Serum Insulin-like Growth Factor-1 Is a Biomarker of Testosterone Production and Intact Acrosome in Asian Elephants (Elephas maximus)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Blood Collection, Semen Collection and Processing Procedures
2.3. Semen Evaluation
2.3.1. Sperm Membrane Integrity Assessment
2.3.2. Sperm Functional Membrane Integrity Assessment
2.3.3. Sperm DNA Integrity Assessment
2.3.4. Acrosome Integrity Assessment
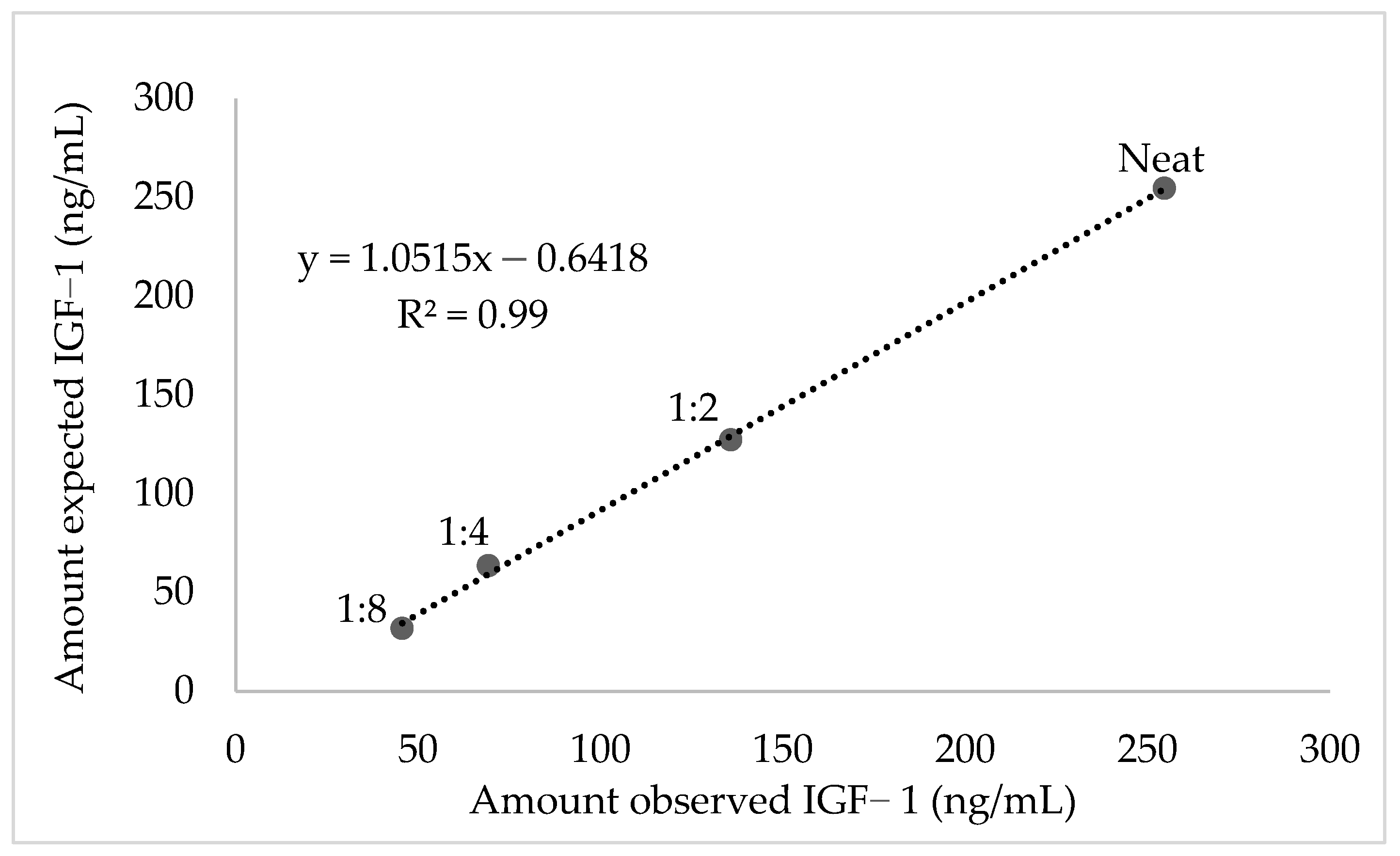
2.4. Validation and Evaluation of Serum IGF-1
2.5. Evaluation of Serum Testosterone
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kontogeorgopoulos, N. The role of tourism in elephant welfare in Northern Thailand. J. Tour. 2009. [Google Scholar]
- Thitaram, C. Breeding Management of Captive Asian Elephant (Elephas maximus) in Range Countries and Zoos. Jpn. J. Zoo Wildl. Med. 2012, 17, 91–96. [Google Scholar] [CrossRef] [Green Version]
- Toin, P.; Brown, J.L.; Punyapornwithaya, V.; Bansiddhi, P.; Somgird, C.; Thitaram, C. Reproductive performance of captive Asian elephants (Elephas maximus) in large tourist camps in Thailand. Anim. Reprod. Sci. 2020, 222, 106606. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.L.; Göritz, F.; Pratt-Hawkes, N.; Hermes, R.; Galloway, M.; Graham, L.H.; Gray, C.; Walker, S.L.; Gomez, A.; Moreland, R.; et al. Successful artificial insemination of an Asian elephant at the National Zoological Park. Zoo Biol. 2004, 23, 45–63. [Google Scholar] [CrossRef]
- Thongtip, N.; Saikhun, J.; Mahasawangkul, S.; Kornkaewrat, K.; Pongsopavijitr, P.; Songsasen, N.; Pinyopummin, A. Potential factors affecting semen quality in the Asian elephant (Elephas maximus). Reprod. Biol. Endocrinol. 2008, 6, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiso, W.K.; Brown, J.L.; Siewerdt, F.; Schmitt, D.L.; Olson, D.; Crichton, E.G.; Pukazhenthi, B.S. Liquid semen storage in elephants (Elephas maximus and Loxodonta africana): Species differences and storage optimization. J. Androl. 2011, 32, 420–431. [Google Scholar] [CrossRef] [Green Version]
- Cannarella, R.; Condorelli, R.A.; La Vignera, S.; Calogero, A.E. Effects of the insulin-like growth factor system on testicular differentiation and function: A review of the literature. Andrology 2018, 6, 3–9. [Google Scholar] [CrossRef] [Green Version]
- Müller, L.; Kowalewski, M.P.; Reichler, I.M.; Kollár, E.; Balogh, O. Different expression of leptin and IGF 1 in the adult and prepubertal testis in dogs. Reprod. Domest. Anim. 2017, 52, 187–192. [Google Scholar] [CrossRef] [Green Version]
- Roser, J.F. Endocrine and paracrine control of sperm production in stallions. Anim. Reprod. Sci. 2001, 68, 139–151. [Google Scholar] [CrossRef]
- Cailleau, J.; Vermeire, S.; Verhoeven, G. Independent control of the production of insulin-like growth factor I and its binding protein by cultured testicular cells. Mol. Cell. Endocrinol. 1990, 69, 79–89. [Google Scholar] [CrossRef]
- Lejeune, H.; Chuzel, F.; Thomas, T.; Avallet, O.; Habert, R.; Durand, P.; Saez, J. Paracrine regulation of Leydig cells. Ann. Endocrinol. 1996, 57, 55–63. [Google Scholar]
- Vannelli, B.G.; Barni, T.; Orlando, C.; Natali, A.; Serio, M.; Balboni, G.C. Insulin-like growth factor-1 (IGF-I) and IGF-I receptor in human testis: An immunohistochemical study. Fertil. Steril. 1988, 49, 666–669. [Google Scholar] [CrossRef]
- Griffeth, R.J.; Bianda, V.; Nef, S. The emerging role of insulin-like growth factors in testis development and function. Basic Clin. Androl. 2014, 24, 12. [Google Scholar] [CrossRef] [Green Version]
- Le Gac, F.; Loir, M.; Le Bail, P.-Y.; Ollitrault, M. Insulin-like growth factor (IGF-I) mRNA and IGF-I receptor in trout testis and in isolated spermatogenic and Sertoli cells. Mol. Reprod. Dev. 1996, 44, 23–35. [Google Scholar] [CrossRef]
- Yoon, M.J.; Berger, T.; Roser, J.F. Localization of insulin-like growth factor-I (IGF-I) and IGF-I receptor (IGF-IR) in equine testes. Reprod. Domest. Anim. 2011, 46, 221–228. [Google Scholar] [CrossRef]
- Henricks, D.M.; Kouba, A.J.; Lackey, B.R.; Boone, W.R.; Gray, S.L. Identification of insulin-like growth factor I in bovine seminal plasma and its receptor on spermatozoa: Influence on sperm motility. Biol. Reprod. 1998, 59, 330–337. [Google Scholar] [CrossRef] [Green Version]
- Naz, R.K.; Padman, P. Identification of insulin-like growth factor (IGF)-1 receptor in human sperm cell. Arch. Androl. 1999, 43, 153–159. [Google Scholar] [CrossRef]
- Leheup, B.P.; Grigong, G. Immunohistochemical Localization of Insulin-like Growth Factor I (IGF-I) in the Rat Epididymis. J. Androl. 1993, 14, 159–163. [Google Scholar] [CrossRef]
- Yoon, M.; Jiang, J.; Chung, K.H.; Roser, J.F. Immunolocalization of insulin-like growth factor-I (IGF-I) and its receptors (IGF-IR) in the equine epididymis. J. Reprod. Dev. 2015, 61, 30–34. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; Suman; Pawaria, S.; Dalal, J.; Bhardwaj, S.; Patil, S.; Jerome, A.; Sharma, R.K. Serum and seminal plasma IGF-1 associations with semen variables and effect of IGF-1 supplementation on semen freezing capacity in buffalo bulls. Anim. Reprod. Sci. 2019, 204, 101–110. [Google Scholar] [CrossRef]
- Weerakoon, W.W.P.N.; Sakase, M.; Kawate, N.; Hannan, M.A.; Kohama, N.; Tamada, H. Plasma IGF-I, INSL3, testosterone, inhibin concentrations and scrotal circumferences surrounding puberty in Japanese Black beef bulls with normal and abnormal semen. Theriogenology 2018, 114, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Park, Y.S.; Lee, J.S.; Seo, J.T. Serum and seminal plasma insulin-like growth factor-1 in male infertility. Clin. Exp. Reprod. Med. 2016, 43, 97–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zangeronimo, M.; Silva, D.M.; Solis-Murgas, L.; Sousa, R.V.; Rocha, L.L.; Pereira, B.; Faria, B.G.; Veras, G. Identification of insulin-like growth factor-I in boar seminal plasma and its influence on sperm quality. Arch. Zootec. 2013, 62. [Google Scholar] [CrossRef] [Green Version]
- Selvaraju, S.; Reddy, I.J.; Nandi, S.; Rao, S.B.N.; Ravindra, J.P. Influence of IGF-I on buffalo (Bubalus bubalis) spermatozoa motility, membrane integrity, lipid peroxidation and fructose uptake in vitro. Anim. Reprod. Sci. 2009, 113, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Makarevich, A.V.; Spalekova, E.; Olexikova, L.; Kubovicova, E.; Hegedusova, Z. Effect of insulin-like growth factor I on functional parameters of ram cooled-stored spermatozoa. Zygote 2014, 22, 305. [Google Scholar] [CrossRef]
- Hirai, M.; Boersma, A.; Hoeflich, A.; Wolf, E.; Foll, J.; Aumuller, T.R.; Braun, J. Objectively measured sperm motility and sperm head morphometry in boars (Sus scrofa): Relation to fertility and seminal plasma growth factors. J. Androl. 2001, 22, 104–110. [Google Scholar]
- Glander, H.J.; Kratzsch, J.; Weisbrich, C.; Birkenmeier, G. Insulin-like growth factor-I and alpha 2-macroglobulin in seminal plasma correlate with semen quality. Hum. Reprod. 1996, 11, 2454–2460. [Google Scholar] [CrossRef] [Green Version]
- Macpherson, M.L.; Simmen, R.C.; Simmen, F.A.; Hernandez, J.; Sheerin, B.R.; Varner, D.D.; Loomis, P.; Cadario, M.E.; Miller, C.D.; Brinsko, S.P.; et al. Insulin-like growth factor-I and insulin-like growth factor binding protein-2 and -5 in equine seminal plasma: Association with sperm characteristics and fertility. Biol. Reprod. 2002, 67, 648–654. [Google Scholar] [CrossRef]
- Schmitt, D.; Hildebrandt, T. Manual collection and characterization of semen from Asian elephants (Elephas maximus). Anim. Reprod. Sci. 1998, 53, 309–314. [Google Scholar] [CrossRef]
- Björndahl, L.; Söderlund, I.; Kvist, U. Evaluation of the one-step eosin-nigrosin staining technique for human sperm vitality assessment. Hum. Reprod. 2003, 18, 813–816. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Llano, B.; Lorenzo, J.L.; Yenes, P.; Trejo, A.; García-Casado, P. A short hypoosmotic swelling test for the prediction of boar sperm fertility. Theriogenology 2001, 56, 387–398. [Google Scholar] [CrossRef]
- Buranaamnuay, K.; Mahasawangkul, S.; Saikhun, K. The in vitro quality of frozen-thawed Asian elephant (Elephas maximus) spermatozoa in semen supplemented with Equex STM paste and oxytocin during and after cryopreservation. Reprod. Biol. 2013, 13, 169–171. [Google Scholar] [CrossRef]
- Tejada, R.I.; Mitchell, J.C.; Norman, A.; Marik, J.J.; Friedman, S. A test for the practical evaluation of male fertility by acridine orange (AO) fluorescence. Fertil. Steril. 1984, 42, 87–91. [Google Scholar] [CrossRef]
- Thuwanut, P.; Chatdarong, K.; Techakumphu, M.; Axnér, E. The effect of antioxidants on motility, viability, acrosome integrity and DNA integrity of frozen-thawed epididymal cat spermatozoa. Theriogenology 2008, 70, 233–240. [Google Scholar] [CrossRef]
- Axnér, E.; Hermansson, U.; Linde-Forsberg, C. The effect of Equex STM paste and sperm morphology on post-thaw survival of cat epididymal spermatozoa. Anim. Reprod Sci. 2004, 84, 179–191. [Google Scholar] [CrossRef]
- Cheng, F.P.; Fazeli, A.; Voorhout, W.F.; Marks, A.; Bevers, M.M.; Colenbrander, B. Use of peanut agglutinin to assess the acrosomal status and the zona pellucida-induced acrosome reaction in stallion spermatozoa. J. Androl. 1996, 17, 674–682. [Google Scholar]
- Rotwein, P. Diversification of the insulin-like growth factor 1 gene in mammals. PLoS ONE 2017, 12, e0189642. [Google Scholar] [CrossRef]
- Stiller, J.; Jasensky, A.-K.; Hennies, M.; Einspanier, R.; Kohn, B. Validation of an enzyme-linked immunosorbent assay for measurement of feline haptoglobin. J. Vet. Diagn. Investig. 2016, 28. [Google Scholar] [CrossRef]
- Axnér, E.; Holst, B. Concentrations of anti-Müllerian hormone in the domestic cat. Relation with spay or neuter status and serum estradiol. Theriogenology 2014, 83. [Google Scholar] [CrossRef]
- Brown, J.; Walker, S.; Steinman, K. Endocrine Manual for the Reproductive Assessment of Domestic and Non-Domestic Species; Endocrine Research Laboratory, Department of Reproductive Sciences, Conservation and Research Center, Nation Zoological Park Smithsonian Institution: Front Royal, VA, USA, 2004; Volume 1, p. 93. [Google Scholar]
- Khonmee, J.; Brown, J.L.; Li, M.-Y.; Somgird, C.; Boonprasert, K.; Norkaew, T.; Punyapornwithaya, V.; Lee, W.-M.; Thitaram, C. Effect of time and temperature on stability of progestagens, testosterone and cortisol in Asian elephant blood stored with and without anticoagulant. Conserv. Physiol. 2019, 7, coz031. [Google Scholar] [CrossRef]
- Morfeld, K.A.; Meehan, C.L.; Hogan, J.N.; Brown, J.L. Assessment of Body Condition in African (Loxodonta africana) and Asian (Elephas maximus) Elephants in North American Zoos and Management Practices Associated with High Body Condition Scores. PLoS ONE 2016, 11, e0155146. [Google Scholar] [CrossRef] [Green Version]
- Imrat, P.; Mahasawangkul, S.; Gosálvez, J.; Suthanmapinanth, P.; Sombutputorn, P.; Jansittiwate, S.; Thongtip, N.; Pinyopummin, A.; Colenbrander, B.; Holt, W. Effect of cooled storage on quality and DNA integrity of Asian elephant (Elephas maximus) spermatozoa. Reprod. Fertil. Dev. 2012, 24, 1105–1116. [Google Scholar] [CrossRef]
- Holt, W.V.; Harrison, R.A. Bicarbonate Stimulation of Boar Sperm Motility via a Protein Kinase A—Dependent Pathway: Between-Cell and Between-Ejaculate Differences Are Not Due to Deficiencies in Protein Kinase A Activation. J. Androl. 2002, 23, 557–565. [Google Scholar]
- Ditchkoff, S.S.; Spicer, L.J.; Masters, R.E.; Lochmiller, R.L. Concentrations of insulin-like growth factor-I in adult male white-tailed deer (Odocoileus virginianus): Associations with serum testosterone, morphometrics and age during and after the breeding season. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2001, 129, 887–895. [Google Scholar] [CrossRef]
- Tardif, S.; Laforest, J.P.; Cormier, N.; Bailey, J.L. The importance of porcine sperm parameters on fertility in vivo. Theriogenology 1999, 52, 447–459. [Google Scholar] [CrossRef]
- Menkveld, R.; Rhemrev, J.P.; Franken, D.R.; Vermeiden, J.P.; Kruger, T.F. Acrosomal morphology as a novel criterion for male fertility diagnosis: Relation with acrosin activity, morphology (strict criteria), and fertilization in vitro. Fertil. Steril. 1996, 65, 637–644. [Google Scholar] [CrossRef]
- Minelli, A.; Liguori, L.; Collodel, G.; Lattaioli, P.; Castellini, C. Effects of the purified IGF-I complex on the capacitation and acrosome reaction of rabbit spermatozoa. J. Exp. Zool. 2001, 290, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Selvaraju, S.; Nandi, S.; Subramani, T.S.; Raghavendra, B.S.; Rao, S.B.N.; Ravindra, J.P. Improvement in buffalo (Bubalus bubalis) spermatozoa functional parameters and fertility in vitro: Effect of insulin-like growth factor-I. Theriogenology 2010, 73, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Juul, A.; Bang, P.; Hertel, N.T.; Main, K.; Dalgaard, P.; Jørgensen, K.; Müller, J.; Hall, K.; Skakkebaek, N.E. Serum insulin-like growth factor-I in 1030 healthy children, adolescents, and adults: Relation to age, sex, stage of puberty, testicular size, and body mass index. J. Clin. Endocrinol. Metab. 1994, 78, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Yagci, A.; Zik, B. Immunohistochemical Localization of Insulin-Like Growth Factor-I Receptor (IGF-IR) in the Developing and Mature Rat Testes. Anat. Histol. Embryol. 2006, 35, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Sengupta, P.; Muhamad, S. Male reproductive hormones and semen quality. Asian Pac. J. Reprod. 2019, 8, 189–194. [Google Scholar] [CrossRef]
- Yoon, M.J.; Roser, J.F. A synergistic effect of insulin-like growth factor (IGF-I) on equine luteinizing hormone (eLH)-induced testosterone production from cultured Leydig cells of horses. Anim. Reprod. Sci. 2011, 126, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Gelber, S.J.; Hardy, M.P.; Mendis-Handagama, S.M.; Casella, S.J. Effects of insulin-like growth factor-I on androgen production by highly purified pubertal and adult rat Leydig cells. J. Androl. 1992, 13, 125–130. [Google Scholar] [PubMed]
- Ashton, W.S.; Degnan, B.M.; Daniel, A.; Francis, G.L. Testosterone increases insulin-like growth factor-1 and insulin-like growth factor-binding protein. Ann. Clin. Lab. Sci. 1995, 25, 381–388. [Google Scholar]
- Pitetti, J.-L.; Calvel, P.; Zimmermann, C.; Conne, B.; Papaioannou, M.D.; Aubry, F.; Cederroth, C.R.; Urner, F.; Fumel, B.; Crausaz, M.; et al. An essential role for insulin and IGF1 receptors in regulating sertoli cell proliferation, testis size, and FSH action in mice. Mol. Endocrinol. (Baltim. Md.) 2013, 27, 814–827. [Google Scholar] [CrossRef]
- Baker, J.; Hardy, M.P.; Zhou, J.; Bondy, C.; Lupu, F.; Bellvé, A.R.; Efstratiadis, A. Effects of an Igf1 gene null mutation on mouse reproduction. Mol. Endocrinol. 1996, 10, 903–918. [Google Scholar]
- Selvaraju, S.; Sivasubramani, T.; Raghavendra, B.S.; Raju, P.; Rao, S.B.N.; Dineshkumar, D.; Ravindra, J.P. Effect of dietary energy on seminal plasma insulin-like growth factor-I (IGF-I), serum IGF-I and testosterone levels, semen quality and fertility in adult rams. Theriogenology 2012, 78, 646–655. [Google Scholar] [CrossRef]
- Tran, L.V.; Malla, B.A.; Sharma, A.N.; Kumar, S.; Tyagi, N.; Tyagi, A.K. Effect of omega-3 and omega-6 polyunsaturated fatty acid enriched diet on plasma IGF-1 and testosterone concentration, puberty and semen quality in male buffalo. Anim. Reprod. Sci. 2016, 173, 63–72. [Google Scholar] [CrossRef]
- Brito, L.F.; Barth, A.D.; Rawlings, N.C.; Wilde, R.E.; Crews, D.H., Jr.; Mir, P.S.; Kastelic, J.P. Effect of improved nutrition during calfhood on serum metabolic hormones, gonadotropins, and testosterone concentrations, and on testicular development in bulls. Domest. Anim. Endocrinol. 2007, 33, 460–469. [Google Scholar] [CrossRef]
- Hess, M.F.; Roser, J.F. The effects of age, season and fertility status on plasma and intratesticular insulin-like growth factor I concentration in stallions. Theriogenology 2001, 56, 723–733. [Google Scholar] [CrossRef]
- Salardi, S.; Cacciari, E.; Ballardini, D.; Righetti, F.; Capelli, M.; Cicognani, A.; Zucchini, S.; Natali, G.; Tassinari, D. Relationships Between Growth Factors (Somatomedin-C and Growth Hormone) and Body Development, Metabolic Control, and Retinal Changes in Children and Adolescents With IDDM. Diabetes 1986, 35, 832–836. [Google Scholar] [CrossRef]
- Schulte, B.A. Behavior and social life. Biol. Med. Surg. Elephants 2006, 35–44. [Google Scholar]
- Govoni, K.E.; Goodman, D.; Maclure, R.M.; Penfold, L.M.; Zinn, S.A. Serum concentrations of insulin-like growth factor-i and insulin-like growth factor binding protein-2 and -3 in eight hoofstock species. Zoo Biol. 2011, 30, 275–284. [Google Scholar] [CrossRef]
- McClain, A.K.; McCarrel, T.M. The effect of four different freezing conditions and time in frozen storage on the concentration of commonly measured growth factors and enzymes in equine platelet-rich plasma over six months. BMC Vet. Res. 2019, 15, 1–9. [Google Scholar] [CrossRef]
| ID | Age (y) | BCS | Body Weight (kg) | Ejaculate Number | Mean IGF-1 (ng/mL) | Mean Testosterone (pg/mL) | Fertility History |
|---|---|---|---|---|---|---|---|
| E1 | 12 | 3 | 2690 | 2 | 437.6 | 1645.0 | No |
| E2 | 20 | 3.5 | 3490 | 4 | 300.4 | 138.3 | No |
| E3 | 27 | 3.5 | 4055 | 2 | 271.4 | 1914.6 | No |
| E4 | 47 | 3 | 3460 | 4 | 215.0 | Not detected | No |
| E5 | 27 | 3 | 3000 | 2 | 283.9 | 576.5 | No |
| E6 | 46 | 3 | 3210 | 2 | 541.6 | 2996.8 | Yes |
| E7 | 40 | 3 | 4070 | 1 | 323.7 | 652.77 | No |
| Parameters | Mean | Serum IGF-1 (r) | p Value | Serum Testosterone (r) | p Value |
|---|---|---|---|---|---|
| Semen volume (mL) | 32.1 ± 13.3 | 0.12 | 0.66 | 0.16 | 0.60 |
| Semen pH | 7.3 ± 0.7 | 0.27 | 0.29 | 0.05 | 0.87 |
| Sperm concentration (×106/mL) | 1209.2 ± 403.4 | 0.07 | 0.80 | 0.24 | 0.41 |
| % Motility | 32.6 ± 22.8 | 0.09 | 0.74 | 0.18 | 0.55 |
| Progressive motility (1–5) | 3.2 ± 1.4 | −0.15 | 0.58 | 0.03 | 0.92 |
| % Membrane integrity | 58.6 ± 21.0 | 0.07 | 0.80 | 0.16 | 0.61 |
| % Intact functional membrane | 26.5 ± 16.8 | 0.09 | 0.74 | 0.11 | 0.73 |
| % Normal DNA integrity | 70.0 ± 23.5 | 0.13 | 0.61 | 0.02 | 0.94 |
| % Intact acrosome | 44.5 ± 18.3 | 0.53 | 0.028 * | 0.22 | 0.47 |
| Serum testosterone (pg/mL) | 1190.1 ± 1081.8 | 0.73 | 0.004 ** | - | - |
| Serum IGF-1 (ng/mL) | 32 6.3 ± 114.6 | - | - | 0.73 | 0.004 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Suwimonteerabutr, J.; Angkawanish, T.; Chatdarong, K. Serum Insulin-like Growth Factor-1 Is a Biomarker of Testosterone Production and Intact Acrosome in Asian Elephants (Elephas maximus). Animals 2022, 12, 1570. https://doi.org/10.3390/ani12121570
Yang Y, Suwimonteerabutr J, Angkawanish T, Chatdarong K. Serum Insulin-like Growth Factor-1 Is a Biomarker of Testosterone Production and Intact Acrosome in Asian Elephants (Elephas maximus). Animals. 2022; 12(12):1570. https://doi.org/10.3390/ani12121570
Chicago/Turabian StyleYang, Yuqing, Junpen Suwimonteerabutr, Taweepoke Angkawanish, and Kaywalee Chatdarong. 2022. "Serum Insulin-like Growth Factor-1 Is a Biomarker of Testosterone Production and Intact Acrosome in Asian Elephants (Elephas maximus)" Animals 12, no. 12: 1570. https://doi.org/10.3390/ani12121570
APA StyleYang, Y., Suwimonteerabutr, J., Angkawanish, T., & Chatdarong, K. (2022). Serum Insulin-like Growth Factor-1 Is a Biomarker of Testosterone Production and Intact Acrosome in Asian Elephants (Elephas maximus). Animals, 12(12), 1570. https://doi.org/10.3390/ani12121570







