Simple Summary
A retrospective study was performed to identify pulmonary alterations and/or pneumonia in aborted bovine fetuses (n = 37) and to associate the presence of infectious disease antigens and nucleic agents with patterns and/or alterations of pulmonary disease. Immunohistochemical (IHC) assays were performed to identify antigens of selected agents associated with bovine respiratory disease: bovine alphaherpesvirus 1 (BoAHV1), bovine viral diarrhea virus (BVDV), bovine parainfluenza virus 3 (BPIV-3), bovine respiratory syncytial virus (BRSV), and Mycoplasma bovis. Molecular assays were performed to identify nucleic acids of a panel of reproductive disease agents and bovine gammaherpesvirus 6 (BoGHV6) in the lungs of 12 fetuses. Only interstitial pneumonia (12/37) and suppurative bronchopneumonia (1/37) were observed; pneumonia was not observed in 65% of the tissues evaluated. The most frequent intralesional agents were BRSV (16.2%; 6/37), BVDV (13.5%; 5/37), and BoAHV1 (8.1%; 3/37). Interstitial pneumonia was associated with BRSV (n = 3), BoAHV1 (n = 3), and BVDV (n = 2); suppurative bronchopneumonia contained a Gram-positive bacterium and intralesional antigens of BVDV and BRSV. Nucleic acid detection identified at least one disease agent in 75% of the fetuses examined. Reproductive pathogens detected included Leptospira spp., (n = 3), BVDV, Neospora caninum, and Brucella abortus (n = 2). BoGHV6 DNA was identified in the lungs of two fetuses with interstitial pneumonia. Single (n = 7), dual (n = 3), triple (n = 4), and quadruple (n = 1) mixed infections were identified while infectious agents were not identified in 59.5% (22/37) of the examined lungs. Single fetal infections were associated with BRSV, BVDV (n = 2), Leptospira spp., BoAHV1, and BoGHV6 (n = 1). These results indicate that the fetuses suffered intrauterine infection through transplacental transmission. The identification of BRSV and BoGHV6 in multiple fetuses with associated pulmonary alterations warrants further investigation relative to the role of these agents in fetopathy and possible direct and/or indirect effects on fetal survival.
Abstract
This study investigated the occurrence of selected pathogens of bovine respiratory disease in fetal pulmonary tissue of cattle and associated these with patterns of disease. Fetal pulmonary (n = 37) tissues were evaluated by histopathology; immunohistochemical assays identified intralesional antigens of bovine alphaherpesvirus 1 (BoAHV1), bovine viral diarrhea virus (BVDV), bovine parainfluenza virus 3 (BPIV-3), bovine respiratory syncytial virus (BRSV), and Mycoplasma bovis. Molecular assays were performed to amplify reproductive disease pathogens and bovine gammaherpesvirus 6 (BoGHV6) from 12 lungs. The 2 patterns of pulmonary diseases were interstitial pneumonia (12/37) and suppurative bronchopneumonia (1/37). The frequency of the intralesional antigens identified was BRSV (16.2%; 6/37), BVDV (13.5%; 5/37), BoAHV1 (8.1%; 3/37), M. bovis (5.4%; 2/37), and BPIV-3 (2.7%; 1/37). Interstitial pneumonia was associated with BRSV (n = 3), BoAHV1 (n = 3), and BVDV (n = 2); suppurative bronchopneumonia contained a Gram-positive bacterium and BVDV and BRSV. Reproductive pathogens detected included Leptospira spp., (n = 3), BVDV, Neospora caninum, and Brucella abortus (n = 2). BoGHV6 DNA was identified in the lungs of two fetuses with interstitial pneumonia. These findings suggest that these fetuses were infected transplacentally by several pathogens. The role of some of these pathogens herein identified must be further elucidated in the possible participation of fetal disease.
1. Introduction
The bovine respiratory disease (BRD) complex is a multifactorial and multi-etiological disease associated with several bacterial and viral agents, together with risk factors or stressors, that favor the development of pneumonic conditions, resulting in varying rates of morbidity and mortality in cattle of all age groups [1,2,3]. Frequent stressors of BRD include weaning, comingling, transportation, abrupt dietary alterations [1,3], and several management factors at feedlots [1]. In Brazil, information relative to the occurrence of infectious agents associated with BRD is scarce and insipient [4] when compared with the data existing in North America [1,2,5,6] and Australia [5]. Consequently, it is difficult to correlate productive losses due to the BRD in feedlot cattle since the available data may not reflect the real situation of cattle health, as well as morbidity and mortality indices in Brazil [6].
The viral agents frequently associated with BRD include bovine alphaherpesvirus 1 (BoAHV1), bovine viral diarrhea virus (BVDV), bovine parainfluenza virus 3 (BPIV-3), bovine respiratory syncytial virus (BRSV), and bovine coronavirus (BCoV) [7,8,9]. Bacterial agents associated with BRD include Histophilus somni, Mannheimia haemolytica, Mycoplasma bovis, and Pasteurella multocida [4,8,10,11]. Our group has identified all of these agents in feedlot and dairy cattle with BRD from several geographical regions of Brazil [10,12,13,14,15] and has contributed to the understanding of disease patterns associated with the development of BRD [10,15].
Although numerous reports have investigated the infectious agents associated with BRD in feedlot cattle worldwide [4,7,8,15], there are comparatively fewer studies with histologic details involving fetal lungs of cattle [16,17,18] as compared with the innumerous studies describing the lesions observed in several fetal organs. Infectious agents previously associated with fetal lungs and/or pneumonia in cattle include Brucella abortus [19,20], M. bovis [17], BPIV-3 [18], BoAHV1 [16,21], and BVDV [10]. Most of these studies have identified the associated agents by in situ diagnostic methods, such as immunohistochemistry (IHC) [10,16,17], in situ hybridization (ISH) [17], as well as molecular identification [16,18] and culture and isolation [19,21] in conjunction with histopathologic evidence of pulmonary disease. The IHC and ISH diagnostic strategies demonstrate the intralesional presence of agent-specific antigens associated with histopathological evidence of lesions [22,23], with the obtained results being a strong indication of an associated disease process within the affected tissues [23], thereby providing evidence of the related disease agent with the pattern of pulmonary disease [15]. Furthermore, diagnostic IHC is recommended to identify a wide range of infectious reproductive agents in cattle [24].
This study investigated the presence of selected infectious agents of BRD in aborted bovine fetal lungs to determine whether these pathogens were associated with pneumonia and/or other pulmonary alterations.
2. Materials and Methods
2.1. Sample Collection, Study Location, and Inclusion Criteria
Retrospective studies were performed on aborted bovine fetuses submitted for histopathologic diagnosis at the Laboratory of Animal of Pathology, Universidade Estadual de Londrina (UEL), Paraná, southern Brazil, and at the Veterinary Diagnostic Laboratory, Universidade Federal de Minas Gerais (UFMG), midwestern Brazil, from 2009 to 2019.
All files within the registry were reviewed to identify fetal bovine tissues submitted for diagnosis. Subsequently, only cases that contained the pathologic data and the correlated paraffin blocks and/or glass slides of fetuses with pulmonary tissue were included in this study. Additionally, when necessary, histological slides were redone by using the Hematoxylin and eosin staining technique. Furthermore, the Giemsa and Gram Brown–Brenn histochemical stains were performed on selected pulmonary tissues to identify intralesional organisms.
2.2. Histopathology and Immunohistochemistry
All pulmonary tissues were initially screened to identify the typical histopathological patterns of interstitial pneumonia, bronchopneumonia, granulomatous pneumonia, and embolic pneumonia [25]. Thereafter, the selected pulmonary tissues were reviewed for predetermined histopathologic patterns of pulmonary disease or histological alterations and categorized as: 0, normal lung; 1, circulatory, reversible, and irreversible cellular alterations; 2, interstitial pneumonia; and 3, suppurative bronchopneumonia, as previously described [15]. Additionally, the histological elements identified in each pulmonary alteration were observed and tabulated.
Subsequently, pulmonary tissues were submitted to IHC assays designed to identify specific agents known to be associated with BRD: BVDV, BoAHV1, BRSV, BPIV-3, and M. bovis [4,10], as previously described [10]. These agents were selected due to the availability of monoclonal and/or polyclonal antibodies; a list of the antibodies used in this study with the respective dilutions and methods of antigen retrieval is provided (Table 1).

Table 1.
Antibodies, dilutions, and antigen retrieval methods used in immunohistochemical assays.
Positive controls consisted of pulmonary fragments known to contain antigens of BVDV, BoAHV1, BRSV, BPIV-3, and M. bovis [10]. Negative control was performed by replacing the primary antibody with diluent; positive and negative controls were included in each IHC assay. The data obtained were tabulated and analyzed.
2.3. Molecular Detection of Agents Associated with Reproductive Diseases in Cattle
The extracted nucleic acids from the lungs of fetuses number 9, 12, 17, 19, 23, 24, and 27–32 were used in molecular assays designed to identify a panel of infectious agents associated with reproductive disease in cattle, using PCR and/or RT-PCR assays as previously described [26] in a thermocycler (Proflex PCR System, Applied Biosystems; Marsiling Ind Estate Road 3, Singapore). These included PCR/RT-PCR assays to detect BoAHV1 [27], BVDV [28], Listeria monocytogenes [29], Histophilus somni [30], Neospora caninum [31], Leptospira spp. [32], and Brucella abortus [33]. Additionally, the extracted nucleic acids of these fetuses maintained at −80 °C (except numbers 9 and 12) were used in nested-PCR (nPCR) assays designed to amplify the bovine gammaherpesvirus 6 (BoGHV6) polymerase gene [34], since there is emerging evidence that BoGHV6 may be a potential pathogen of bovine fetuses [35]. Only fetuses submitted frozen and/or refrigerated were used for molecular detection; all other fetuses were submitted in formalin solution for histopathologic evaluation and, thus, were not used for molecular identification.
Positive controls consisted of nucleic acids extracted from Madin–Darby bovine kidney cell culture inoculated with BVDV (NADL strain) and BoAHV1 (Los Angeles strain) and field strains of previous cases of OvGHV2 [36], L. monocytogenes [37], H. somni [38], N. caninum [31], Leptospira spp., B. abortus [39], and BoGHV6 [35]. Nuclease-free water (Invitrogen Corp., Carlsbad, CA, USA) was used as negative control in all PCR and RT-PCR assays; positive and negative controls were included in all molecular assays. PCR/RT-PCR products were resolved by electrophoresis in 2% agarose gels, stained with ethidium bromide, and examined under ultraviolet light.
2.4. Sequencing and Phylogenetic Analysis of BoHV-6 Polymerase Gene
The nPCR products of BoGHV6 nPCR assays were purified using the PureLink® Quick Gel Extraction and PCR Purification Combo Kit (Invitrogen® Life Technologies, Carlsbad, CA, USA), quantified by using a Qubit® Fluorometer (Invitrogen® Life Technologies, Eugene, OR, USA), and submitted to sequencing in both directions with the forward and reverse primers used in the respective molecular assays in an ABI3500 Genetic Analyzer sequencer with the BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems®, Foster City, CA, USA).
Sequence quality analyses and consensus sequences were obtained using PHRED and CAP3 software (http://asparagin.cenargen.embrapa.br/phph/, accessed on 21 April 2022), respectively. Similarity searches of the BoGHV6 polymerase gene were performed with nucleotide (nt) sequences deposited in GenBank using the Basic Local Alignment Search Tool software (https://blast.ncbi.nlm.nih.gov/Blast.cgi, accessed on 21 April 2022). Sequencing was done only for BoGHV6 since there is still controversy as to the role of this pathogen in infections [35].
2.5. Animal Ethics
This study followed the animal use rules of the National Council for Animal Control in Experiments and was approved by the Ethics Committee on Animal Usage, Universidade Estadual de Londrina (CEUA/UEL; protocol, 835.2019.45).
3. Results
3.1. Histopathological Findings and Pulmonary Patterns
During the period (2009–2019), 45 fetuses were submitted for routine post-mortem evaluations at UEL, southern Brazil, and three at UFMG, midwestern Brazil. However, only 34 fetuses from UEL, Paraná, and those from UFMG (n = 3), fulfilled the selection criteria and were included since they contained paraffin blocks, pulmonary tissues, and the biological data of the submitted animal.
Normal pulmonary tissue was observed in 48.8% (18/37) of the cases, circulatory and/or cellular alterations were diagnosed in 18.9% (7/37), 29.7% (11/37) had interstitial (Figure 1A), with one case (1/37; 2.7%) of fetal suppurative bronchopneumonia being identified (Table 2). Accordingly, pneumonia was identified in 35.1% (12/37) of the fetal tissues, while 64.9% (25/37) of the fetal lungs did not show histologic evidence of pneumonia.
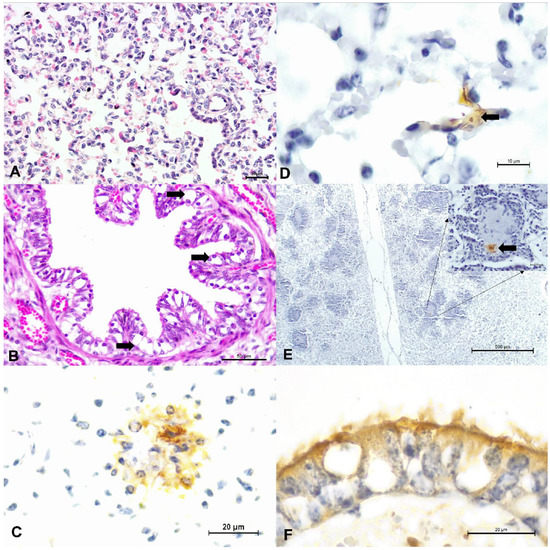
Figure 1.
Principal histopathologic and immunohistochemical findings observed in fetal lungs of cattle. There is interstitial pneumonia (A) and degeneration (arrows) of bronchial epithelium (B). Observe positive intracytoplasmic immunoreactivity to antigens of BRSV (C), BVDV within alveolar epithelium (D), and within a region of suppurative bronchopneumonia (E); BVDV immunoreactivity is highlighted at the insert. There is positive intracytoplasmic immunoreactivity to BoAHV1 within degenerated bronchial epithelium (F). (A,B), Hematoxylin and eosin stain; (C–F), immunoperoxidase counterstained with Hematoxylin. Bars, (A,C,F), and insert, 20 µm; (B), 50 µm; (D), 10 um; (E), 500 µm.

Table 2.
Patterns of pulmonary disease observed in fetuses from southern and midwestern Brazil.
The histological changes (n = 52) observed in the lungs evaluated with and without pneumonia are presented in Table 3; since some of these alterations occurred simultaneously in the same fetal lung. The most frequent histological change was pulmonary congestion (27%; 14/52), followed by ballooning degeneration (Figure 1B) of the bronchial epithelium (19.2%; 10/52) and bronchiolar (13.5%; 7/52). Additionally, the fetus with suppurative bronchopneumonia contained accumulations of an intralesional Gram-positive coccoid bacteria.

Table 3.
Histological findings observed within the patterns of pulmonary lesions observed in 37 fetuses.
3.2. Relationship between Immunohistochemical Identification of Infectious Agents and Pulmonary Alterations
Table 4 demonstrates the relationship between pulmonary changes and IHC detection of intralesional agents. Positive immunoreactivity to antigens associated with BRD were observed in 29.7% (11/37) of the fetal lungs in the above-mentioned categories: 0, normal lung (n = 0); 1, pulmonary tissue with circulatory alterations (n = 5); 2, interstitial pneumonia (n = 6); and 3, suppurative bronchopneumonia (n = 1); 70.3% (26/37) of the fetal pulmonary fragments did not contain any of the analyzed agents. The most frequent intralesional agents identified was BRSV (16.2%; 6/37), followed by BVDV (13.5%; 5/37), BoAHV1 (8.1%; 3/37), M. bovis (5.4%; 2/37), and BPIV-3 (2.7%; 1/37).

Table 4.
Principal histopathological findings, patterns of pneumonia, and infectious agents observed in the lungs of bovine fetuses by immunohistochemistry and molecular detection.
The association between intralesional accumulation of tissue antigens and the categories of pulmonary alterations identified in the fetal lungs by IHC is graphically presented (Figure 2). All antibodies used showed patchy, intracytoplasmic immunoreactivity within several histologic pulmonary elements. Interstitial pneumonia was associated with intralesional antigens of BRSV (n = 3), BoAHV1 (n = 3), BVDV (n = 2), and M. bovis (n = 1). However, in 46.2% (6/13) of lungs with interstitial pneumonia, none of the agents investigated was identified. The suppurative bronchopneumonia observed in fetus #37 contained intralesional antigens of BVDV and BRSV, with accumulations of Gram-positive bacteria. Circulatory (category 1) alterations were associated with the intralesional accumulations of antigens of BRSV (n = 3), BVDV (n = 2), BPIV-3, and M. bovis (n = 1).
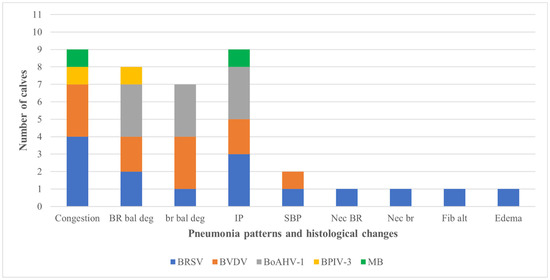
Figure 2.
Relationship between pneumonic patterns and histological changes with the associated infected agent identified by immunohistochemistry in the lungs of aborted bovine fetuses. Footnote: BoAHV1: bovine alphaherpesvirus 1; BPIV-3: bovine parainfluenza virus 3; BRSV: bovine respiratory syncytial virus; BVDV: bovine viral diarrhea virus; M. bovis: Mycoplasma bovis. IP, interstitial pneumonia; BR bal deg, bronchial epithelial ballooning degeneration; SBP, suppurative bronchopneumonia; br bal deg, bronchiolar epithelial ballooning degeneration; BR nec, bronchial epithelial necrosis; br nec, bronchiolar epithelial necrosis; Fib Alt, fibrinoid alteration.
Positive immunoreactivity to BRSV antigens was observed within degenerated and normal bronchial and bronchiolar epithelial cells, alveolar epithelium, and epithelial cells of the mixed bronchial gland (Figure 1C). Positive immunoreactivity to BVDV was observed within normal and degenerated bronchial epithelial cells and also within the suppurative exudate of a fetus with bronchopneumonia (Figure 1D,E). Intralesional BoAHV1 antigens were detected within normal bronchial and bronchiolar epithelial cells, chondrocytes of hyaline cartilage, and capillary endothelium (Figure 1F). Infections due to M. bovis resulted in positive immunolabelling within normal, degenerated, and necrotic bronchial and bronchiolar epithelial cells, necrotic epithelial cells of the mixed bronchial glands, endothelial cells, and alveolar macrophages. BPIV-3 antigens revealed positive intracytoplasmic immunoreactivity within normal bronchiolar epithelial cells and alveolar macrophages.
3.3. Molecular Identification of Pathogens and Relationship with Histologic Pulmonary Alterations
The results of the molecular detection of a panel of reproductive disease agents and BoGHV6 from the lungs of the fetuses are presented in Table 3, with at least one infectious agent being amplified from most (75%; 9/12) of the fetuses evaluated. The agents detected were Leptospira spp., (n = 3), BVDV (n = 2), N. caninum (n = 2), and B. abortus (n = 2), while BoGHV6 was identified in the lungs of 4 fetuses. Sequence analysis of the amplicons confirmed the identity of the products obtained from the BoGHV6 nPCR assay.
Furthermore, Leptospira spp. was the only agent identified in a lung (#9) without pneumonia and was also observed in a fetal lung with interstitial pneumonia that was concomitantly infected by B. abortus. Additionally, BVDV, N. caninum, and B. abortus were associated with pulmonary congestion (Table 3). Additionally, 2 (#24 and 32) of the 4 fetal lungs infected by BoGHV6 had a histologic diagnosis of interstitial pneumonia, with 1 lung (#32) being simultaneously infected by BRSV and BoAHV1 as demonstrated by IHC, while the other fetal lung (#24) was only infected by BoGHV6, suggesting a possible association between interstitial fetal pneumonia and BoGHV6.
Moreover, when all IHC and molecular analyses were considered, single (n = 7), dual (n = 3), triple (n = 4), and quadruple (n = 1) infections were identified. Alternatively, tissue antigens and/or nucleic acids were not identified in 59.5% (22/37) of the fetal lungs. Single fetal infections were associated with BRSV, BVDV (n = 2), Leptospira spp., BoAHV1, and BoGHV6 (n = 1). The only quadruple infection identified was associated with the simultaneous identification of antigens of BRSV and M. bovis by IHC and the nucleic acids of N. caninum and BoGHV6 via PCR in a congested fetal lung (#27).
4. Discussion
This study demonstrates the presence of pathogens known to produce respiratory diseases in cattle within the lungs of aborted bovine fetuses, amplified BoGHV6 DNA from some of these, and associates these pathogens with or without pulmonary alterations in bovine fetuses, demonstrating clear evidence that these fetuses were infected [24]. Furthermore, some well-known pathogens of reproductive disease in cattle, such as BVDV, BoAHV1, Leptospira spp., B. abortus, and M. bovis, were also identified and associated with pulmonary alteration(s), collaborating with the results of previous studies that have demonstrated B. abortus [19,20], M. bovis [17], BoAHV1 [16,21], BVDV [10] in the fetal lungs of cattle. Collectively, fetal infection by these pathogens was probably due to intrauterine infection resulting from transplacental transmission [40,41]. Consequently, most of the disease agents herein identified may be classified either as primary or secondary fetopathic agents of cattle [24,40] and could have produced direct or indirect effects on fetal survival [41]. The primary fetopathy agents herein identified (BVDV, BoAHV1, N. caninum, B. abortus, and Leptospira spp.) can cross the placental barrier, producing fetal disease in healthy cows, whereas the secondary or opportunistic agent (H. somni) crosses the placental barrier when there is placental damage, alteration to the microflora of the reproductive tract, or in immunocompromised cows [40].
The novelty of this study is the identification of antigens of two classical respiratory pathogens (BRSV and BPIV-3) within fetal lungs, the association of BRSV with interstitial pneumonia in two fetuses, while there was double infection by these viruses in one congested fetal lung. As far as the authors are aware, this is a novel description of BRSV-associated lesions in bovine fetuses, while there is a previous report of the participation of BPIV-3 in the development of fetal interstitial pneumonia in cattle [18].
Furthermore, the amplification of BoGHV6 DNA from multiple fetuses with interstitial pneumonia and other pulmonary alterations supports the theory that this pathogen should be considered an agent of fetal disease in cattle [35]. This is supported by previous studies which have identified this virus in an aborted fetus from Canada [42], within uterine secretions of a cow from Belgium [43], and in cows with endometritis from the UK [44]. Recently, we have identified BoGHV6 in several bovine fetuses that were simultaneously infected with H. somni and other well-known pathogenic abortive agents of cattle, while one fetus with myocarditis contained only BoGHV6 DNA [36]. Collectively, these are adequate emerging evidence to suggest a possible association of BoGHV6 with fetal disease and possible fetal death in cattle [35]. Furthermore, BoGHV6 and BRSV should be added to the list of secondary fetopathic agents of cattle [40] until the direct and/or indirect effects of these infections on fetal survival [41] and the associated pathogenesis with possible disease manifestations are fully investigated. Nevertheless, the IHC detection of tissue antigens of BoGHV6, using monoclonal or polyclonal antibodies, would definitely determine the participation of this pathogen in the development of fetal pneumonia. Consequently, it would be interesting to determine whether BoGHV6 is just an innocent bystander or an inductor of fetal disease in cattle [35] since this pathogen was frequently identified in mixed infections during this investigation. However, the current role of BoGHV6 in the development of disease is unknown, so caution must be used in the interpretation of these results until experimental studies have demonstrated the participation of this pathogen in the development of disease processes.
This study used two methods to associate the pathogens with infection: molecular and IHC detection/identification. Although molecular detection by PCR/RT-PCR is more sensitive than IHC, intralesional detection of these pathogens by IHC is strong evidence of their association with the disease process [23]. Molecular detection of an infectious agent should not be definitively interpreted as the cause of a specific disease process but is fundamental to differentiate between vaccine and field strain of disease agents [22]. Additionally, the IHC identification and molecular detection of tissue antigens and nucleic acids, respectively, with related histologic evidence of disease in fetal tissues, are suggestive of causal association [40]. Diagnostic IHC was used in previous studies to effectively associate intralesional organisms within fetal lungs of cattle [10,16,17], and as indicated previously, would be needed to definitely associate BoGHV6 with fetal pathology.
Most of the immunohistochemical findings associated with BRD pathogens herein identified in the fetal lungs were previously observed in the lungs of feedlot and dairy cattle with histological evidence of several patterns of pulmonary disease [10,15]. Intralesional immunoreactivity for M. bovis was observed within several epithelial cells of the lung; a previous investigation using IHC demonstrated positive immunoreactivity with the epithelial cells of the alveolar wall but with multifocal identification of M. bovis proteins by ISH [17]. Collectively, these results suggest that the distribution of M. bovis antigens and/or proteins within the lungs of bovine fetuses seems to be multifocal and not restricted to a specific histologic element of the lung. However, the classical pulmonary pattern of necrosuppurative or suppurative bronchopneumonia associated with pulmonary infections due to M. bovis [10] was not observed during this study when compared to a previous report of M. bovis-associated pulmonary diseases in a fetus [17]. The fetuses herein infected by M. bovis had histologic evidence of interstitial pneumonia and pulmonary congestion and contained other disease pathogens, including N. caninum, BVDV, and BoAHV1, suggesting that M. bovis may not have been directly related to the development of the principal pattern of pulmonary disease identified in these fetuses.
During this study, single, double, triple, and quadruple infections were observed by the identification of intralesional antigens and/or nucleic acids of primary and secondary agents. Single infections were predominant, as frequently described in fetal deaths related to infectious diseases [40], and also occurred in other studies investigating fetal pulmonary diseases of cattle [17,18,19]. Alternatively, the multiple concomitant infections identified in this study are not frequently identified in fetal pathology [40], with only one previous description of spontaneous dual infections in bovine fetuses [16]. Although the reason for the identification of mixed infections in bovine fetuses is unclear, high environmental infectious challenges, such as the endemicity of a disease pathogen, were proposed [40]. Pathogen endemicity seems plausible to the dynamics of reproductive disease pathogens in Brazil, considering that BVDV, BoAHV1, Leptospira spp., B. abortus [26,45,46], and to some extent H. somni [26,47], are frequently associated with fetal pathology and/or abortions in cattle from this country. Additionally, the frequency of concomitant fetal pulmonary infections herein identified may suggest that simultaneous infections in bovine fetuses may be more common than previously described [40].
5. Conclusions
In conclusion, molecular and IHC detection confirmed the presence of several agents associated with pulmonary and reproductive diseases of cattle within fetal lungs that had histologic evidence of interstitial pneumonia and/or pulmonary alterations. Collectively, these assays have demonstrated the occurrence of primary and secondary fetopathy agents in these fetuses and indicate intrauterine/transplacental infection. The amplification of BoGHV6, BRSV, and BPIV-3 from the lungs of several fetuses with histologic evidence of pulmonary alteration, particularly with interstitial pneumonia, suggests that these pathogens should be considered as putative fetopathy agents of cattle.
Nevertheless, the role of BoGHV6 in the development of disease processes is not fully known and must be confirmed by experimental and in situ studies. Finally, the relative frequency of simultaneous infections herein identified may indicate that concomitant fetal infectious may be more frequent than previously diagnosed.
Author Contributions
T.E.S.d.O., G.S.S. and S.A.H. contributed substantially to the conception and design of the study, drafted the initial manuscript, and contributed to the analysis and interpretation of all pathological and immunohistochemical data. J.T.T.F., R.P.M. and D.C.S. participated in the realization of all molecular analyses. J.T.T.F., E.F.F. and A.A.A. contributed to the analysis of all molecular data. T.E.S.d.O., G.S.S., I.F.P. and L.E.S. participated in the realization of all pathological and immunohistochemical stains and analyses. R.L.S., J.A.N.L., A.A.A. and S.A.H. contributed towards the obtention of fetuses used during this study. E.F.F. and L.G.P.-G. contributed towards the provision of the antibodies used in the IHC analyses. S.A.H. coordinated and supervised the realization of all aspects of this study and ensured the validation of all data herein utilized. All authors have read, critically analyzed, and approved the final draft of this manuscript, and have agreed to be accountable for all aspects of the study in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors have read and agreed to the published version of the manuscript.
Funding
Brazilian National Council of Scientific and Technological Development (CNPq; Brazil); Coordination for the Improvement of Higher Education Personnel (CAPES; Brazil); National Institute of Science and Technology for Dairy Production Chain (INCT—LEITE).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Animal Ethics Committees for Animal Usage of the Universidade Estadual de Londrina (CEUA/UEL; protocol, 835.2019.45).
Informed Consent Statement
Not applicable.
Data Availability Statement
Details of all molecular sequences identified during this study are deposited in GenBank.
Acknowledgments
Graduate (Oliveira, T.E.S.) and undergraduate (Scuisato, G.S. and Pelaquim, I.F.) student fellowships were received from the Brazilian National Council of Scientific and Technological Development (CNPq; Brazil). Silva, L.E. is the recipient of a graduate student fellowship from the Coordination for the Improvement of Higher Education Personnel (CAPES; Brazil). Flores, E.F., Santos, R.L., Lisbôa, J.A.N., Alfieri, A.A. and Headley, S.A., are recipients of the Brazilian National Council of Scientific and Technological Development (CNPq; Brazil) fellowships.
Conflicts of Interest
The authors declared no potential conflict of interest with respect to the research, authorship, and/or publication of this article.
References
- Taylor, J.D.; Fulton, R.W.; Lehenbauer, T.W.; Step, D.L.; Confer, A.W. The epidemiology of bovine respiratory disease: What is the evidence for predisposing factors? Can. Vet. J. 2010, 51, 1095–1102. [Google Scholar] [PubMed]
- Griffin, D.; Chengappa, M.M.; Kuszak, J.; McVey, D.S. Bacterial Pathogens of the Bovine Respiratory Disease Complex. Vet. Clin. N. Am. Food Anim. Pract. 2010, 26, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Chai, J.; Capik, S.F.; Kegley, B.; Richeson, J.T.; Powell, J.G.; Zhao, J. Bovine respiratory microbiota of feedlot cattle and its association with disease. Vet. Res. 2022, 53, 4. [Google Scholar] [CrossRef] [PubMed]
- Headley, S.A.; Okano, W.; Balbo, L.C.; Marcasso, R.A.; Oliveira, T.E.; Alfieri, A.F.; Filho, L.C.N.; Michelazzo, M.Z.; Rodrigues, S.C.; Baptista, A.L.; et al. Molecular survey of infectious agents associated with bovine respiratory disease in a beef cattle feedlot in southern Brazil. J. Vet. Diagn. Investig. 2018, 30, 249–251. [Google Scholar] [CrossRef] [PubMed]
- Gagea, M.I.; Bateman, K.G.; van Dreumel, T.; McEwen, B.J.; Carman, S.; Archambault, M.; Shanahan, R.A.; Caswell, J.L. Diseases and pathogens associated with mortality in Ontario beef feedlots. J. Vet. Diagn. Investig. 2006, 18, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Francoz, D.; Buczinski, S.; Bélanger, A.M.; Forté, G.; Labrecque, O.; Tremblay, D.; Wellemans, V.; Dubuc, J. Respiratory Pathogens in Québec Dairy Calves and Their Relationship with Clinical Status, Lung Consolidation, and Average Daily Gain. J. Vet. Intern. Med. 2015, 29, 381–387. [Google Scholar] [CrossRef]
- Cusack, P.M.V.; McMeniman, N.; Lean, I.J. The medicine and epidemiology of bovine respiratory disease in feedlots. Aust. Vet. J. 2003, 81, 480–487. [Google Scholar] [CrossRef]
- Baptista, A.L.; Rezende, A.L.; de Almeida Fonseca, P.; Massi, R.P.; Nogueira, G.M.; Magalhães, L.Q.; Headley, S.A.; Menezes, G.L.; Alfieri, A.A.; Saut, J.P.E. Bovine respiratory disease complex associated mortality and morbidity rates in feedlot cattle from southeastern Brazil. J. Infect. Dev. Ctries. 2017, 11, 791–799. [Google Scholar] [CrossRef]
- Fulton, R.W.; Blood, K.S.; Panciera, R.J.; Payton, M.E.; Ridpath, J.F.; Confer, A.W.; Saliki, J.T.; Burge, L.T.; Welsh, R.D.; Johnson, B.J.; et al. Lung pathology and infectious agents in fatal feedlot pneumonias and relationship with mortality, disease onset, and treatments. J. Vet. Diagn. Investig. 2009, 21, 464–477. [Google Scholar] [CrossRef]
- Fulton, R.W.; Purdy, C.; Confer, A.W.; Saliki, J.; Loan, R.W.; Briggs, R.E.; Burge, L.J. Bovine viral diarrhea viral infections in feeder calves with respiratory disease: Interactions with Pasteurella spp., parainfluenza-3 virus, and bovine respiratory syncytial virus. Can. J. Vet. Res. 2000, 64, 151. [Google Scholar]
- Oliveira, T.E.S.; Pelaquim, I.F.; Flores, E.F.; Massi, R.P.; Valdiviezo, M.J.J.; Pretto-Giordano, L.G.; Alfieri, A.A.; Saut, J.P.E.; Headley, S.A. Mycoplasma bovis and viral agents associated with the development of bovine respiratory disease in adult dairy cows. Transbound. Emerg. Dis. 2019, 67, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Tortorelli, G.; Carrillo Gaeta, N.; Mendonça Ribeiro, B.; Miranda Marques, L.; Timenetsky, J.; Gregory, L. Evaluation of mollicutes microorganisms in respiratory disease of cattle and their relationship to clinical signs. J. Vet. Intern. Med. 2017, 31, 1215–1220. [Google Scholar] [CrossRef] [PubMed]
- Headley, S.A.; Alfieri, A.F.; Oliveira, V.H.; Beuttemmuller, E.A.; Alfieri, A.A. Histophilus somni is a potential threat to beef cattle feedlots in Brazil. Vet. Rec. 2014, 175, 249. [Google Scholar] [CrossRef] [PubMed]
- Headley, S.A.; Balbo, L.C.; Alfieri, A.F.; Saut, J.P.E.; Baptista, A.L.; Alfieri, A.A. Bovine respiratory disease associated with Histophilus somni and bovine respiratory syncytial virus in a beef cattle feedlot from Southeastern Brazil. Semin. Ciênc. Agrár. 2017, 38, 283–294. [Google Scholar] [CrossRef]
- Castro, M.M.; Oliveira, T.E.S.; Headley, S.A. Bovine respiratory disease in Brasil: A short review. Semin. Ciênc. Agrár. 2021, 42, 2081–2110. [Google Scholar] [CrossRef]
- Oliveira, T.E.S.; Scuisato, G.S.; Pelaquim, I.F.; Cunha, C.W.; Cunha, L.S.; Flores, E.F.; Pretto-Giordano, L.G.; Lisbôa, J.A.N.; Alfieri, A.A.; Saut, J.P.E.; et al. The participation of a Malignant Catarrhal Fever Virus and Mycoplasma bovis in the development of single and mixed infections in beef and dairy cattle with bovine respiratory disease. Front. Vet. Sci. 2021, 8, 691448. [Google Scholar] [CrossRef]
- Crook, T.; Benavides, J.; Russell, G.; Gilray, J.; Maley, M.; Willoughby, K. Bovine herpesvirus 1 abortion: Current prevalence in the United Kingdom and evidence of hematogenous spread within the fetus in natural cases. J. Vet. Diagn. Investig. 2012, 24, 662–670. [Google Scholar] [CrossRef]
- Hermeyer, K.; Peters, M.; Brügmann, M.; Jacobsen, B.; Hewicker-Trautwein, M. Demonstration of Mycoplasma bovis by immunohistochemistry and in situ hybridization in an aborted bovine fetus and neonatal calf. J. Vet. Diagn. Investig. 2012, 24, 364–369. [Google Scholar] [CrossRef]
- Macías-Rioseco, M.; Mirazo, S.; Uzal, F.A.; Fraga, M.; Silveira, C.; Maya, L.; Riet-Correa, F.; Arbiza, J.; Colina, R.; Anderson, M.L.; et al. Fetal pathology in an aborted Holstein fetus infected with Bovine Parainfluenza Virus-3 Genotype A. Vet. Pathol. 2019, 56, 277–281. [Google Scholar] [CrossRef]
- López, A.; Hitos, F.; Pérez, A.; Navarro-Fierro, R.R. Lung lesions in bovine fetuses aborted by Brucella abortus. Can. J. Comp. Med. 1984, 48, 275–277. [Google Scholar]
- Xavier, M.N.; Paixão, T.A.; Poester, F.P.; Lage, A.P.; Santos, R.L. Pathological, immunohistochemical and bacteriological study of tissues and milk of cows and fetuses experimentally infected with Brucella abortus. J. Comp. Pathol. 2009, 140, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.B.; Quinn, P.J. Observations on abortions in cattle: A comparison of pathological, microbiological and immunological findings in aborted foetuses and foetuses collected at abattoirs. Can. J. Comp. Med. 1975, 39, 270–290. [Google Scholar] [PubMed]
- Maes, R.K.; Langohr, I.M.; Wise, A.G.; Smedley, R.C.; Thaiwong, T.; Kiupel, M. Beyond H&E: Integration of Nucleic Acid–Based analyses into diagnostic pathology. Vet. Pathol. 2013, 51, 238–256. [Google Scholar] [CrossRef] [PubMed]
- Fulton, R.W.; Confer, A.W. Laboratory test descriptions for bovine respiratory disease diagnosis and their strengths and weaknesses: Gold standards for diagnosis, do they exist? Can. Vet. J. 2012, 53, 754–761. [Google Scholar]
- Anderson, M.L. Infectious causes of bovine abortion during mid- to late-gestation. Theriogenology 2007, 68, 474–486. [Google Scholar] [CrossRef]
- Panciera, R.J.; Confer, A.W. Pathogenesis and pathology of bovine pneumonia. Vet. Clin. Food Anim. Pract. 2010, 26, 191–214. [Google Scholar] [CrossRef]
- Headley, S.A.; Voltarelli, D.; de Oliveira, V.H.; Bronkhorst, D.E.; Alfieri, A.F.; Filho, L.C.; Okano, W.; Alfieri, A.A. Association of Histophilus somni with spontaneous abortions in dairy cattle herds from Brazil. Trop. Anim. Health Prod. 2015, 47, 403–413. [Google Scholar] [CrossRef]
- Claus, M.P.; Alfieri, A.F.; Folgueras-Flatschart, A.V.; Wosiacki, S.R.; Medici, K.C.; Alfieri, A.A. Rapid detection and differentiation of bovine herpesvirus 1 and 5 glycoprotein C gene in clinical specimens by multiplex-PCR. J. Virol. Methods 2005, 128, 183–188. [Google Scholar] [CrossRef]
- Vilček, Š.; Herring, A.J.; Herring, J.A.; Nettleton, P.F.; Lowings, J.P.; Paton, D.J. Pestiviruses isolated from pigs, cattle and sheep can be allocated into at least three genogroups using polymerase chain reaction and restriction endonuclease analysis. Arch. Virol. 1994, 136, 309–323. [Google Scholar] [CrossRef]
- Wesley, I.V.; Larson, D.J.; Harmon, K.M.; Luchansky, J.B.; Schwartz, A.R. A case report of sporadic ovine listerial menigoencephalitis in Iowa with an overview of livestock and human cases. J. Vet. Diagn. Investig. 2002, 14, 314–321. [Google Scholar] [CrossRef]
- Angen, O.; Ahrens, P.; Tegtmeier, C. Development of a PCR test for identification of Haemophilus somnus in pure and mixed cultures. Vet. Microbiol. 1998, 63, 39–48. [Google Scholar] [CrossRef]
- Marques, F.A.; Headley, A.S.; Figueredo-Pereira, V.; Taroda, A.; Barros, L.D.; Cunha, I.A.; Munhoz, K.; Bugni, F.M.; Zulpo, D.L.; Igarashi, M.; et al. Neospora caninum: Evaluation of vertical transmission in slaughtered beef cows (Bos indicus). Parasitol. Res. 2011, 108, 1015–1019. [Google Scholar] [CrossRef] [PubMed]
- Merien, F.; Amouriaux, P.; Perolat, P.; Baranton, G.; Saint Girons, I. Polymerase chain reaction for detection of Leptospira spp. in clinical samples. J. Clin. Microbiol. 1992, 30, 2219–2224. [Google Scholar] [CrossRef] [PubMed]
- Baily, G.G.; Krahn, J.B.; Drasar, B.S.; Stoker, N.G. Detection of Brucella melitensis and Brucella abortus by DNA amplification. J. Trop. Med. Hyg. 1992, 95, 271–275. [Google Scholar] [PubMed]
- Oliveira, C.H.; Oliveira, F.G.; Gasparini, M.R.; Galinari, G.C.; Lima, G.K.; Fonseca, A.A., Jr.; Barbosa, J.D.; Barbosa-Stancioli, E.F.; Leite, R.C.; Dos Reis, J.K.P. Bovine herpesvirus 6 in buffaloes (Bubalus bulalis) from the Amazon region, Brazil. Trop. Anim. Health Prod. 2015, 47, 465–468. [Google Scholar] [CrossRef]
- Headley, S.A.; Fritzen, J.T.T.; Bon, V.R.; Xavier, A.A.C.; Dall Agnol, A.M.; Zucoloto, N.Z.; Silva, F.H.P.; Figueiredo, J.R.X.; Alfieri, A.F.; Okano, W.; et al. Detection of bovine gammaherpesvirus 6 in tissues of aborted fetuses from dairy cows concomitantly infected by Histophilus somni. Microb. Pathog. 2022, 169, 105621. [Google Scholar] [CrossRef]
- Headley, S.; Oliveira, T.; Li, H.; Lisbôa, J.; Queiroz, G.; Fritzen, J.; Flores, E.; Alfieri, A.; Cunha, C. Immunohistochemical detection of intralesional antigens of Ovine Gammaherpesvirus-2 in cattle with Sheep-associated Malignant Catarrhal Fever. J. Comp. Pathol. 2020, 174, 86–98. [Google Scholar] [CrossRef]
- Headley, S.A.; Fritzen, J.T.T.; Queiroz, G.R.; Oliveira, R.A.M.; Alfieri, A.F.; Santis, G.W.D.; Lisbôa, J.A.N.; Alfieri, A.A. Molecular characterization of encephalitic bovine listeriosis from southern Brazil. Trop. Anim. Health Prod. 2014, 46, 19–25. [Google Scholar] [CrossRef]
- Headley, S.A.; Oliveira, V.H.; Figueira, G.F.; Bronkhorst, D.E.; Alfieri, A.F.; Okano, W.; Alfieri, A.A. Histophilus somni-induced infections in cattle from southern Brazil. Trop. Anim. Health Prod. 2013, 45, 1579–1588. [Google Scholar] [CrossRef]
- Headley, S.A.; Pereira, A.H.T.; Balbo, L.C.; Di Santia, G.W.; Bracarense, A.P.F.R.L.; Filho, L.F.C.C.; Schade, J.; Okano, W.; Pereira, P.F.; Morotti, F.; et al. Histophilus somni-associated syndromes in sheep from Southern Brazil. Braz. J. Microbiol. 2018, 49, 591–600. [Google Scholar] [CrossRef]
- Mee, J.F.; Jawor, P.; Stefaniak, T. Role of infection and immunity in bovine perinatal mortality: Part 1. Causes and current diagnostic approaches. Animals 2021, 11, 1033. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, T.J. 24—Specific Infectious Diseases Causing Infertility and Subfertility in Cattle. In Veterinary Reproduction and Obstetrics, 10th ed.; Noakes, D.E., Parkinson, T.J., England, G.C.W., Eds.; W.B. Saunders: St. Louis, MO, USA, 2019; pp. 434–466. [Google Scholar]
- Gagnon, C.A.; Allam, O.; Drolet, R.; Tremblay, D. Quebec: Detection of bovine lymphotropic herpesvirus DNA in tissues of a bovine aborted fetus. Can. Vet. J. 2010, 51, 1021–1022. [Google Scholar] [PubMed]
- Garigliany, M.M.; Bayrou, C.; Cassart, D.; Jolly, S.; Desmecht, D. Bovine lymphotropic herpesvirus detected in Belgium. Vet. Rec. 2013, 172, 535–536. [Google Scholar] [CrossRef] [PubMed]
- Cobb, S.P.; Banks, M.; Russell, C.; Thorne, M. Bovine lymphotrophic herpesvirus in a UK dairy herd. Vet. Rec. 2006, 158, 807–808. [Google Scholar] [CrossRef] [PubMed]
- Antoniassi, N.A.B.; Juffo, G.D.; Santos, A.S.; Pescador, C.A.; Corbellini, L.G.; Driemeier, D. Causas de aborto bovino diagnosticadas no Setor de Patologia Veterinária da UFRGS de 2003 a 2011. Pesqui. Vet. Barsileira 2013, 33, 155–160. [Google Scholar] [CrossRef][Green Version]
- Silva, T.M.A.; Oliveira, R.G.; MolI, J.P.D.S.; Xavier, M.N.; Paixão, T.A.; Cortez, A.; Heinemann, M.B.; Richtzenhain, L.J.; Santos, A.P.L.R.D. Etiologic diagnosis of bovine infectious abortion by PCR. Ciênc. Rural. 2009, 39, 2563–2570. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).