Impact of Super-High Density Olive Orchard Management System on Soil Free-Living and Plant-Parasitic Nematodes in Central and South Italy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Sites
2.2. Soil Sampling Design
2.3. Soil Chemical Analysis
2.4. Soil Nematode Community Analysis
2.5. Statistical Analysis
3. Results
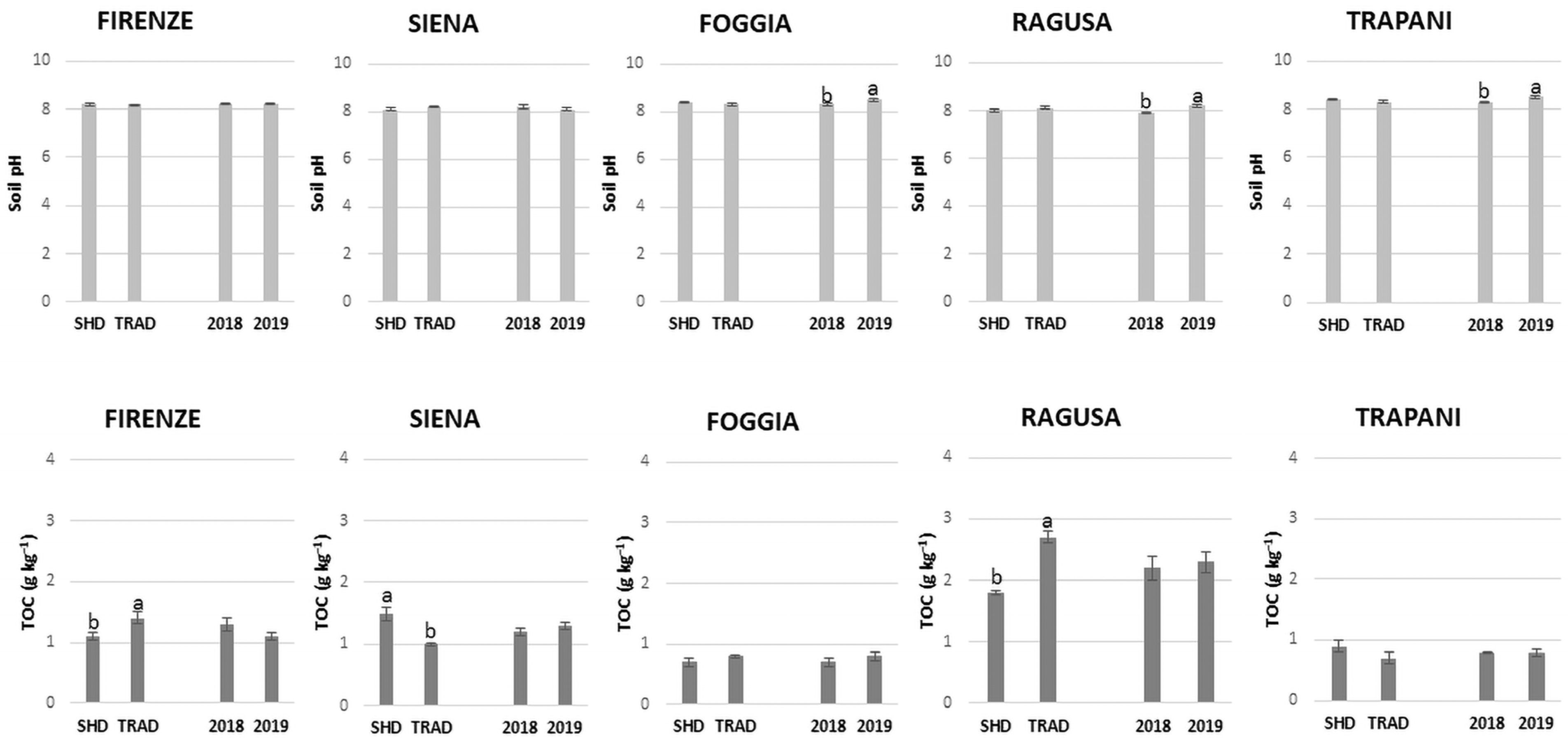
3.1. Soil Chemical Properties
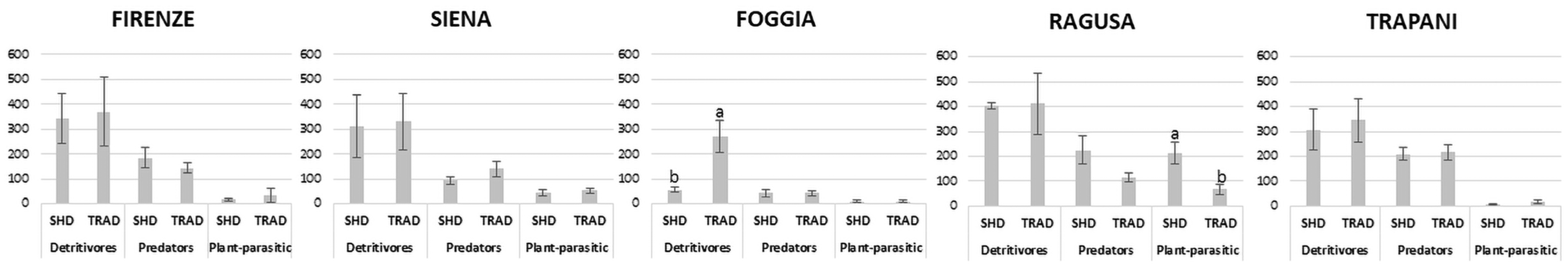
3.2. Soil Nematode Structure
3.3. Soil Nematode Indicators
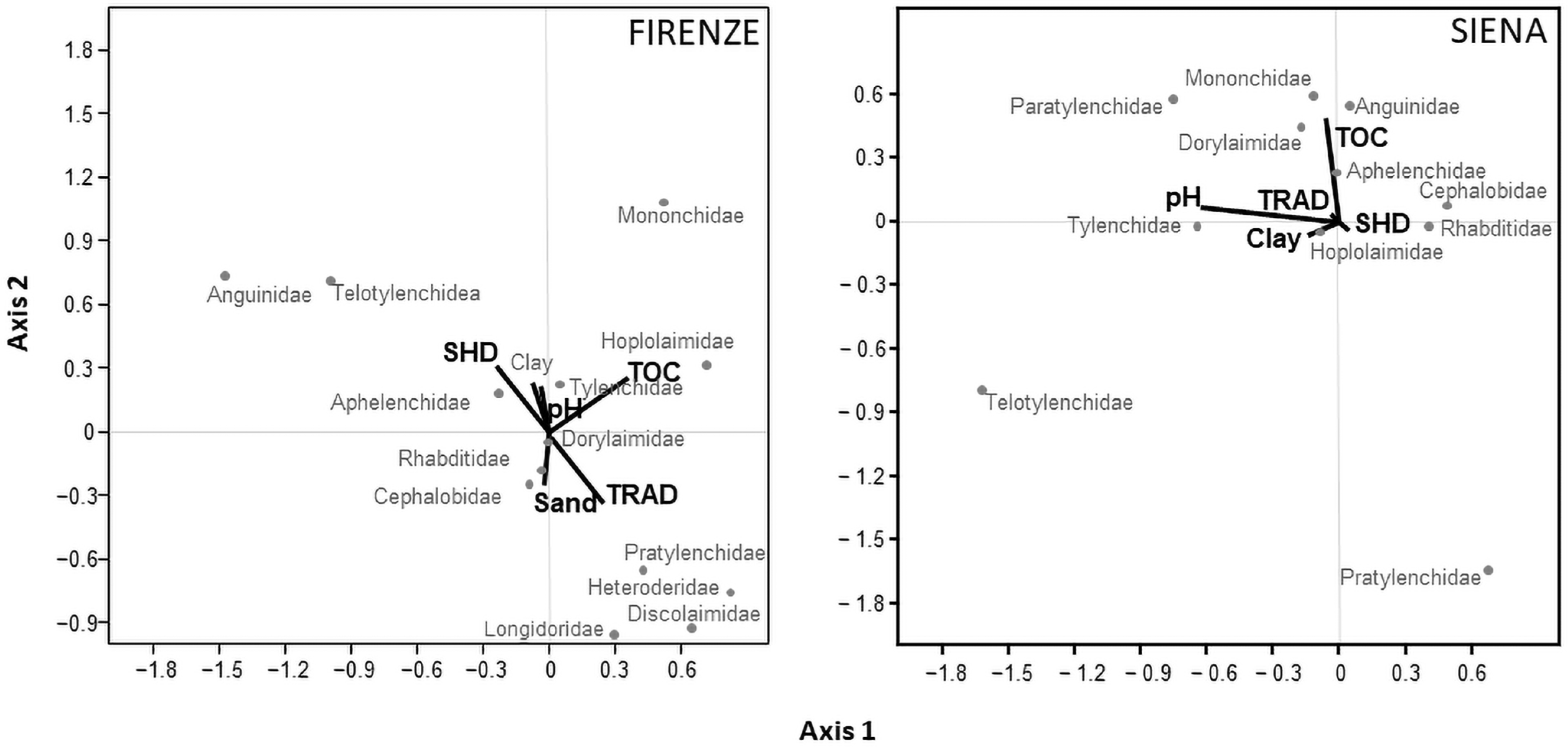
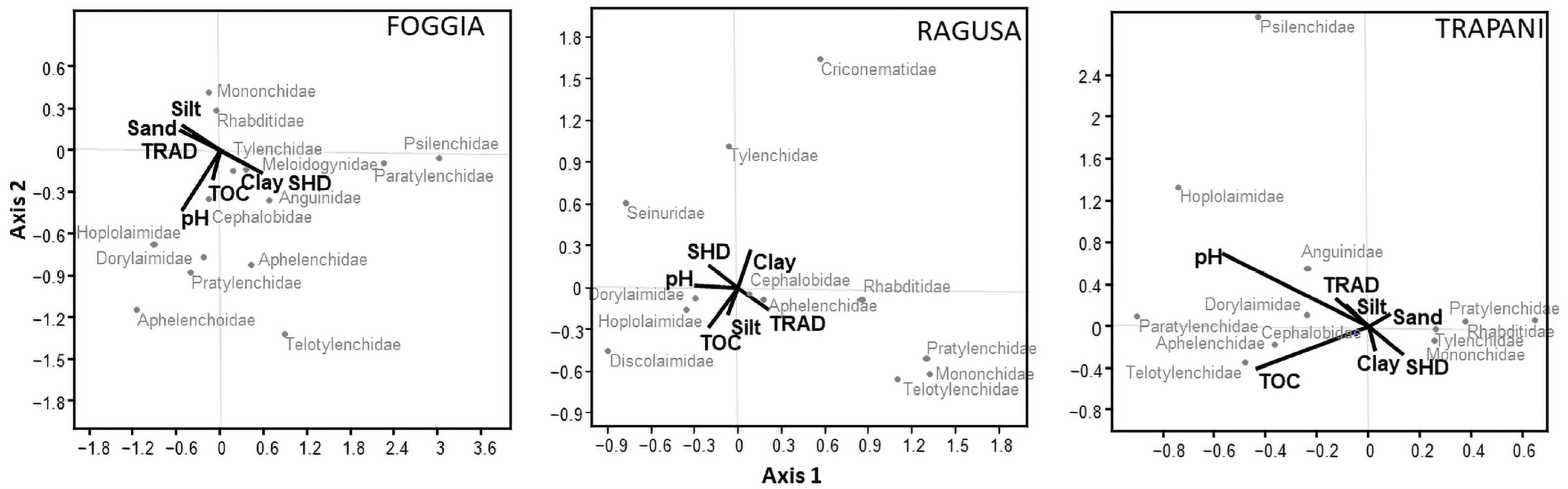
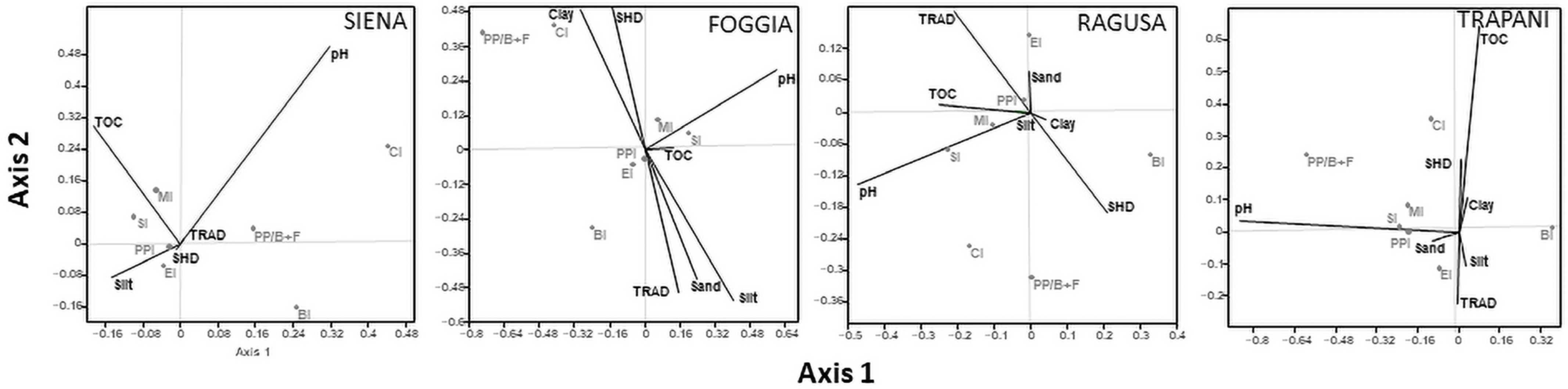
3.4. Relationship among Environmental Variables and Nematode Community Structure
4. Discussion
4.1. Effect of the SHD Olive Orchard Systems on Soil Fertility
4.2. Effect of SHD Olive Orchard Systems on Soil Nematode Community Structure
4.3. Soil and Management Factors Influencing Soil Nematode Structure
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pupo D’Andrea, M.R. L’olio d’oliva. In Annuario Dell’Agricoltura Italiana 2019; CREA Consiglio per la ricerca in agricoltura e l.analisi dell.economia agraria: Roma, Italy, 2021; Volume LXXIII, p. 560. ISBN 9788833851044. [Google Scholar]
- Russo, C.; Cappelletti, G.M.; Nicoletti, G.M.; Di Noia, A.E.; Michalopoulos, G. Comparison of European olive production systems. Sustainability 2016, 8, 825. [Google Scholar] [CrossRef] [Green Version]
- Castillo, P.; Nico, A.I.; Navas-Cortés, J.A.; Landa, B.B.; Jiménez-Díaz, R.M.; Vovlas, N. Plant-parasitic nematodes attacking olive trees and their management. Plant Dis. 2010, 94, 148–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koenning, S.; Overstreet, C.; Noling, J.; Donald, P.; Becker, J.; Fortnum, B. Survey of crop losses in response to phytoparasitic nematodes in the United States for 1994. J. Nematol. 1999, 31, 587–618. [Google Scholar]
- Scognamiglio, M.; Talame, F.P.; Giandomenico, N. Indagine sui nematodi viventi nella rizosfera dell’olivo (1 contributo). Boll. Lab. Ent. Agr. 1968, 26, 205–226. [Google Scholar]
- Lamberti, F.; Baines, R. Infectivity of the three biotypes of the citrus nematode (Tylenchulus semipenetrans) on two varieties of olive. Plant. Dis. Rep. 1970, 54, 717–718. [Google Scholar]
- Scognamiglio, M.; Talame, F.P.; d’Errico, F.P. Indagine sui nematodi viventi nella rizosfera dell’olivo (2 contributo). Boll. Lab. Ent. Agr. 1971, 29, 43–59. [Google Scholar]
- Inserra, R.N.; Vovlas, N. The biology of Rotylenchus macrodoratus. J. Nematol. 1980, 12, 97–102. [Google Scholar]
- Vovlas, N.; Inserra, R.N. Notes on Helicotylenchus dihystera on Olive in Sicily. Inform. Fitopatol. 1981, 31, 23–25. [Google Scholar]
- Lamberti, F.; Sasanelli, N.; D’Addabbo, T.; Ambrico, A.; Ciccarese, F.; Schiavona, D. Relationship between plant-parasitic nematodes and Verticillium dahlia on olive [Olea europaea L.]. Nemato. Mediterr. 2001, 29, 3–9. [Google Scholar]
- Ragozzino, A.; d’Errico, G. Interactions between nematodes and fungi: A concise review. Redia 2011, 44, 123–125. [Google Scholar]
- Ali, N.; Chapuis, E.; Tavoillot, J.; Mateille, T. Plant-parasitic nematodes associated with olive tree (Olea europaea L.) with a focus on the Mediterranean Basin: A review. Comptes Rendus Biol. 2014, 337, 423–442. [Google Scholar] [CrossRef] [PubMed]
- Archidona-Yuste, A.; Wiegand, T.; Castillo, P.; Navas-Cortés, J.A. Spatial structure and soil properties shape the local community structure of plant-parasitic nematodes in cultivated olive trees in southern Spain. Agric. Ecosyst. Environ. 2020, 287, 106688. [Google Scholar] [CrossRef]
- Ferris, H.; Bongers, T.; De Goede, R. A framework for soil food web diagnostics: Extension of the nematode faunal analysis concept. Appl. Soil Ecol. 2001, 18, 13–29. [Google Scholar] [CrossRef]
- Irshad, U.; Villenave, C.; Brauman, A.; Plassard, C. Grazing by nematodes on rhizosphere bacteria enhances nitrate and phosphorus availability to Pinus pinaster seedlings. Soil Biol. Biochem. 2011, 43, 2121–2126. [Google Scholar] [CrossRef]
- Salome, C.; Coll, P.; Lardo, E.; Villenave, C.; Blanchart, E.; Hinsinger, P.; Marsden, C.; Le Cadre, E. Relevance of use-invariant soil properties to assess soil quality of vulnerable ecosystems: The case of Mediterranean vineyards. Ecol. Indic. 2014, 43, 83–93. [Google Scholar] [CrossRef]
- Landi, S.; Papini, R.; d’Errico, G.; Brandi, G.; Rocchini, A.; Roversi, P.F.; Bazzoffi, P.; Mocali, S. Effect of different set-aside management systems on soil nematode community and soil fertility in North, Central and South Italy. Agric. Ecosyst. Environ. 2018, 261, 251–260. [Google Scholar] [CrossRef]
- Schreck, E.; Gontier, L.; Dumat, C.; Geret, F. Ecological and physiological effects of soil management practices on earthworm communities on French vineyards. Eur. J. Soil Biol. 2012, 52, 8–15. [Google Scholar] [CrossRef] [Green Version]
- Connor, D.J.; Gómez-del-Campo, M.; Rousseaux, M.C.; Searles, P.S. Structure, management and productivity od hedgerow olive orchards: A review. Sci. Hortic. 2014, 169, 71–93. [Google Scholar] [CrossRef]
- Palomares-Rius, J.E.; Castillo, P.; Montes-Borrego, M.; Müller, H.; Landa, B.B. Nematode community populations in the rhizosphere of cultivated olive differs according to the plant genotype. Soil Biol. Biochem. 2012, 45, 168–171. [Google Scholar] [CrossRef]
- Sánchez-Moreno, S.; Nicola, N.L.; Ferris, H.; Zalom, F.G. Effects of agricultural management on nematode-mite assemblages: Soil food web indices as predictors of mite community composition. Appl. Soil Ecol. 2009, 41, 107–117. [Google Scholar] [CrossRef]
- Palomares-Rius, J.E.; Castillo, P.; Montes-Borrego, M.; Navas-Cortés, J.A.; Landa, B.B. Soil properties and olive cultivar determine the structure and diversity of plant-parasitic nematode communities infesting olive orchards soils in Southern Spain. PLoS ONE 2015, 10, e0116890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Archidona-Yuste, A.; Cantalapiedra-Navarrete, C.; Liébanas, G.; Rapoport, H.F.; Castillo, P.; Palomares-Rius, J.E. Diversity of root-knot nematodes of the genus Meloidogyne Göeldi, 1892 (Nematoda: Meloidogynidae) associated with olive plants and environmental cues regarding their distribution in southern Spain. PLoS ONE 2018, 13, e0198236. [Google Scholar] [CrossRef] [PubMed]
- Warde, D.A. Impacts of disturbance on detritus food webs in agro-ecosystems of contrasting tillage and weed management practices. Adv. Ecol. Res. 1995, 26, 379–387. [Google Scholar]
- Indorante, S.J.; Hammer, R.D.; Koening, P.G.; Follmer, L.R. Particle-size analysis by modified pipette procedure. Soil Sci. Soc. Am. J. 1990, 54, 560–563. [Google Scholar] [CrossRef]
- Yeomans, J.C.; Bremner, J.M. A rapid and precise method for routine determination of organic carbon in soil. Commun. Soil Sci. Plant Anal. 1988, 19, 1467–1476. [Google Scholar] [CrossRef]
- Lu, Q.; Liu, T.; Wang, N.; Dou, Z.; Wang, K.; Zuo, Y. A review of soil nematodes as biological indicators for the assessment of soil health. Front. Sci. Eng. 2020, 7, 275–281. [Google Scholar] [CrossRef] [Green Version]
- Bongers, T. The maturity index: An ecological measure of environmental disturbance based on nematode species composition. Oecologia 1990, 83, 14–19. [Google Scholar] [CrossRef]
- Ferris, H. Form and function: Metabolic footprints of nematodes in the soil food web. Eur. J. Soil Biol. 2010, 46, 97–104. [Google Scholar] [CrossRef]
- Ferris, H.; Tuomisto, H. Unearthing the role of biological diversity in soil health. Soil Biol. Biochem. 2015, 85, 101–109. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.; Ryan, P. PAST—Paleontological statistics, ver. 1. 89. Paleontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Clark, K.R. Non-parametric multivariate analysis of changes in community structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Loveland, P.; Legendre, L. Is there a critical level of organic production practices on soil quality indicators? J. Environ. Qual. 1998, 28, 1601–1609. [Google Scholar]
- Francaviglia, R.; Ledda, L.; Farina, R. Organic carbon and ecosystem services in agricultural soils of the Mediterranean Basin. Sustain. Agric. Rev. 2018, 28, 183–210. [Google Scholar]
- Vignozzi, N.; Agnelli, A.E.; Brandi, G.; Gagnarli, E.; Goggioli, D.; Lagomarsino, A.; Pellegrini, S.; Simoncini, S.; Simoni, S.; Valboa, G.; et al. Soil ecosystem functions in a high-density olive orchard management by different soil conservation practices. Appl. Soil Ecol. 2019, 134, 64–76. [Google Scholar] [CrossRef]
- Landi, S.; d’Errico, G.; Simoncini, S.; L’Abate, G.; Priori, S. Nematodes communities as indicators of soil quality in vineyard system: A case of study in degraded areas. J. Environ. Qual. 2018, 31, 41–46. [Google Scholar] [CrossRef]
- Cadet, P.; Berry, S.; Spaul, V. Mapping of interactions between soil factors and nematodes. Eur. J. Doil Biol. 2004, 40, 77–86. [Google Scholar] [CrossRef]
- Barker, K.R.; Koenning, S.R. Developing sustainable systems for nematode management. Annu. Rev. Phytopathol. 1998, 36, 165–205. [Google Scholar] [CrossRef] [Green Version]
- Landi, S.; Pennacchio, F.; Papini, R.; d’Errico, G.; Torrini, G.; Strangi, A.; Barabaschi, D.; Roversi, P.F. Evaluation of sheep grazing effects on nematode community, insect infestation and soil fertility in sweet chestnut orchards: A case of study. Redia 2016, 99, 117–126. [Google Scholar] [CrossRef]
- Lal, R. Soil organic matter and water retention. Agron. J. 2020, 112, 3265–3277. [Google Scholar] [CrossRef]
- Widmer, T.; Mitkowski, N.; Abawi, G. Soil organic matter and management of plant-parasitic nematodes. J. Nematol. 2002, 34, 289. [Google Scholar]
- Ali, N.; Tavoillot, J.; Besnard, G.; Khadari, B.; Dmowska, E.; Winiszewska, G.; Fottati-Gaschignard, O.; Ater, M.; Hamza, M.A.; El Mousadik, A.; et al. How anthropogenic changes may affect soil-borne parasite diversity? Plant-parasitic nematode communities associated with olive trees in Morocco as a case study. BMC Ecol. 2017, 17, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landi, S.; d’Errico, G.; Binazzi, F.; Di Salvatore, U.; Gardin, L.; Marchi, M.; Mazza, G.; Roversi, P.F.; Simoncini, S.; Torrini, G.; et al. The short-term impact of different silvicultural thinnings on soil nematode and microarthropod biodiversity in artificial black pine stands. Forests 2020, 11, 1212. [Google Scholar] [CrossRef]
| Sites | Regions | Geographical Position | Climate Parameters | Soil Texture USDA | Olive Orchard Features | ||||
|---|---|---|---|---|---|---|---|---|---|
| Coordinates | Altitude a.s.l (m) | KöppenClimate Types | Mean Air Temperature (°C) | Mean Annual Precipitation (mm) | Olive Tree Cultivar | Soil Management | |||
| Firenze (FIR) | Tuscany | 43.800183 N; 11.403579 E | 260 | Cfa | 14.7 | 940 | Clay | SHD: Leccio del Corno, Tosca, Diana TRAD: Frantoio, Leccino, Moraiolo | Conventional tillage Mineral fertilization |
| Siena (SIE) | Tuscany | 43.275739 N; 11.603974 E | 280 | Csa | 14.5 | 880 | Silty Clay Loam | SHD: Frantoio, Leccino, Moraiolo, Pendolino, Leccio del Corno, Correggiolo, Maurino selezione Vittoria TRAD: Frantoio, Leccino, Moraiolo, Pendolino | Green cover Organic fertilization |
| Foggia (FOG) | Apulia | 41.5653940 N; 15.736466 E | 582 | Cfa | 14.5 | 715 | Clay Loam | SHD: Arbequina TRAD: Ogliarola Garganica | Conventional tillage Mineral fertilization |
| Ragusa (RAG) | Sicily | 37.0786790 N; 14.665837 E | 380 | Csa | 17.0 | 541 | Clay Loam | SHD: Tonda Iblea, Nocellara dell’Etna, Biancolilla, Moresca, Nocellara del Belice, Arbequina, Picual TRAD: Tonda Iblea | Conventional tillage Organic fertilization |
| Trapani(TRP) | Sicily | 37.972381 N; 12.683364 E | 220 | Csa | 17.6 | 680 | Clay Loam | SHD: Arbequina TRAD: Biancolilla, Cerasuola, Nocellara del Belice | Green manure Organic fertilization |
| Management | Year | Significant Effects | |||||
|---|---|---|---|---|---|---|---|
| SHD | TRAD | 2018 | 2019 | M * | Y ** | M + Y | |
| Firenze | |||||||
| MI | 2.0 ± 0.1 | 2.1 ± 0.1 | 1.9 ± 0.1 b | 2.3 ± 0.1 a | 0.48 | 0.02 | 0.47 |
| PPI | 2.5 ± 0.1 | 2.5 ± 0.1 | 2.4 ± 0.1 | 2.5 ± 0.1 | 0.94 | 0.27 | 0.25 |
| BI | 37.7 ± 7.3 | 39.1 ± 10.6 | 55.2 ± 9.7 a | 21.5 ± 4.6 b | 0.90 | 0.006 | 0.35 |
| EI | 79.1 ± 2.1 | 77.8 ± 2.5 | 82.2 ± 2.2 a | 74.7 ± 1.9 b | 0.66 | 0.02 | 1.00 |
| SI | 62.9 ± 6.4 | 69.8 ± 3.5 | 59.9 ± 6.4 b | 72.8 ± 2.6 a | 0.34 | 0.08 | 0.83 |
| CI | 12.3 ± 2.5 | 10.4 ± 2.5 | 13.3 ± 3.0 | 9.4 ± 1.5 | 0.59 | 0.28 | 0.44 |
| Pp/(B+F) | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.81 | 0.02 | 0.20 |
| Siena | |||||||
| MI | 2.0 ± 0.2 | 2.2 ± 0.12 | 1.8 ± 0.1 b | 2.5±0.1 a | 0.33 | 0.0006 | 0.30 |
| PPI | 2.7 ± 0.1 | 2.7 ± 0.1 | 2.6 ± 0.1 b | 2.8±0.04 a | 0.99 | 0.007 | 0.63 |
| BI | 19.7 ± 4.7 | 22.4 ± 5.6 | 32.3 ± 5.3 a | 9.8±1.4 b | 0.65 | 0.0008 | 0.54 |
| EI | 79.9 ± 3.6 | 79.0 ± 4.2 | 82.0 ± 5.0 | 76.9 ± 2.0 | 0.88 | 0.38 | 0.76 |
| SI | 67.9 ± 5.5 | 72.1 ± 5.2 | 59.8 ± 5.6 b | 80.1 ± 2.8 a | 0.52 | 0.005 | 0.62 |
| CI | 10.4 ± 3.9 | 10.6 ± 4.2 | 15.0 ± 5.4 | 6.0 ± 0.8 | 0.97 | 0.13 | 0.81 |
| Pp/(B+F) | 0.9 ± 0.2 | 1.7 ± 0.5 | 0.9 ± 0.2 | 1.7 ± 0.5 | 0.11 | 0.13 | 0.007 |
| Foggia | |||||||
| MI | 1.8 ± 0.1 | 1.7 ± 0.1 | 1.5 ± 0.06 b | 2.1 ± 0.1 a | 0.13 | 0.00001 | 0.01 |
| PPI | 2.0 ± 0.3 | 2.1 ± 0.2 | 2.2 ± 0.1 | 1.8 ± 0.3 | 0.79 | 0.3 | 0.30 |
| BI | 8.7 ± 1.1 b | 14.5 ± 2.6 a | 13.9 ± 2.6 a | 9.3 ± 1.5 b | 0.01 | 0.04 | 0.0005 |
| EI | 81.6 ± 2.4 | 87.8 ± 2.4 | 87.6 ± 2.1 | 81.8 ± 2.7 | 0.06 | 0.08 | 0.16 |
| SI | 54.4 ± 6.0 | 57.3 ± 6.3 | 40.3 ± 2.9 b | 71.4 ± 4.9 a | 0.62 | 0.00001 | 0.64 |
| CI | 12.5 ± 2.1 a | 5.6 ± 2.2 b | 9.6 ± 2.2 | 8.5 ± 2.5 | 0.02 | 0.71 | 0.04 |
| Pp/(B+F) | 0.2 ± 0.07 b | 0.06 ± 0.03 a | 0.2 ± 0.1 | 0.1 ± 0.04 | 0.05 | 0.57 | 0.10 |
| Ragusa | |||||||
| MI | 2.2 ± 0.2 | 2.1 ± 0.1 | 1.9 ± 0.1 b | 2.4 ± 0.1 a | 0.72 | 0.006 | 0.60 |
| PPI | 2.9 ± 0.06 | 2.8 ± 0.1 | 2.7 ± 0.1 b | 3.0 ± 0.03 a | 0.50 | 0.02 | 0.98 |
| BI | 61.7 ± 18.5 | 37.7 ± 7.0 | 79.6 ± 15.7 a | 19.9 ± 2.9 b | 0.12 | 0.0007 | 0.12 |
| EI | 72.7 ± 4.9 | 78.7 ± 3.8 | 74.6 ± 5.3 | 76.9 ± 3.4 | 0.37 | 0.73 | 0.93 |
| SI | 60.0 ± 7.5 | 66.1 ± 7.0 | 46.8 ± 6.7 b | 79.3 ± 3.8 a | 0.45 | 0.0006 | 0.84 |
| CI | 9.4 ± 4.4 | 6.9 ± 1.7 | 10.4 ± 4.3 | 6.0 ± 1.7 | 0.59 | 0.36 | 0.18 |
| Pp/(B+F) | 1.9 ± 0.4 a | 1.0 ± 0.4 b | 1.0 ± 0.3 b | 2.0 ± 0.4 a | 0.05 | 0.05 | 0.06 |
| Trapani | |||||||
| MI | 2.2 ± 0.1 | 2.2 ± 0.1 | 2.1±0.1 b | 2.3 ± 0.1 a | 0.72 | 0.03 | 0.84 |
| PPI | 2.1 ± 0.04 | 2.3 ± 0.1 | 2.0 ± 0.01 b | 2.4 ± 0.1 a | 0.06 | 0.00001 | 0.05 |
| BI | 59.9 ± 8.6 | 63.3 ± 11.2 | 85.3 ± 5.9 a | 37.8 ± 7.9 b | 0.74 | 0.0001 | 0.50 |
| EI | 64.9 ± 3.7 | 69.5 ± 3.9 | 65.7 ± 5.0 | 68.7 ± 2.2 | 0.42 | 0.61 | 0.85 |
| SI | 63.3 ± 3.0 | 64.7 ± 2.6 | 58.0 ± 1.9 b | 70.0 ± 2.4 a | 0.68 | 0.01 | 0.78 |
| CI | 16.2 ± 2.3 | 13.5 ± 2.8 | 13.5 ± 3.0 | 16.2 ± 2.0 | 0.48 | 0.47 | 0.43 |
| Pp/(B+F) | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.2 ± 0.1 | 0.71 | 0.54 | 0.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Landi, S.; d’Errico, G.; Papini, R.; Cutino, I.; Simoncini, S.; Rocchini, A.; Brandi, G.; Rizzo, R.; Gugliuzza, G.; Germinara, G.S.; et al. Impact of Super-High Density Olive Orchard Management System on Soil Free-Living and Plant-Parasitic Nematodes in Central and South Italy. Animals 2022, 12, 1551. https://doi.org/10.3390/ani12121551
Landi S, d’Errico G, Papini R, Cutino I, Simoncini S, Rocchini A, Brandi G, Rizzo R, Gugliuzza G, Germinara GS, et al. Impact of Super-High Density Olive Orchard Management System on Soil Free-Living and Plant-Parasitic Nematodes in Central and South Italy. Animals. 2022; 12(12):1551. https://doi.org/10.3390/ani12121551
Chicago/Turabian StyleLandi, Silvia, Giada d’Errico, Rossella Papini, Ilaria Cutino, Stefania Simoncini, Andrea Rocchini, Giorgio Brandi, Roberto Rizzo, Giovanni Gugliuzza, Giacinto Salvatore Germinara, and et al. 2022. "Impact of Super-High Density Olive Orchard Management System on Soil Free-Living and Plant-Parasitic Nematodes in Central and South Italy" Animals 12, no. 12: 1551. https://doi.org/10.3390/ani12121551
APA StyleLandi, S., d’Errico, G., Papini, R., Cutino, I., Simoncini, S., Rocchini, A., Brandi, G., Rizzo, R., Gugliuzza, G., Germinara, G. S., Nucifora, S., Mazzeo, G., & Roversi, P. F. (2022). Impact of Super-High Density Olive Orchard Management System on Soil Free-Living and Plant-Parasitic Nematodes in Central and South Italy. Animals, 12(12), 1551. https://doi.org/10.3390/ani12121551









