.; Christoffersen, B.Ø.; Moesgaard, S.G.; Larsen, N.; Boye, M. Characterisation of Gut Microbiota in Ossabaw and Göttingen Minipigs as Models of Obesity and Metabolic Syndrome. PLoS ONE 2013, 8, e56612. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Bian, G.; Su, Y.; Zhu, W. Comparison of Faecal Microbial Community of Lantang, Bama, Erhualian, Meishan, Xiaomeishan, Duroc, Landrace, and Yorkshire Sows. Asian Australas. J. Anim. Sci. 2014, 27, 898–906. [Google Scholar] [CrossRef] [PubMed]
- Hildebrand, F.; Nguyen, T.L.A.; Brinkman, B.; Yunta, R.; Cauwe, B.; Vandenabeele, P.; Liston, A.; Raes, J. Inflammation-Associated Enterotypes, Host Genotype, Cage and Inter-Individual Effects Drive Gut Microbiota Variation in Common Laboratory Mice. Genome Biol. 2013, 14, R4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; Huang, X.; Fang, S.; Xin, W.; Huang, L.; Chen, C. Uncovering the Composition of Microbial Community Structure and Metagenomics among Three Gut Locations in Pigs with Distinct Fatness. Sci. Rep. 2016, 6, 27427. [Google Scholar] [CrossRef] [Green Version]






| Model | Equation | Parameters | Wi | Day at Inflection | Maximum Daily Gain |
|---|---|---|---|---|---|
| Logistic | A, B, K | A/2 | (lnB)/K | ||
| Gompertz | A, B, K | A/e | (lnB)/K | KWi | |
| Von Bertalanffy | A, B, K | 8A/27 | (ln3B)/K | ||
| Richards | A, B, K, m |
| Model | Breed | A | B | K | m | Wi | Day at Inflection | Maximum Daily Gain |
|---|---|---|---|---|---|---|---|---|
| Logistic | D | 163.2 | 34.12 | 0.02664 | - | 81.60 | 132.5 | 1.087 |
| L | 123.3 | 37.93 | 0.03359 | - | 61.65 | 108.2 | 1.035 | |
| Y | 131.8 | 36.63 | 0.03195 | - | 65.90 | 112.7 | 1.053 | |
| Gompertz | D | 278.9 | 4.690 | 0.01002 | - | 102.60 | 154.2 | 1.028 |
| L | 156.3 | 5.277 | 0.01606 | - | 57.50 | 103.6 | 0.923 | |
| Y | 169.6 | 5.100 | 0.01500 | - | 62.39 | 108.6 | 0.936 | |
| Von Bertalanffy | D | 573.1 | 0.8623 | 0.00440 | - | 169.81 | 216.1 | 1.120 |
| L | 195.3 | 0.9574 | 0.01013 | - | 57.87 | 104.2 | 0.879 | |
| Y | 247.3 | 0.8958 | 0.00803 | - | 73.27 | 123.1 | 0.883 | |
| Richards | D | 278.9 | 0.000868 | 0.01003 | 0.000185 | 102.61 | 154.1 | 0.699 |
| L | 137.3 | 4.041 | 0.02268 | 0.3740 | 58.71 | 104.9 | 0.656 | |
| Y | 139.5 | 10.84 | 0.02568 | 0.6295 | 64.23 | 110.8 | 0.681 |
| Predictor Variables | Correlation | Direct Effects (D) | p Value | Indirect Effects (I) | Total Effects (D + I) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BL | BH | CC | AC | HC | CW | CD | CBC | Total (I) | |||||
| BL | 0.973 | 0.507 | <0.01 | −0.057 | 0.505 | −0.169 | 0.081 | −0.059 | 0.062 | 0.102 | 0.4656 | 0.9728 | |
| BH | 0.931 | −0.061 | 0.200 | 0.471 | 0.501 | −0.164 | 0.081 | −0.058 | 0.061 | 0.101 | 0.9923 | 0.9313 | |
| CC | 0.970 | 0.532 | <0.01 | 0.482 | −0.057 | −0.177 | 0.082 | −0.064 | 0.065 | 0.107 | 0.4379 | 0.9695 | |
| AC | 0.937 | −0.182 | <0.01 | 0.474 | −0.055 | 0.517 | 0.080 | −0.065 | 0.064 | 0.104 | 1.1188 | 0.9373 | |
| HC | 0.924 | 0.088 | 0.045 | 0.465 | −0.056 | 0.494 | −0.165 | −0.058 | 0.061 | 0.095 | 0.8361 | 0.9241 | |
| CW | 0.822 | −0.073 | 0.030 | 0.410 | −0.049 | 0.469 | −0.162 | 0.070 | 0.060 | 0.096 | 0.8944 | 0.8216 | |
| CD | 0.924 | 0.069 | 0.139 | 0.459 | −0.054 | 0.505 | −0.170 | 0.078 | −0.064 | 0.102 | 0.8553 | 0.9242 | |
| CBC | 0.888 | 0.120 | <0.01 | 0.433 | −0.052 | 0.474 | −0.158 | 0.069 | −0.058 | 0.059 | 0.7678 | 0.8875 | |
| Predictor Variables | Correlation | Direct Effects (D) | p Value | Indirect Effects (I) | Total Effects (D + I) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BL | BH | CC | AC | HC | CW | CD | CBC | Total (I) | |||||
| BL | 0.863 | 0.203 | <0.01 | 0.001 | 0.364 | −0.058 | 0.254 | 0.040 | 0.026 | 0.034 | 0.660 | 0.8632 | |
| BH | 0.901 | 0.001 | 0.983 | 0.1643 | 0.412 | −0.065 | 0.272 | 0.047 | 0.028 | 0.044 | 0.900 | 0.9012 | |
| CC | 0.957 | 0.454 | <0.01 | 0.1628 | 0.001 | −0.071 | 0.286 | 0.051 | 0.029 | 0.043 | 0.503 | 0.9569 | |
| AC | 0.930 | −0.074 | 0.302 | 0.1613 | 0.001 | 0.435 | 0.284 | 0.051 | 0.029 | 0.042 | 1.004 | 0.9299 | |
| HC | 0.946 | 0.312 | <0.01 | 0.1655 | 0.001 | 0.416 | −0.067 | 0.049 | 0.029 | 0.040 | 0.635 | 0.9465 | |
| CW | 0.847 | 0.060 | 0.152 | 0.1352 | 0.001 | 0.392 | −0.064 | 0.256 | 0.025 | 0.042 | 0.787 | 0.8469 | |
| CD | 0.895 | 0.033 | 0.496 | 0.1589 | 0.001 | 0.405 | −0.065 | 0.279 | 0.046 | 0.038 | 0.863 | 0.8955 | |
| CBC | 0.827 | 0.051 | 0.214 | 0.1363 | 0.001 | 0.380 | −0.061 | 0.247 | 0.048 | 0.024 | 0.776 | 0.8266 | |
| Groups | Observed Species | Shannon | Simpson | Chao1 | Ace | Good’s Coverage | PD Whole Tree |
|---|---|---|---|---|---|---|---|
| DG | 934.0 ± 26.54 | 6.12 ± 0.16 | 0.9393 ± 0.0070 | 1007.71 ± 30.95 | 988.36 ± 31.53 | 0.9977 ± 0.0001 | 71.5 ± 4.2 |
| LG | 1057.8 ± 208.06 | 6.11 ± 0.85 | 0.9232 ± 0.0484 | 1157.34 ± 201.06 | 1133.14 ± 200.7 | 0.9970 ± 0.0004 | 83.90 ± 13.38 |
| YG | 1078.8 ± 114.87 | 6.09 ± 0.38 | 0.9234 ± 0.0220 | 1195.22 ± 129.71 | 1163.51 ± 120.66 | 0.9966 ± 0.0003 | 86.90 ± 6.36 |
| DF | 886.8 ± 51.14 | 6.42 ± 0.25 | 0.9556 ± 0.0103 | 955.03 ± 58.56 | 937.04 ± 53.67 | 0.9979 ± 0.0001 | 78.00 ± 11.29 |
| LF | 1080.6 ± 158.33 | 5.97 ± 0.65 | 0.9114 ± 0.0500 | 1190.42 ± 181.05 | 1161.67 ± 172.1 | 0.9967 ± 0.0006 | 88.11 ± 10.83 |
| YF | 1181.2 ± 116.54 | 6.47 ± 0.51 | 0.9487 ± 0.0177 | 1302.62 ± 138.18 | 1266.83 ± 124.51 | 0.9965 ± 0.0003 | 90.87 ± 7.94 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; Chen, J.; Gan, M.; Chen, L.; Zhao, Y.; Zhu, Y.; Niu, L.; Zhang, S.; Zhu, L.; Shen, L. Gut Microbiota Composition and Diversity in Different Commercial Swine Breeds in Early and Finishing Growth Stages. Animals 2022, 12, 1607. https://doi.org/10.3390/ani12131607
Ma J, Chen J, Gan M, Chen L, Zhao Y, Zhu Y, Niu L, Zhang S, Zhu L, Shen L. Gut Microbiota Composition and Diversity in Different Commercial Swine Breeds in Early and Finishing Growth Stages. Animals. 2022; 12(13):1607. https://doi.org/10.3390/ani12131607
Chicago/Turabian StyleMa, Jianfeng, Jingyun Chen, Mailin Gan, Lei Chen, Ye Zhao, Yan Zhu, Lili Niu, Shunhua Zhang, Li Zhu, and Linyuan Shen. 2022. "Gut Microbiota Composition and Diversity in Different Commercial Swine Breeds in Early and Finishing Growth Stages" Animals 12, no. 13: 1607. https://doi.org/10.3390/ani12131607
APA StyleMa, J., Chen, J., Gan, M., Chen, L., Zhao, Y., Zhu, Y., Niu, L., Zhang, S., Zhu, L., & Shen, L. (2022). Gut Microbiota Composition and Diversity in Different Commercial Swine Breeds in Early and Finishing Growth Stages. Animals, 12(13), 1607. https://doi.org/10.3390/ani12131607









