Temporal Changes of Fish Diversity and Driver Factors in a National Nature Reserve, China
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Study Area
2.2. Samplings Methods
2.3. Data Analysis
3. Results
3.1. Species Composition of Fish
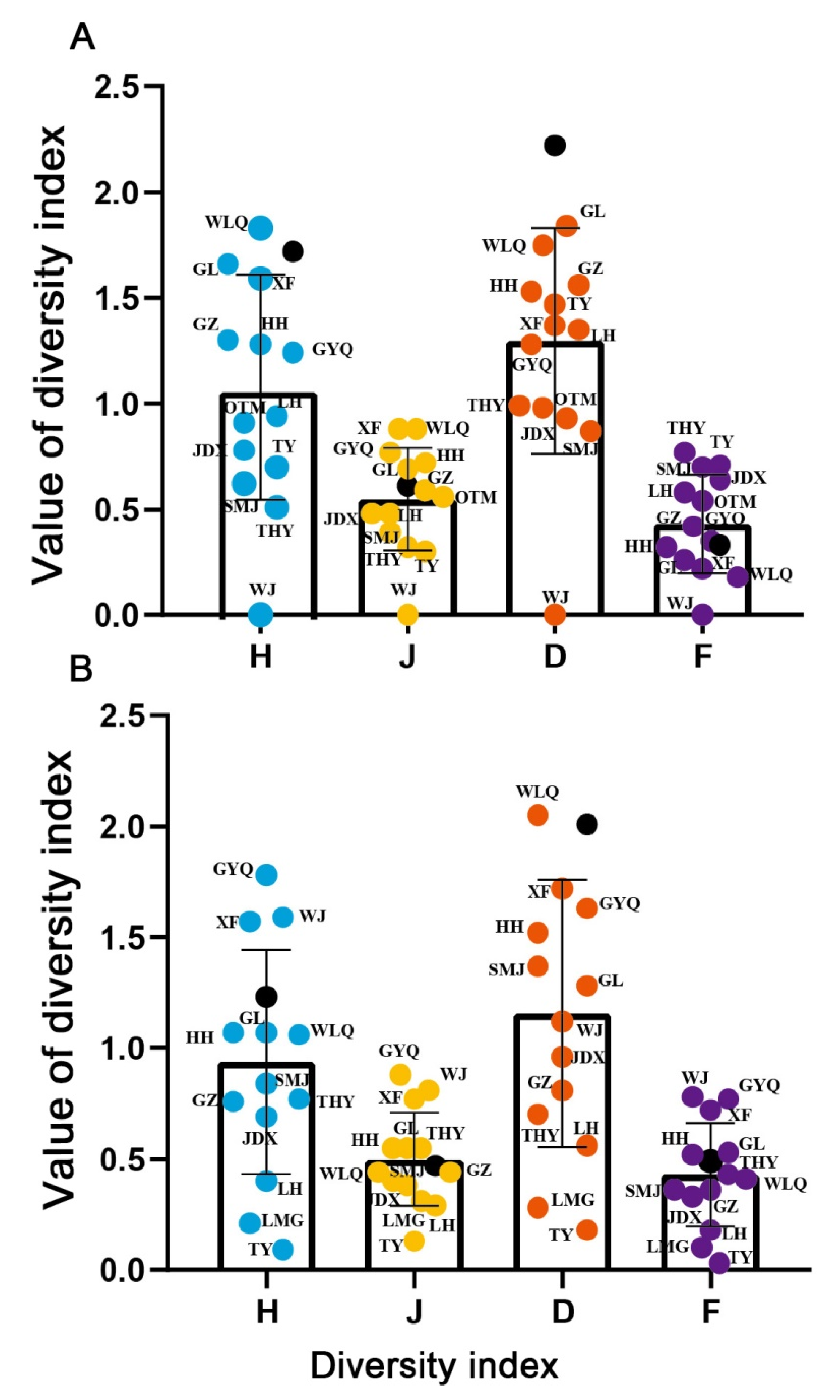
3.2. Diversity of Fish
3.3. Community Structure of Fish
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bruton, M.N. Have fishes had their chips? The dilemma of threatened fishes. Environ. Biol. Fish. 1995, 43, 1–27. [Google Scholar] [CrossRef]
- Duncan, J.R.; Lockwood, J.L. Extinction in a field of bullets: A search for causes in the decline of the world’s freshwater fishes. Biol. Conserv. 2001, 102, 97–105. [Google Scholar] [CrossRef]
- Arthington, A.H.; Dulvy, N.K.; Gladstone, W.; Winfield, I.J. Fish conservation in freshwater and marine realms: Status, threats and management. Aquat. Conserv. 2016, 26, 838–857. [Google Scholar] [CrossRef] [Green Version]
- Oberdorff, T.; Pont, D.; Hugueny, B.; Porcher, J. Development and validation of a fish-based index for the assessment of ‘river health’ in France. Freshw. Biol. 2002, 47, 1720–1734. [Google Scholar] [CrossRef]
- Li, T.; Huang, X.; Jiang, X.; Wang, X. Assessment of ecosystem health of the Yellow River with fish index of biotic integrity. Hydrobiologia 2018, 814, 31–43. [Google Scholar] [CrossRef]
- Flecker, A. Biodiversity Conservation in Running WatersIdentifying the major factors that threaten destruction of riverine species and ecosystems. BioScience 1993, 43, 32–43. [Google Scholar]
- Fu, C.; Wu, J.; Chen, J.; Wu, Q.; Lei, G. Freshwater fish biodiversity in the Yangtze River basin of China: Patterns, threats and conservation. Biodivers. Conserv. 2003, 12, 1649–1685. [Google Scholar] [CrossRef]
- Liu, X.; Qin, J.; Xu, Y.; Ouyang, S.; Wu, X. Biodiversity decline of fish assemblages after the impoundment of the Three Gorges Dam in the Yangtze River Basin, China. Rev. Fish Biol. Fish. 2019, 29, 177–195. [Google Scholar] [CrossRef]
- Bongaarts, J. Summary for policymakers of the global assessment report on biodiversity and ecosystem services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services. Popul. Dev. Rev. 2019, 45, 680–681. [Google Scholar] [CrossRef] [Green Version]
- Agostinho, A.A.; Bonecker, C.C.; Gomes, L.C. Effects of water quantity on connectivity: The case of the upper Paraná River floodplain. Ecohydrol. Hydrobiol. 2009, 9, 99–113. [Google Scholar] [CrossRef]
- Lévêque, C.; Oberdorff, T.; Paugy, D.; Stiassny, M.; Tedesco, P. Global diversity of fish (Pisces) in freshwater. Hydrobiologia 2007, 595, 545–567. [Google Scholar] [CrossRef]
- Cheng, S.T.; Herricks, E.E.; Tsai, W.P.; Chang, F.J. Assessing the natural and anthropogenic influences on basin-wide fish species richness. Sci. Total Environ. 2016, 572, 825–836. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Zheng, P.; Cao, L.; Pan, B. Effects of long-term floodplain disconnection on multiple facets of lake fish biodiversity: Decline of alpha diversity leads to a regional differentiation through time. Sci. Total Environ. 2020, 763, 144177. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, L.; Tang, W.; Heino, J.; Jiang, X. Effects of dam construction and fish invasion on the species, functional and phylogenetic diversity of fish assemblages in the Yellow River Basin. J. Environ. Manag. 2021, 293, 112863. [Google Scholar] [CrossRef]
- Brosse, S.; Grenouillet, G.; Gevrey, M.; Khazraie, K.; Tudesque, L. Small-scale gold mining erodes fish assemblage structure in small neotropical streams. Biodivers. Conserv. 2011, 20, 1013–1026. [Google Scholar] [CrossRef]
- Schmera, D.; Heino, J.; Podani, J.; Ers, T.; Dolédec, S. Functional diversity: A review of methodology and current knowledge in freshwater macroinvertebrate research. Hydrobiologia 2017, 787, 27–44. [Google Scholar] [CrossRef] [Green Version]
- Webb, C.O.; Ackerly, D.; Mcpeek, M.A.; Donoghue, M.J. Phylogenies and community ecology. Annu. Rev. Ecol. Syst. 2002, 33, 475–505. [Google Scholar] [CrossRef] [Green Version]
- Graham, C.H.; Fine, P. Phylogenetic beta diversity: Linking ecological and evolutionary processes across space in time. Ecol. Lett. 2010, 11, 1265–1277. [Google Scholar] [CrossRef]
- Winter, M.; Schweiger, O.; Klotz, S.; Nentwig, W.; Andriopoulos, P.; Arianoutsou, M.; Basnou, C.; Delipetrou, P.; Didziulis, V.; Hejda, M. Plant extinctions and introductions lead to phylogenetic and taxonomic homogenization of the European flora. Proc. Natl. Acad Sci. USA 2009, 106, 21721–21725. [Google Scholar] [CrossRef] [Green Version]
- Strecker, A.L.; Olden, J.D.; Whittier, J.B.; Paukert, C.P. Defining conservation priorities for freshwater fishes according to taxonomic, functional, and phylogenetic diversity. Ecol. Appl. 2011, 21, 3002–3013. [Google Scholar] [CrossRef] [Green Version]
- Pool, T.K.; Grenouillet, G.; Villéger, S.; Ricciardi, A. Species contribute differently to the taxonomic, functional, and phylogenetic alpha and beta diversity of freshwater fish communities. Divers. Distrib. 2015, 20, 1235–1244. [Google Scholar] [CrossRef]
- Su, G.; Logez, M.; Xu, J.; Tao, S.; Brosse, S. Human impacts on global freshwater fish biodiversity. Science 2021, 371, 835–838. [Google Scholar] [CrossRef] [PubMed]
- Xun, G.; Quanan, W. The function and significance of reservation in the conserving biological diversity. Guihaia 1993, 4, 359–366. [Google Scholar]
- Liu, X.; Wang, L. Biodiversity Study and Research in Mount Lu Nature Reserve, Jiangxi Province; Science Press: Beijing, China, 2010. [Google Scholar]
- Jin, T.; Zhao, M.; Wu, J.; Li, J.; Li, H. Distribution characteristics and influencing factors of glomalin-related soil protein in the rhizosphere of common tree species in evergreen broad-leaved forest of Lushan. Chin. J. Ecol. 2021, 40, 11. [Google Scholar]
- Zhang, L.; Li, B.; Yang, W. Forest vegetation classification and its variation in Lushan Nature Reserve using Proba-V vegetation products. Prog. Geogr. 2021, 40, 10. [Google Scholar] [CrossRef]
- Huang, L.; Wu, Z.; Hu, M.; Li, Q.; Zong, D.S.; Wan, Z.Q.; Zhao, W.Q. Species Diversity of Fish of Lushan Nature Reserve in Jiangxi Province. J. Nanchang Univ. Nat. Sci. 2008, 32, 161–164. [Google Scholar]
- Chen, Y.Y. Fauna Sinica, Osteichthyes; Science Press: Beijing, China, 1998. [Google Scholar]
- Chu, X.; Zheng, B.; Dai, D. Fauna Sinica: Osteichthyes Siluriformes; Science Press: Beijing, China, 1999. [Google Scholar]
- Yue, P. Fauna Sinica: Osteichthyes Cypriniformes III; Science Press: Beijing, China, 2000. [Google Scholar]
- Froese, R.; Pauly, D.; FishBase. World Wide Web Electronic Publication. 2014. Available online: http://www.fishbase.org (accessed on 5 May 2022).
- Chao, A.; Ma, K.H.; Hsieh, T.C. iNEXT (iNterpolation and EXTrapolation) Online: Software for Interpolation and Extrapolation of Species Diversity. Program and User’s Guide. 2016. Available online: http://chao.stat.nthu.edu.tw/wordpress/software_download/ (accessed on 1 March 2022).
- Pianka, E.R. Ecology of the Agamid Lizard Amphibolurus isolepis in Western Australia. Copeia 1971, 3, 527–536. [Google Scholar] [CrossRef]
- Magurran, A.E. Ecological Diversity and Its Measurement; Princeton University Press: London, UK, 1988. [Google Scholar]
- Peet, R.K. The Measurement of Species Diversity. Annu. Rev. Ecol. Syst. 1974, 5, 285–307. [Google Scholar] [CrossRef]
- Baselga, A. Partitioning the turnover and nestedness components of beta diversity. Glob. Ecol. Biogeogr. 2010, 19, 134–143. [Google Scholar] [CrossRef]
- Carvalho, J.C.; Cardoso, P.; Gomes, P. Determining the relative roles of species replacement and species richness differences in generating beta-diversity patterns. Glob. Ecol. Biogeogr. 2012, 21, 760–771. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2009. [Google Scholar]
- Baselga, A.; Orme, C. betapart: An R package for the study of beta diversity. Methods Ecol. Evol. 2012, 3, 808–812. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P. Vegan: Community Ecology Package. R Package Version 2.3-2. 2011. Available online: https://cran.r-project.org (accessed on 15 February 2022).
- Clarke, K.R.; Gorley, R.N. PRIMER v6: User Manual/Tutorial; PRIMER-E: Plymouth, UK, 2006. [Google Scholar]
- Clarke, K.R. Non-parametric multivariate analyses of changes in community structure. Austral. Ecol. 2010, 18, 117–143. [Google Scholar] [CrossRef]
- Webb, C.O. picante: R tools for integrating phylogenies and ecology. Bioinformatics 2010, 26, 1463–1464. [Google Scholar]
- Barbarossa, V.; Schmitt, R.; Huijbregts, M.; Zarfl, C.; Schipper, A.M. Impacts of current and future large dams on the geographic range connectivity of freshwater fish worldwide. Proc. Natl. Acad. Sci. USA 2020, 117, 3648–3655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Díaz, G.; Górski, K.; Heino, J.; Arriagada, P. The Longest Fragment Drives Fish Beta Diversity in Fragmented River Networks: Implications for River Management And Conservation. Sci. Total Environ. 2021, 766, 144323. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.; He, D.; Perrett, L.; Wang, H.; Hu, W.; Deng, W.; Wu, Y. Fish and fisheries in the Upper Mekong: Current assessment of the fish community, threats and conservation. Rev. Fish Biol. Fish. 2009, 19, 465. [Google Scholar] [CrossRef]
- Liu, X.; Qin, J.; Xu, Y.; Zhou, M.; Wu, X.; Ouyang, S. Biodiversity pattern of fish assemblages in Poyang Lake Basin: Threat and conservation. Ecol. Evol. 2019, 9, 11672–11683. [Google Scholar] [CrossRef]
- Zhang, H.; Jarić, I.; Roberts, D.L.; He, Y.; Du, H.; Wu, J.; Wang, C.; Wei, Q. Extinction of one of the world’s largest freshwater fishes: Lessons for conserving the endangered Yangtze fauna. Sci. Total Environ. 2020, 710, 136242. [Google Scholar] [CrossRef]
- Bunn, S.E.; Arthington, A.H. Basic Principles and Ecological Consequences of Altered Flow Regimes for Aquatic Biodiversity. Environ. Manag. 2002, 30, 492–507. [Google Scholar] [CrossRef] [Green Version]
- Reidy, L.C.; Christer, N.; James, R.; Ng, R.Y. Implications of dam obstruction for global freshwater fish diversity. BioScience 2012, 62, 539–548. [Google Scholar]
- Lansac-Tôha, F.M.; Heino, J.; Quirino, B.A.; Moresco, G.A.; Peláez, O.; Meira, B.R.; Rodrigues, L.C.; Jati, S.; Lansac-Tôha, F.A.; Velho, L.F.M. Differently dispersing organism groups show contrasting beta diversity patterns in a dammed subtropical river basin. Sci. Total Environ. 2019, 691, 1271–1281. [Google Scholar] [CrossRef] [PubMed]
- Bonecker, C.C.; Simões, N.; Minte-Vera, C.V.; Lansac-Tôha, F.A.; Velho, L.F.M.; Agostinho, A. Temporal changes in zooplankton species diversity in response to environmental changes in an alluvial valley. Limnol. Ecol. Manag. Inl. Waters 2013, 43, 114–121. [Google Scholar] [CrossRef]
- Winemiller, K.O.; Mcintyre, P.B.; Castello, L.; Fluet-Chouinard, E.; Saenz, L. Balancing Hydropower and Biodiversity in the Amazon, Congo, and Mekong. Science 2016, 351, 128–129. [Google Scholar] [CrossRef] [Green Version]
- Vitule, J.R.S.; Skora, F. Homogenization of freshwater fish faunas after the elimination of a natural barrier by a dam in Neotropics. Divers. Distrib. 2012, 18, 111–120. [Google Scholar] [CrossRef]
- Petesse, M.L.; Petrere, M. Tendency towards homogenization in fish assemblages in the cascade reservoir system of the Tietê river basin, Brazil. Ecol. Eng. 2012, 48, 109–116. [Google Scholar] [CrossRef]
- Daga, V.S.; Skóra, F.; Padial, A.A.; Abilhoa, V.; Gubiani, É.A.; Vitule, J. Homogenization dynamics of the fish assemblages in Neotropical reservoirs: Comparing the roles of introduced species and their vectors. Hydrobiologia 2015, 746, 327–347. [Google Scholar] [CrossRef]
- Couto, T.B.; Olden, J.D. Global proliferation of small hydropower plants—Science and policy. Front. Ecol. Environ. 2018, 6, 91–100. [Google Scholar] [CrossRef]
- Grill, G.; Lehner, B.; Thiem, M.; Geenen, B. Mapping the world’s free-flowing rivers. Nature 2019, 569, 215–221. [Google Scholar] [CrossRef]
- Reid, A.J.; Carlson, A.K.; Creed, I.F.; Eliason, E.J.; Cooke, S.J. Emerging threats and persistent conservation challenges for freshwater biodiversity. Biol. Rev. 2018, 94, 849–873. [Google Scholar] [CrossRef] [Green Version]
- Tickner, D.; Opperman, J.J.; Abell, R.; Acreman, M.; Young, L. Bending the Curve of Global Freshwater Biodiversity Loss—An Emergency Recovery Plan. BioScience 2020, 70, 330–342. [Google Scholar] [CrossRef]
- Gthe, E.; Pedersen, A.B.; Larsen, P.W.; Graeber, D.; Kristensen, E.A.; Friberg, N. Environmental and spatial controls of taxonomic versus trait composition of stream biota. Freshw. Biol. 2017, 62, 397–413. [Google Scholar] [CrossRef]
- Heino, J.; Tolonen, K.T. Ecological drivers of multiple facets of beta diversity in a lentic macroinvertebrate metacommunity. Limnol. Oceanogr. 2017, 62, 2431–2444. [Google Scholar] [CrossRef]
- Mouchet, M.A.; Burns, M.; Garcia, A.M.; Vieira, J.P.; Mouillot, D. Invariant scaling relationship between functional dissimilarity and co-occurrence in fish assemblages of the Patos Lagoon estuary (Brazil): Environmental filtering consistently overshadows competitive exclusion. Oikos 2013, 122, 247–257. [Google Scholar] [CrossRef]
- Sá-Oliveira, J.C.; Hawes, J.E.; Isaac-Nahum, V.J.; Peres, C.A. Upstream and downstream responses of fish assemblages to an eastern Amazonian hydroelectric dam. Freshw. Biol. 2015, 60, 2037–2050. [Google Scholar] [CrossRef]
- Guo, Q.; Liu, X.; Ao, X.; Qin, J.; Wu, X.; Ouyang, S. Fish diversity in the middle and lower reaches of the Ganjiang River of China: Threats and conservation. PLoS ONE 2018, 13, e205116. [Google Scholar] [CrossRef] [Green Version]
- Methratta, E.T.; Link, J.S. Ontogenetic variation in habitat association for four groundfish species in the Gulf of Maine—Georges Bank region. Mar. Ecol. Prog. Ser. 2007, 338, 169–181. [Google Scholar] [CrossRef]
- Jones, J.B.; Campana, S.E. Stable Oxygen Isotope Reconstruction of Ambient Temperature during the Collapse of a Cod (Gadus Morhua) Fishery. Ecol. Appl. 2009, 19, 1500–1514. [Google Scholar] [CrossRef] [Green Version]
- Bruno, D.; Belmar, O.; Maire, A.; Morel, A.; Dumont, B.; Datry, T. Structural and functional responses of invertebrate communities to climate change and flow regulation in alpine catchments. Glob. Chang. Biol. 2019, 25, 1612–1628. [Google Scholar] [CrossRef] [Green Version]
- Olden, J.D.; Kennard, M.K.; Leprieur, F.; Tedesco, P.A.; Winemiller, K.O.; García-Berthou, E. Conservation biogeography of freshwater fishes: Past progress and future directions. Divers. Distrib. 2010, 16, 496–513. [Google Scholar] [CrossRef]
- Soininen, J.; Heino, J.; Wang, J. A meta-analysis of nestedness and turnover components of beta diversity across organisms and ecosystems. Glob. Ecol. Biogeogr. 2018, 27, 96–109. [Google Scholar] [CrossRef] [Green Version]



| River | Codes | 2008 | 2021 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| MPD Randomization | MPD Observed | SD | NRI | MPD Randomization | MPD Observed | SD | NRI | ||
| Taohuayuan | THY | 0.061 | 0.060 | 0.008 | 0.173 | 0.122 | 0.109 | 0.019 | 0.658 |
| Guizong | GZ | 0.159 | 0.149 | 0.017 | 0.587 | 0.102 | 0.101 | 0.014 | 0.076 |
| Xiufeng | XF | 0.210 | 0.189 | 0.021 | 0.980 | 0.200 | 0.196 | 0.020 | 0.230 |
| Guanyinqiao | GYQ | 0.172 | 0.155 | 0.020 | 0.819 | 0.220 | 0.223 | 0.021 | −0.145 |
| Gaolong | GL | 0.201 | 0.176 | 0.018 | 1.334 | 0.137 | 0.123 | 0.017 | 0.812 |
| Tongyuan | TY | 0.080 | 0.063 | 0.010 | 1.694 | 0.010 | 0.011 | 0.002 | −0.554 |
| Jiandaoxia | JDX | 0.098 | 0.096 | 0.012 | 0.180 | 0.091 | 0.098 | 0.013 | −0.536 |
| Lianhua | LH | 0.115 | 0.114 | 0.014 | 0.115 | 0.051 | 0.056 | 0.008 | −0.676 |
| Weijia | WJ | NA | NA | NA | NA | 0.215 | 0.210 | 0.022 | 0.211 |
| Shimenjian | SMJ | 0.083 | 0.076 | 0.011 | 0.615 | 0.101 | 0.106 | 0.013 | −0.426 |
| Wuliqiao | WLQ | 0.222 | 0.207 | 0.019 | 0.837 | 0.116 | 0.112 | 0.013 | 0.285 |
| Haihui | HH | 0.178 | 0.164 | 0.022 | 0.624 | 0.146 | 0.119 | 0.019 | 1.372 |
| Shanshang | OTM | 0.125 | 0.138 | 0.017 | −0.823 | — | — | — | — |
| Longmengou | LMG | — | — | — | — | 0.029 | 0.031 | 0.006 | −0.337 |
| Total | 0.188 | 0.171 | 0.017 | 1.002 | 0.131 | 0.124 | 0.014 | 0.510 | |
| 2008 | ||||||
|---|---|---|---|---|---|---|
| Species | A group–B group (average dissimilarity = 100) | A group–C group (average dissimilarity = 100) | B group–C group (average dissimilarity = 78.12) | |||
| Average dissimilarity | Contribution rate (%) | Average dissimilarity | Contribution rate (%) | Average dissimilarity | Contribution rate (%) | |
| Zacco platypus | 46.51 | 46.51 | 45.16 | 45.16 | 33.45 | 42.82 |
| Rhynchocypris oxycephala | 11.41 | 11.41 | — | — | 9.08 | 11.63 |
| Acrossocheilus parallens | 4.74 | 4.74 | 14.16 | 14.16 | 5.11 | 6.54 |
| Rhinogobius giurinus | 15.56 | 15.56 | — | — | 8.50 | 10.89 |
| Rhodeus ocellatus | — | — | 14.51 | 14.51 | 5.57 | 7.13 |
| Misgurnus anguillicaudatus | 8.31 | 8.31 | 3.77 | 3.77 | 4.68 | 5.99 |
| Hemibarbus maculatus | 3.40 | 3.40 | 7.14 | 7.14 | 3.59 | 4.59 |
| Opsariichthys bidens | 3.26 | 3.26 | — | — | 2.95 | 3.78 |
| Liobagrus anguillicauda | — | — | 5.46 | 5.46 | — | — |
| 2021 | ||||||
| Species | A group–B group (average dissimilarity = 87.2) | A group–C group (average dissimilarity = 86.74) | B group–C group (average dissimilarity = 49.94) | |||
| Average dissimilarity | Contribution rate (%) | Average dissimilarity | Contribution rate (%) | Average dissimilarity | Contribution rate (%) | |
| Zacco platypus | 34.57 | 39.64 | 47.43 | 54.68 | 26.73 | 53.53 |
| Opsariichthys bidens | 14.63 | 16.77 | 11.70 | 13.49 | 2.35 | 4.71 |
| Acrossocheilus parallens | 10.45 | 11.99 | — | — | 7.99 | 16.01 |
| Rhodeus ocellatus | 7.94 | 9.10 | 8.41 | 9.70 | — | — |
| Rhinogobius giurinus | 7.20 | 8.26 | 4.76 | 5.49 | 2.78 | 5.57 |
| Misgurnus anguillicaudatus | 5.33 | 6.11 | 6.79 | 7.83 | 1.84 | 3.69 |
| Liobagrus anguillicauda | — | — | — | — | 1.48 | 2.96 |
| Monopterus albus | — | — | — | — | 1.23 | 2.47 |
| Odontobutis sinensis | — | — | — | — | 1.19 | 2.39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, D.; Wan, Z.; Jia, C.; Zhu, Y.; Zou, Q.; Ye, F.; Feng, Y.; Zhang, M.; Wu, X.; Liu, X.; et al. Temporal Changes of Fish Diversity and Driver Factors in a National Nature Reserve, China. Animals 2022, 12, 1544. https://doi.org/10.3390/ani12121544
Luo D, Wan Z, Jia C, Zhu Y, Zou Q, Ye F, Feng Y, Zhang M, Wu X, Liu X, et al. Temporal Changes of Fish Diversity and Driver Factors in a National Nature Reserve, China. Animals. 2022; 12(12):1544. https://doi.org/10.3390/ani12121544
Chicago/Turabian StyleLuo, Dongmei, Ziwei Wan, Chenchen Jia, Yiqiu Zhu, Qin Zou, Fangfei Ye, Yan Feng, Mi Zhang, Xiaoping Wu, Xiongjun Liu, and et al. 2022. "Temporal Changes of Fish Diversity and Driver Factors in a National Nature Reserve, China" Animals 12, no. 12: 1544. https://doi.org/10.3390/ani12121544
APA StyleLuo, D., Wan, Z., Jia, C., Zhu, Y., Zou, Q., Ye, F., Feng, Y., Zhang, M., Wu, X., Liu, X., & Ouyang, S. (2022). Temporal Changes of Fish Diversity and Driver Factors in a National Nature Reserve, China. Animals, 12(12), 1544. https://doi.org/10.3390/ani12121544





