Evaluation of a Treadmill-Based Submaximal Fitness Test in Pugs, and Collecting Breed-Specific Information on Brachycephalic Obstructive Airway Syndrome
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Animals
2.2. Submaximal Fitness Test
2.3. Assessment of Respiratory Noises and Breathing Patterns
2.4. Functional BOAS Grading
2.5. Assessment of Body Conformation and Measurements
2.6. Statistical Analysis
3. Results
3.1. Submaximal Exercise Test
3.2. Respiratory Noises and Breathing Patterns
3.3. Functional BOAS Grading
3.4. Results of the Fitness Test in Relation to BOAS Grading
3.5. Body Conformation and Measurements and Their Impact on BOAS Grading
3.6. Owner Questionnaire
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oechtering, T.H.; Oechtering, G.U.; Nöller, C. Strukturelle Besonderheiten der Nase brachyzephaler Hunderassen in der Computertomographie. Tierärztl. Prax. 2007, 35, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Bannasch, D.; Young, A.; Myers, J.; Truve, K.; Dickinson, P.; Gregg, J.; Davis, R.; Bongcam-Rudloff, E.; Webster, M.T.; Lindblad-Toh, K.; et al. Localization of canine brachycephaly using an across breed mapping approach. PLoS ONE 2010, 5, e9632. [Google Scholar] [CrossRef] [PubMed]
- Marchant, T.W.; Johnson, E.J.; McTeir, L.; Johnson, C.I.; Gow, A.; Liuti, T.; Kuehn, D.; Svenson, K.; Bermingham, M.L.; Drögemüller, M.; et al. Canine brachycephaly is associated with a retrotransposon-mediated missplicing of SMOC2. Curr. Biol. 2017, 27, 1573–1584. [Google Scholar] [CrossRef] [PubMed]
- Oechtering, G.U.; Schlüter, C.; Lippert, J.P. Brachyzephalie bei Hund und Katze: Eine „menschengemachte” Obstruktion der oberen Atemwege. Pneumologie 2010, 64, 450–452. [Google Scholar] [CrossRef]
- Lorenz, K. Studies in Animal and Human Behaviour; Methuen and Co.: London, UK, 1971; pp. 154–162. [Google Scholar]
- Asher, L.; Diesel, G.; Summers, J.F.; McGreevy, P.D.; Collins, L.M. Inherited defects in pedigree dogs. Part 1: Disorders related to breed standards. Vet. J. 2009, 182, 402–411. [Google Scholar] [CrossRef]
- O’Neill, D.G.; Jackson, C.; Guy, J.H.; Church, D.B.; McGreevy, P.D.; Thomson, P.C.; Brodbelt, D.C. Epidemiological associations between brachycephaly and upper respiratory tract disorders in dogs attending veterinary practices in England. Canine Genet. Epidemiol. 2015, 2, 10. [Google Scholar] [CrossRef]
- O’Neill, D.G.; Pegram, C.; Crocker, P.; Brodbelt, D.C.; Church, D.B.; Packer, R.M.A. Unravelling the health status of brachycephalic dogs in the UK using multivariable analysis. Sci. Rep. 2020, 10, 17251. [Google Scholar] [CrossRef]
- Liu, N.C.; Troconis, E.L.; Kalmar, L.; Price, D.J.; Wright, H.E.; Adams, V.J.; Sargan, D.R.; Ladlow, J.F. Conformational risk factors of brachycephalic obstructive airway syndrome (BOAS) in pugs, French bulldogs, and bulldogs. PLoS ONE 2017, 12, e0181928. [Google Scholar] [CrossRef]
- Njikam, I.N.; Huault, M.; Pirson, V.; Detilleux, J. The Influence of Phylogenic Origin on the Occurrence of Brachycephalic Airway Obstruction Syndrome in a Large Retrospective Study. Int. J. Appl. Res. Vet. Med. 2009, 7, 138–143. [Google Scholar]
- Aromaa, M.; Lilja-Maula, L.; Rajamaki, M.M. Assessment of welfare and brachycephalic obstructive airway syndrome signs in young, breeding age French bulldogs and pugs, using owner questionnaire, physical examination and walk tests. Anim. Welf. 2019, 28, 287–298. [Google Scholar] [CrossRef]
- Hendricks, J.C. Brachycephalic airway syndrome. Vet. Clin. N. Am. Small Anim. Pract. 1992, 22, 1145–1153. [Google Scholar] [CrossRef]
- Lorinson, D.; Bright, R.M.; White, R.A.S. Brachycephalic airway obstruction syndrome—A review of 118 cases. Canine Pract. 1997, 22, 18–21. [Google Scholar]
- Koch, D.A.; Arnold, S.; Hubler, M.; Montavon, P.M. Brachycephalic syndrome in dogs. Compend. Contin. Educ. Vet. 2003, 25, 48–55. [Google Scholar]
- Holt, D. Upper airway obstruction, stertor and stridor. In Textbook of Respiratory Diseases in Dogs and Cats; King, L., Ed.; Saunders: St. Louis, MO, USA, 2004; pp. 35–42. [Google Scholar]
- Poncet, C.M.; Dupre, G.P.; Freiche, V.G.; Estrada, M.M.; Poubanne, Y.A.; Bouvy, B.M. Prevalence of gastrointestinal tract lesions in 73 brachycephalic dogs with upper respiratory syndrome. J. Small Anim. Pract. 2005, 46, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Riecks, T.W.; Birchard, S.J.; Stephens, J.A. Surgical correction of brachycephalic syndrome in dogs: 62 cases (1991–2004). J. Am. Vet. Med. Assoc. 2007, 230, 1324–1328. [Google Scholar] [CrossRef]
- Packer, R.M.A.; Hendricks, A.; Burn, C.C. Do dog owners perceive the clinical signs related to conformational inherited disorders as ‘normal’ for the breed? A potential constraint to improving canine welfare. Anim. Welf. 2012, 21, 81–93. [Google Scholar] [CrossRef]
- Roedler, F.S.; Pohl, S.; Oechtering, G.U. How does severe brachycephaly affect dog’s lives? Results of a structured preoperative owner questionnaire. Vet. J. 2013, 198, 606–610. [Google Scholar] [CrossRef]
- Haimel, G.; Dupre, G. Brachycephalic airway syndrome: A comparative study between pugs and French bulldogs. J. Small Anim. Pract. 2015, 56, 714–719. [Google Scholar] [CrossRef]
- Schlotthauer, C.F. Congenital defects in the stomach of a dog. J. Am. Vet. Med. Ass. 1929, 75, 370–371. [Google Scholar]
- TASSO, e.V: Die Beliebtesten Hunderassen. Available online: https://www.tasso.net/Service/Wissensportal/TASSO-Fakten/Die-beliebtesten-Hunderassen (accessed on 1 October 2021).
- The American Kennel Club: Most Popular Breeds. Available online: https://www.akc.org/most-popular-breeds/ (accessed on 1 October 2021).
- The Kennel Club: Breed Registration Statistics. Available online: https://www.thekennelclub.org.uk/media-centre/breed-registration-statistics/ (accessed on 1 October 2021).
- Packer, R.M.; Hendricks, A.; Tivers, M.S.; Burn, C.C. Impact of facial conformation on canine health: Brachycephalic Obstructive Airway Syndrome. PLoS ONE 2015, 10, e0137496. [Google Scholar] [CrossRef]
- Ravn-Mølby, E.-M.; Sindahl, L.; Nielsen, S.S.; Bruun, C.S.; Sandøe, P.; Fredholm, M. Breeding French bulldogs so that they breathe well—A long way to go. PLoS ONE 2019, 14, e0226280. [Google Scholar] [CrossRef] [PubMed]
- Lear, S.A.; Brozic, A.; Myers, J.N.; Ignaszewski, A. Exercise stress testing. An overview of current guidelines. Sports Med. 1999, 27, 285–312. [Google Scholar] [CrossRef] [PubMed]
- Enright, P.L. The six-minute walk test. Respir. Care 2003, 48, 783–785. [Google Scholar] [PubMed]
- Guyatt, G.H.; Sullivan, M.J.; Thompson, P.J.; Fallen, E.L.; Pugsley, S.O.; Taylor, D.W.; Berman, L.B. The 6-minute walk: A new measure of exercise capacity in patients with chronic heart failure. Can. Med. Assoc. J. 1985, 132, 919–923. [Google Scholar] [PubMed]
- American Thoracic Society. ATS statement: Guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [Google Scholar] [CrossRef]
- Riggs, J.; Liu, N.C.; Sutton, D.R.; Sargan, D.; Ladlow, J.F. Validation of exercise testing and laryngeal auscultation for grading brachycephalic obstructive airway syndrome in pugs, French bulldogs, and English bulldogs by using whole-body barometric plethysmography. Vet. Surg. 2019, 48, 488–496. [Google Scholar] [CrossRef]
- Bartels, A.; Martin, V.; Bidoli, E.; Steigmeier-Raith, S.; Bruhschwein, A.; Reese, S.; Köstlin, R.; Erhard, M.H. Brachycephalic problems of pugs relevant to animal welfare. Anim. Welf. 2015, 24, 327–333. [Google Scholar] [CrossRef]
- Wall, L.; Mohr, A.; Ripoli, F.L.; Schulze, N.; Penter, C.D.; Hungerbuehler, S.; Bach, J.-P.; Lucas, K.; Nolte, I. Clinical use of submaximal treadmill exercise testing and assessments of cardiac biomarkers NT-proBNP and cTnI in dogs with presymptomatic mitral regurgitation. PLoS ONE 2018, 13, e0199023. [Google Scholar] [CrossRef]
- Iwanuk, N.; Nolte, I.; Wall, L.; Sehn, M.; Raue, J.; Pilgram, A.; Rumstedt, K.; Bach, J.-P. Effect of Pimobendan on NT-proBNP and c troponin I before and after a submaximal exercise test in dogs with preclinical mitral valve disease without cardiomegaly—A randomised, double-blinded trial. BMC Vet. Res. 2019, 15, 237. [Google Scholar] [CrossRef]
- Iwanuk, N.; Wall, L.; Nolte, I.; Raue, J.; Rumstedt, K.; Pilgram, A.; Sehn, M.; Rohn, K.; Bach, J.-P. Effect of pimobendan on physical fitness, lactate and echocardiographic parameters in dogs with preclinical mitral valve disease without cardiomegaly. PLoS ONE 2019, 14, e0223164. [Google Scholar] [CrossRef]
- Ferasin, L.; Marcora, S. Reliability of an incremental exercise test to evaluate acute blood lactate, heart rate and body temperature responses in Labrador retrievers. J. Comp. Physiol. B 2009, 179, 839–845. [Google Scholar] [CrossRef]
- Piccione, G.; Casella, S.; Panzera, M.; Giannetto, C.; Fazio, F. Effect of Moderate Treadmill Exercise on Some Physiological Parameters in Untrained Beagle Dogs. Exp. Anim. 2012, 61, 511–515. [Google Scholar] [CrossRef][Green Version]
- Lee, H.S.; Lee, S.H.; Kim, J.W.; Lee, Y.S.; Lee, B.C.; Oh, H.J.; Kim, J.H. Development of novel continuous and interval exercise programs by applying the FITT-VP principle in dogs. Sci. World J. 2020, 2020, 1–9. [Google Scholar] [CrossRef]
- Essner, A.; Sjostrom, R.; Ahlgren, E.; Lindmark, B. Validity and reliability of Polar (R) RS800CX heart rate monitor, measuring heart rate in dogs during standing position and at trot on a treadmill. Physiol. Behav. 2013, 114, 1–5. [Google Scholar] [CrossRef]
- Rovira, S.; Munoz, A.; Riber, C.; Benito, M. Heart rate, electrocardiographic parameters and arrhythmias during agility exercises in trained dogs. Rev. Med. Vet. 2010, 161, 307–313. [Google Scholar]
- Nelson, R.W.; Couto, C.G. Small Animal Internal Medicine, 5th ed.; Mosby: St. Louis, MO, USA, 2014; pp. 1279–1282. [Google Scholar]
- Venker-van Haagen, A.J. Ear, Nose, Throat, and Tracheobronchial Diseases in Dogs and Cats; Schlütersche Verlagsgesellschaft: Hannover, Germany, 2015; pp. 83–166. [Google Scholar]
- Liu, N.C.; Sargan, D.R.; Adams, V.J.; Ladlow, J.F. Characterisation of brachycephalic obstructive airway syndrome in French bulldogs using whole-body barometric plethysmography. PLoS ONE 2015, 10, e0130741. [Google Scholar] [CrossRef]
- Laflamme, D. Development and validation of a body condition score system for dogs. Canine Pract. 1997, 22, 10–15. [Google Scholar]
- Sutter, N.B.; Mosher, D.S.; Gray, M.M.; Ostrander, E.A. Morphometrics within dog breeds are highly reproducible and dispute Rensch’s rule. Mamm. Genome 2008, 19, 713–723. [Google Scholar] [CrossRef]
- Matwichuk, C.L.; Taylor, S.M.; Shmon, C.L.; Kass, P.H.; Shelton, G.D. Changes in rectal temperature and hematologic, biochemical, blood gas, and acid-base values in healthy Labrador Retrievers before and after strenuous exercise. Am. J. Vet. Res. 1999, 60, 88–92. [Google Scholar]
- Musch, T.I.; Friedman, D.B.; Haidet, G.C.; Stray-Gundersen, J.; Waldrop, T.G.; Ordway, G.A. Arterial blood gases and acid-base status of dogs during graded dynamic exercise. J. Appl. Physiol. 1986, 61, 1914–1919. [Google Scholar] [CrossRef]
- Lilja-Maula, L.; Lappalainen, A.K.; Hyytiainen, H.K.; Kuusela, E.; Kaimio, M.; Schildt, K.; Mölsä, S.; Morelius, M.; Rajamäki, M.M. Comparison of submaximal exercise test results and severity of brachycephalic obstructive airway syndrome in English bulldogs. Vet. J. 2017, 219, 22–26. [Google Scholar] [CrossRef]
- Kohn, B.; Schwarz, G. Praktikum der Hundeklinik, 12th ed.; Enke Verlag: Stuttgart, Germany, 2018; pp. 13–19. [Google Scholar]
- Tipton, C.M.; Carey, R.A.; Eastin, W.C.; Erickson, H.H. A submaximal test for dogs: Evaluation of effects of training, detraining, and cage confinement. J. Appl. Physiol. 1974, 37, 271–275. [Google Scholar] [CrossRef]
- Sneddon, J.C.; Minnaar, P.P.; Grosskopf, J.F.; Groeneveld, H.T. Physiological and blood biochemical responses to submaximal treadmill exercise in Canaan dogs before, during and after training. J. S. Afr. Vet. Assoc. 1989, 60, 87–91. [Google Scholar]
- Rasmussen, C.E.; Vesterholm, S.; Ludvigsen, T.P.; Haggstrom, J.; Pedersen, H.D.; Moesgaard, S.G.; Olsen, L. Holter monitoring in clinically healthy Cavalier King Charles Spaniels, wire-haired Dachshunds, and Cairn Terriers. J. Vet. Intern. Med. 2011, 25, 460–468. [Google Scholar] [CrossRef]
- Hezzell, M.J.; Humm, K.; Dennis, S.G.; Agee, L.; Boswood, A. Relationships between heart rate and age, bodyweight and breed in 10,849 dogs. J. Small Anim. Pract. 2013, 54, 318–324. [Google Scholar] [CrossRef]
- Hammel, H.T.; Wyndham, C.H.; Hardy, J.D. Heat production and heat loss in the dog at 8-degrees-C-36-degrees-C environmental temperature. Am. J. Physiol. Imaging 1958, 194, 99–108. [Google Scholar] [CrossRef]
- Young, D.R.; Mosher, R.; Erve, P.; Spector, H. Body temperature and heat exchange during treadmill running in dogs. J. Appl. Physiol. 1959, 14, 839–843. [Google Scholar] [CrossRef]
- Schmidt-Nielsen, K.; Bretz, W.L.; Taylor, C.R. Panting in dogs: Unidirectional air flow over evaporative surfaces. Science 1970, 169, 1102–1104. [Google Scholar] [CrossRef]
- Oechtering, G.U.; Pohl, S.; Schlueter, C.; Lippert, J.P.; Alef, M.; Kiefer, I.; Ludewig, E.; Schuenemann, R. A novel approach to brachycephalic syndrome. 1. Evaluation of anatomical intranasal airway obstruction. Vet. Surg. 2016, 45, 165–172. [Google Scholar] [CrossRef]
- Goldberg, M.B.; Langman, V.A.; Taylor, C.R. Panting in dogs: Paths of air flow in response to heat and exercise. Respir. Physiol. 1981, 43, 327–338. [Google Scholar] [CrossRef]
- Beerda, B.; Schilder, M.B.H.; van Hooff, J.A.R.A.M.; de Vries, H.W. Manifestations of chronic and acute stress in dogs. Appl. Anim. Behav. Sci. 1997, 52, 307–319. [Google Scholar] [CrossRef]
- Englar, R.E. Stertor and Stridor. In Common Clinical Presentation in Dogs and Cats, 1st ed.; Englar, R.E., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2019; pp. 449–461. [Google Scholar]
- Pink, J.J.; Doyle, R.S.; Hughes, J.M.; Tobin, E.; Bellenger, C.R. Laryngeal collapse in seven brachycephalic puppies. J. Small Anim. Pract. 2006, 47, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, D.M.; Smith, C.A.; Eicker, S.W.; Henderson, K.S.; Dempsey, J.A. The effects of locomotion on respiratory muscle activity in the awake dog. Respir. Physiol. 1989, 78, 145–162. [Google Scholar] [CrossRef]
- Ohnishi, T.; Ogura, J.H.; Nelson, J.R. Effects of nasal obstruction upon the mechanics of the lung in the dog. Laryngoscope 1972, 82, 712–736. [Google Scholar] [CrossRef]
- Dohoo, I.R.; Martin, W.; Stryhn, H. Methods in Epidemiologic Research; VER Inc.: Charlottetown, PE, Canada, 2012; pp. 429–460. [Google Scholar]
- Fédération Cynologique Internationale (FCI). Standard Nr. 253. Available online: http://www.fci.be/de/nomenclature/9-Gesellschafts-und-Begleithunde.html#s11 (accessed on 1 October 2021).
- Monnet, E. Brachycephalic airway syndrome. In Proceedings of the 40th World Small Animal Veterinary Association Congress, Bangkok, Thailand, 15–18 May 2015; pp. 245–247. [Google Scholar]
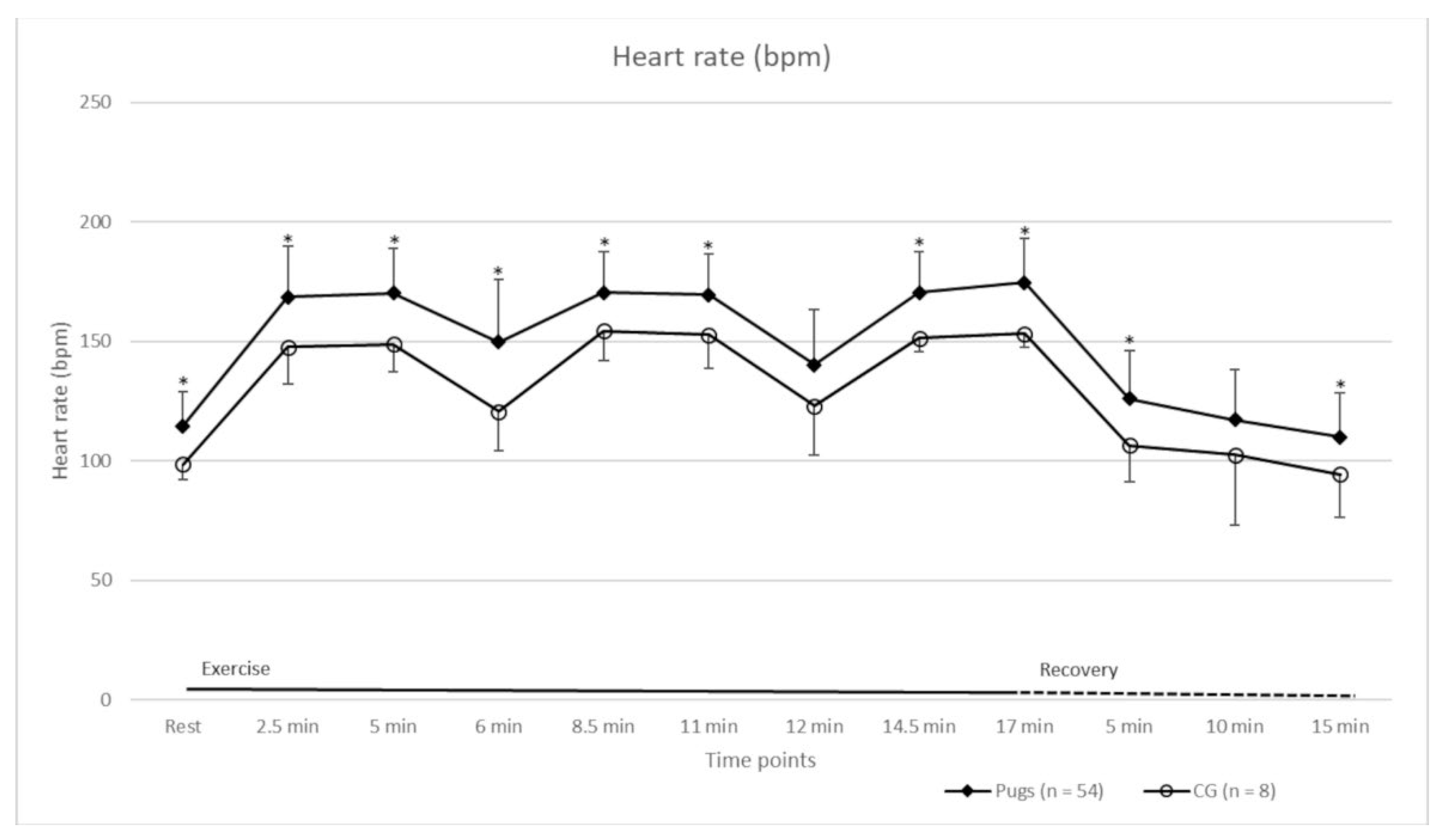
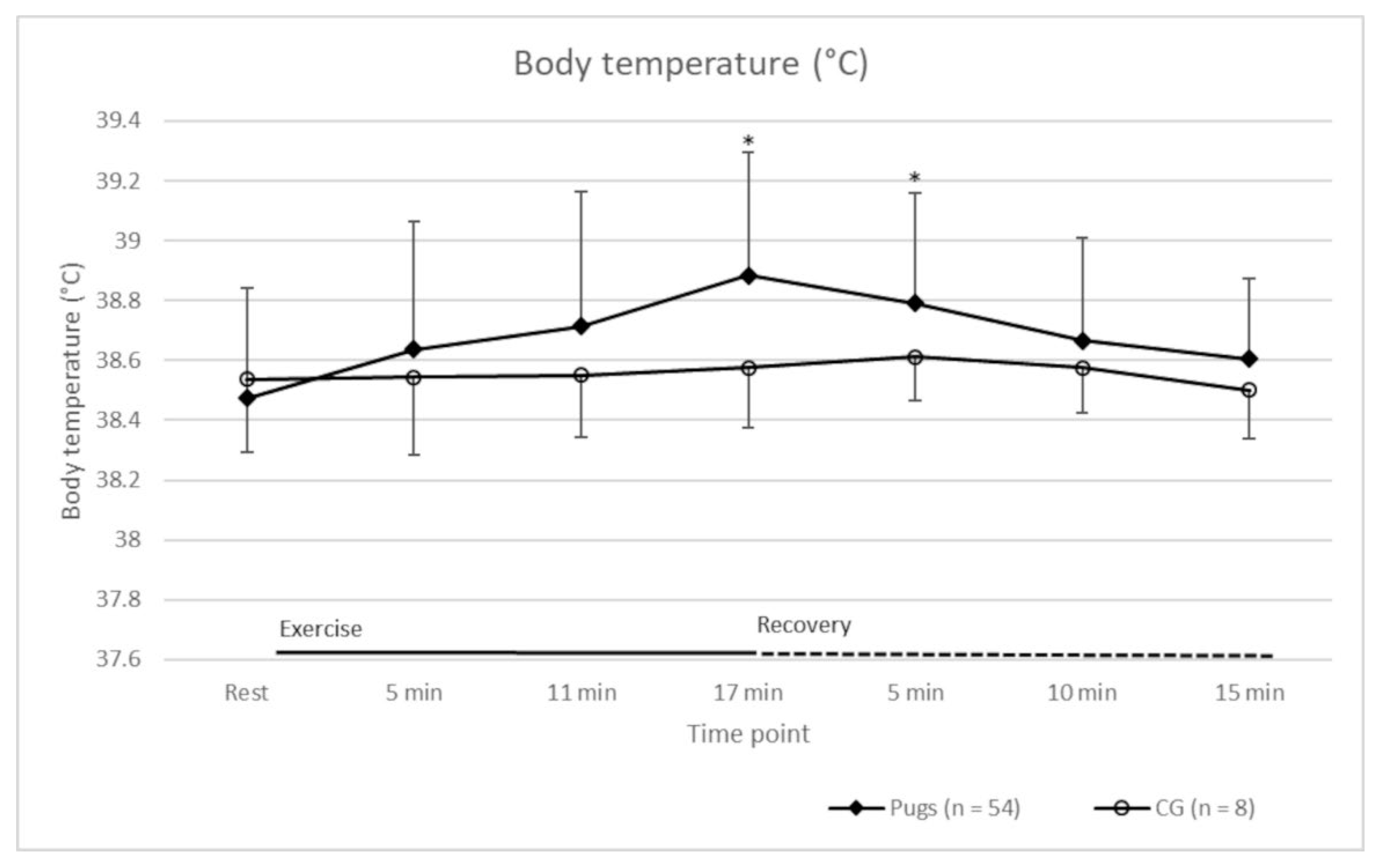


| Time Point (min:sec)/Parameter | At Rest | 02:30 | 05:00 | 06:00 | 08:30 | 11:00 | 12:00 | 14:30 | 17:00 |
|---|---|---|---|---|---|---|---|---|---|
| Heart rate (bpm) | X | X | X | X | X | X | X | X | X |
| Temperature (°C) | X | X | X | X | |||||
| Respiratory rate (breaths min−1) | X | X | X | X |
| Time Point | At Rest | 5 min | 11 min | 17 min | |
|---|---|---|---|---|---|
| Number of subjects (n) | 62 | 52 | 52 | 52 | |
| No RN (%) | 37 | 25 | 27 | 17 | |
| RN audible without stethoscope (%) | |||||
| Intermittent | Mild | 34 | 15 | 13 | 19 |
| Moderate | 2 | 0 | 2 | 2 | |
| Severe | 0 | 0 | 0 | 0 | |
| Constant | Mild | 6 | 31 | 31 | 19 |
| Moderate | 3 | 2 | 11 | 27 | |
| Severe | 0 | 2 | 0 | 0 | |
| RN audible only with stethoscope (%) | |||||
| Intermittent | Mild | 8 | 13 | 0 | 6 |
| Moderate | 0 | 0 | 8 | 0 | |
| Severe | 0 | 0 | 0 | 0 | |
| Constant | Mild | 8 | 10 | 8 | 8 |
| Moderate | 2 | 2 | 0 | 2 | |
| Severe | 0 | 0 | 0 | 0 | |
| Time Point/Grading | BOAS− | BOAS+ | ||
|---|---|---|---|---|
| Grade 0 | Grade 1 | Grade 2 | Grade 3 | |
| 5 min | 14 | 11 | 24 | 7 |
| 10 min | 12 | 7 | 30 | 7 |
| 15 min | 10 | 7 | 32 | 7 |
| Variable/Group | CG | All Pugs | Grade 0 | Grade 1 | Grade 2 | Grade 3 |
|---|---|---|---|---|---|---|
| Number (n) | 10 | 62 | 10 | 7 | 32 | 7 |
| Male (%) | 50 | 46.77 | 70 | 28.57 | 50 | 28.57 |
| Age (years, mean ± SD) | 6.2 ± 2.6 | 4.8 ± 2.4 | 4.5 ± 1.5 | 4.1 ± 1.7 | 4.6 ± 2.1 | 4.9 ± 2.2 |
| Weight (kg, mean ± SD) | 8.8 ± 1.9 | 8.9 ± 1.3 | 9.5 ± 1.1 | 8.2 ± 0.9 | 9.1 ± 1.21 | 7.8 ± 1.4 |
| Speed (km/h, mean ± SD) | 5.3 ± 0.7 | 5.0 ± 0.4 | 5.1 ± 0.5 | 4.9 ± 0.5 | 5.0 ± 0.4 | 4.8 ± 0.6 |
| Groups/Time Point (min:sec) | CG (n = 8) | Grade 0 (n = 10) | Grade 1 (n = 7) | Grade 2 (n = 29) | Grade 3 (n = 7) | |
|---|---|---|---|---|---|---|
| At rest | 103 ± 7 | 102 ± 9 | 116 ± 10 | 118 ± 8 | 120 ± 12 | |
| Exercise | 02:30 | 148 ± 15 | 153 ± 25 | 182 ± 24 | 171 ± 16 | 169 ± 22 |
| 05:00 | 149 ± 12 | 154 ± 17 | 179 ± 20 | 172 ± 17 | 179 ± 19 | |
| 06:00 | 121 ± 16 | 123 ± 19 | 165 ± 13 | 156 ± 27 | 145 ± 14 | |
| 08:30 | 155 ± 12 | 164 ± 17 | 178 ± 18 | 170 ± 17 | 179 ± 11 | |
| 11:00 | 153 ± 14 | 162 ± 15 | 178 ± 17 | 169 ± 18 | 181 ± 13 | |
| 12:00 | 123 ± 20 | 121 ± 24 | 156 ± 16 | 141 ± 19 | 152 ± 30 | |
| 14:30 | 152 ± 6 | 162 ± 14 | 179 ± 18 | 172 ± 18 | 174 ± 7 | |
| 17:00 | 153 ± 6 | 169 ± 17 | 179 ± 21 | 176 ± 18 | 178 ± 18 | |
| Recovery | 05:00 | 107 ± 15 | 102 ± 16 | 131 ± 21 | 132 ± 16 | 134 ± 21 |
| 10:00 | 103 ± 29 | 97 ± 16 | 119 ± 17 | 123 ± 19 | 123 ± 29 | |
| 15:00 | 94 ± 18 | 92 ± 17 | 111 ± 17 | 115 ± 17 | 118 ± 21 | |
| Groups/Time Point (min:sec) | CG (n = 8) | Grade 0 (n = 10) | Grade 1 (n = 7) | Grade 2 (n = 29) | Grade 3 (n = 7) | |
|---|---|---|---|---|---|---|
| At rest | 38.5 ± 0.3 | 38.4 ± 0.4 | 38.5 ± 0.3 | 38.5 ± 0.3 | 38.4 ± 0.5 | |
| Exercise | 05:00 | 38.5 ± 0.3 | 38.4 ± 0.4 | 38.8 ± 0.3 | 38.7 ± 0.5 | 38.6 ± 0.5 |
| 11:00 | 38.6 ± 0.2 | 38.4 ± 0.5 | 38.9 ± 0.3 | 38.8 ± 0.4 | 38.7 ± 0.2 | |
| 17:00 | 38.6 ± 0.2 | 38.6 ± 0.4 | 39.0 ± 0.3 | 39.0 ± 0.5 | 38.9 ± 0.3 | |
| Recovery | 05:00 | 38.6 ± 0.2 | 38.6 ± 0.3 | 38.9 ± 0.3 | 38.9 ± 0.4 | 38.9 ± 0.4 |
| 10:00 | 38.6 ± 0.2 | 38.5 ± 0.3 | 38.7 ± 0.4 | 38.7 ± 0.3 | 38.7 ± 0.4 | |
| 15:00 | 38.5 ± 0.2 | 38.4 ± 0.2 | 38.7 ± 0.3 | 38.7 ± 0.3 | 38.6 ± 0.3 | |
| Recovery Time/Groups | CG (n = 8) | All Pugs (n = 52) | Grade 0 (n = 10) | Grade 1 (n = 7) | Grade 2 (n = 29) | Grade 3 (n = 5) | |
|---|---|---|---|---|---|---|---|
| Heart rate (bpm) | ≤5 min | 5 | 21 | 9 | 4 | 6 | 2 |
| ≤10 min | 1 | 20 | 1 | 1 | 16 | 1 | |
| ≤15 min | 1 | 7 | 0 | 1 | 4 | 2 | |
| >15 min | 1 | 4 | 0 | 1 | 3 | 0 | |
| Body temperature (°C) | ≤5 min | 8 | 45 | 10 | 6 | 23 | 5 |
| ≤10 min | 0 | 2 | 0 | 0 | 2 | 0 | |
| ≤15 min | 0 | 5 | 0 | 1 | 4 | 0 | |
| >15 min | 0 | 0 | 0 | 0 | 0 | 0 | |
| Respiratory rate | ≤5 min | 5 | 15 | 5 | 2 | 5 | 2 |
| ≤10 min | 2 | 17 | 3 | 3 | 10 | 1 | |
| ≤15 min | 0 | 14 | 2 | 2 | 10 | 0 | |
| >15 min | 1 | 6 | 0 | 0 | 4 | 2 |
| Risk Categories | BOAS+ | BOAS− | Univariable Model | Multivariable Model | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95%-CI | p | OR | 95%-CI | p | |||||||
| Low | Up | Low | Up | |||||||||
| n | % | n | % | |||||||||
| total | 25 | 58.1 | 18 | 41.9 | x | x | x | x | x | x | x | x |
| Gender | ||||||||||||
| female (ref) | 11 | 55.0 | 9 | 45.0 | 1 | x | x | x | 1 | x | x | x |
| male | 14 | 60.9 | 9 | 39.1 | 1.27 | 0.38 | 4.29 | 0.697 | 1.49 | 0.08 | 29.33 | 0.786 |
| neuter status | ||||||||||||
| intact (ref) | 16 | 51.6 | 15 | 48.4 | 1 | x | x | x | 1 | x | x | x |
| neutered | 9 | 75.0 | 3 | 25.0 | 2.81 | 0.64 | 12.41 | 0.172 | 0.09 | 0.01 | 1.27 | 0.073 |
| BCS | ||||||||||||
| overweight (ref) | 14 | 70.0 | 6 | 30.0 | 1 | x | x | x | 1 | x | x | x |
| normal weight | 11 | 47.8 | 12 | 52.2 | 0.39 | 0.11 | 1.38 | 0.146 | 2.15 | 0.26 | 17.70 | 0.465 |
| nostril | ||||||||||||
| stenosis (ref) | 13 | 56.5 | 10 | 43.5 | 1 | x | x | x | 1 | x | x | x |
| open | 12 | 60.0 | 8 | 40.0 | 1.15 | 0.34 | 3.90 | 0.818 | 1.06 | 0.15 | 7.30 | 0.956 |
| Continuous variables | ||||||||||||
| age (per 1 month increase) | x | x | x | x | 0.98 | 0.96 | 1.01 | 0.256 | 1.00 | 0.96 | 1.05 | 0.980 |
| body length (per 1 cm increase) | x | x | x | x | 1.24 | 0.94 | 1.63 | 0.127 | 1.35 | 0.84 | 2.16 | 0.211 |
| neck girth (per 1 cm increase) | x | x | x | x | 0.80 | 0.60 | 1.06 | 0.124 | 0.81 | 0.41 | 1.59 | 0.519 |
| eye width (per 1 cm increase) | x | x | x | x | 0.36 | 0.06 | 2.09 | 0.255 | 0.35 | 0.01 | 19.30 | 0.597 |
| chest girth (per 1 cm increase) | x | x | x | x | 0.80 | 0.60 | 1.06 | 0.113 | 0.71 | 0.42 | 1.19 | 0.186 |
| height (per 1 cm increase) | x | x | x | x | 1.16 | 0.89 | 1.52 | 0.270 | 0.90 | 0.48 | 1.68 | 0.720 |
| CFR (per 1% increase) | x | x | x | x | 1.33 | 1.04 | 1.69 | 0.023 | 1.35 | 0.93 | 1.97 | 0.114 |
| Signs/Frequency | Never | Rarely | Regularly | Daily | Constant | No Specification | |
|---|---|---|---|---|---|---|---|
| Regurgitation | Pugs (%) | 92 | 4.8 | 0 | 0 | 1.6 | 1.6 |
| CG (%) | 100 | 0 | 0 | 0 | 0 | 0 | |
| Vomiting | Pugs (%) | 83.9 | 12.9 | 1.6 | 0 | 0 | 1.6 |
| CG (%) | 60 | 40 | 0 | 0 | 0 | 0 | |
| Choking on food, difficulties swallowing | Pugs (%) | 61.3 | 32.3 | 3.9 | 1.6 | 0 | 0 |
| CG (%) | 40 | 60 | 0 | 0 | 0 | 0 | |
| Dyspnoea during feeding | Pugs (%) | 93.6 | 1.6 | 1.6 | 1.6 | 0 | 1.6 |
| CG (%) | 90 | 10 | 0 | 0 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mach, R.; Wiegel, P.S.; Bach, J.-P.; Beyerbach, M.; Kreienbrock, L.; Nolte, I. Evaluation of a Treadmill-Based Submaximal Fitness Test in Pugs, and Collecting Breed-Specific Information on Brachycephalic Obstructive Airway Syndrome. Animals 2022, 12, 1585. https://doi.org/10.3390/ani12121585
Mach R, Wiegel PS, Bach J-P, Beyerbach M, Kreienbrock L, Nolte I. Evaluation of a Treadmill-Based Submaximal Fitness Test in Pugs, and Collecting Breed-Specific Information on Brachycephalic Obstructive Airway Syndrome. Animals. 2022; 12(12):1585. https://doi.org/10.3390/ani12121585
Chicago/Turabian StyleMach, Rebekka, Pia S. Wiegel, Jan-Peter Bach, Martin Beyerbach, Lothar Kreienbrock, and Ingo Nolte. 2022. "Evaluation of a Treadmill-Based Submaximal Fitness Test in Pugs, and Collecting Breed-Specific Information on Brachycephalic Obstructive Airway Syndrome" Animals 12, no. 12: 1585. https://doi.org/10.3390/ani12121585
APA StyleMach, R., Wiegel, P. S., Bach, J.-P., Beyerbach, M., Kreienbrock, L., & Nolte, I. (2022). Evaluation of a Treadmill-Based Submaximal Fitness Test in Pugs, and Collecting Breed-Specific Information on Brachycephalic Obstructive Airway Syndrome. Animals, 12(12), 1585. https://doi.org/10.3390/ani12121585





