The Hard Choice about Dry Pet Food: Comparison of Protein and Lipid Nutritional Qualities and Digestibility of Three Different Chicken-Based Formulations
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Chemicals
2.3. Determination of Moisture Content
2.4. Protein Solubilization
2.5. Determination of Soluble Proteins
2.6. Determination of Nitrogen Content
2.7. Amino Acid Profile
2.8. Determination of Crude Fat Content
2.9. Lipid Profile
2.10. In Vitro Digestibility
2.11. Statistical Analysis
3. Results
3.1. Protein Content
3.2. Amino Acid Profile
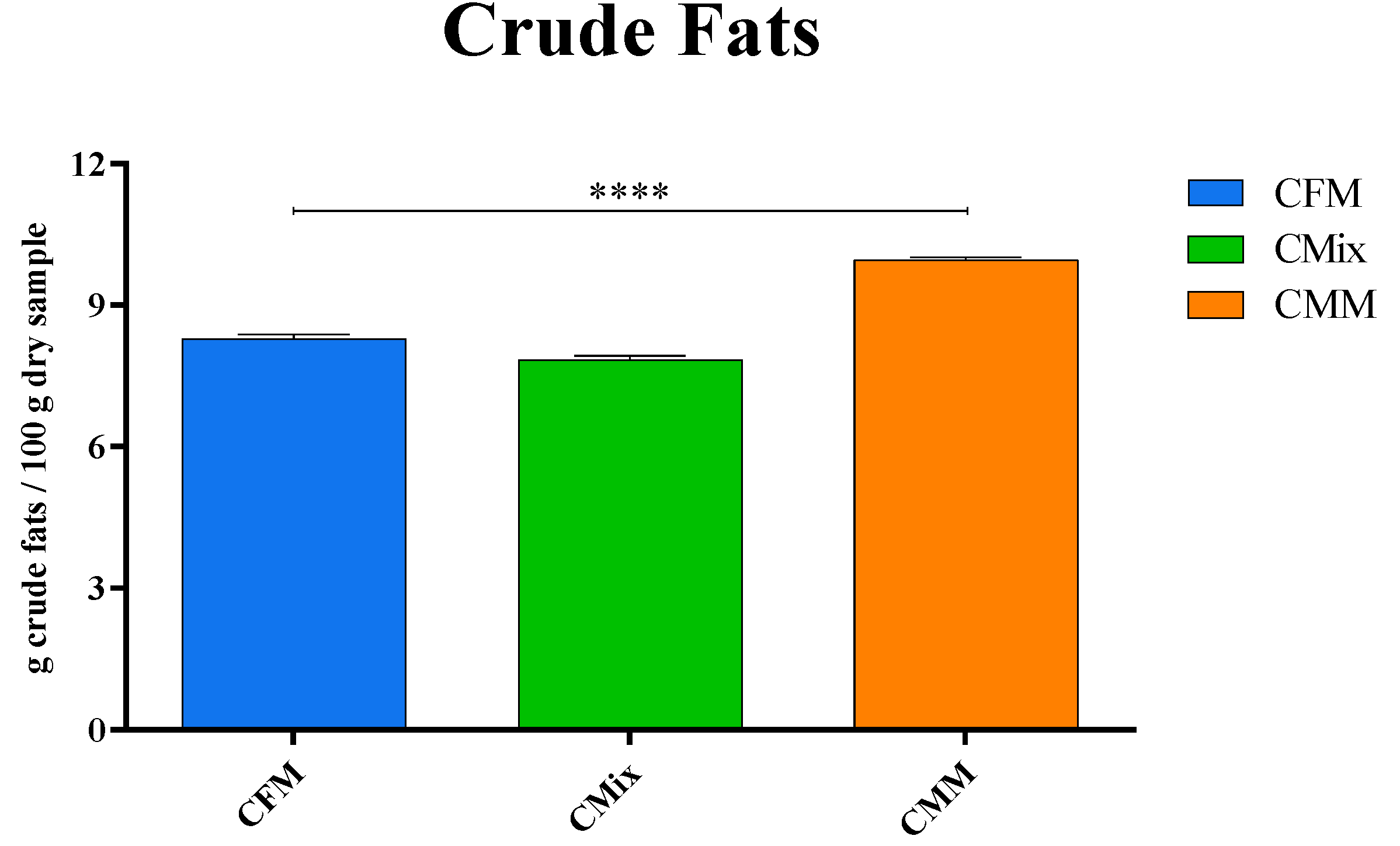
3.3. Determination of Crude Fat Content
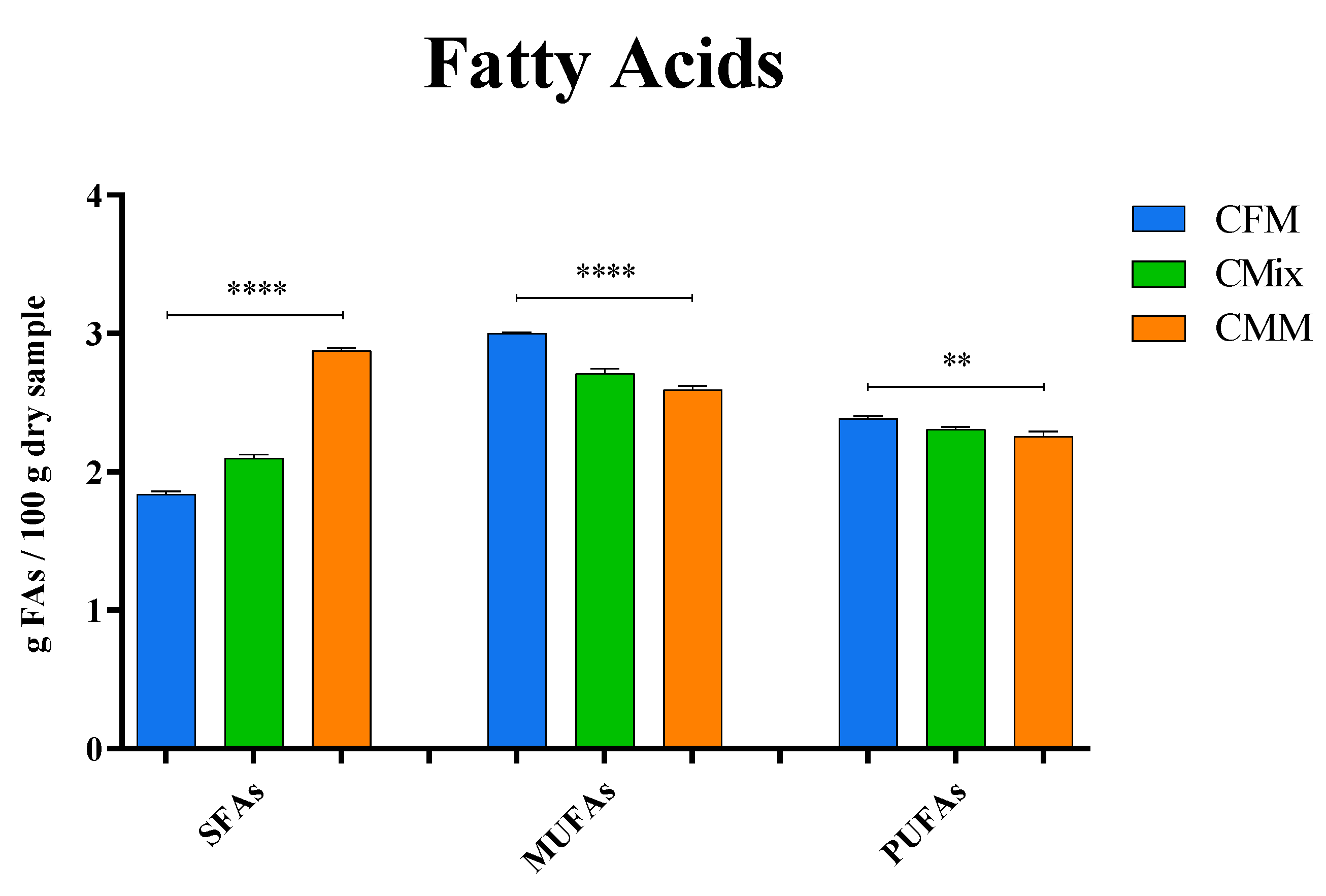
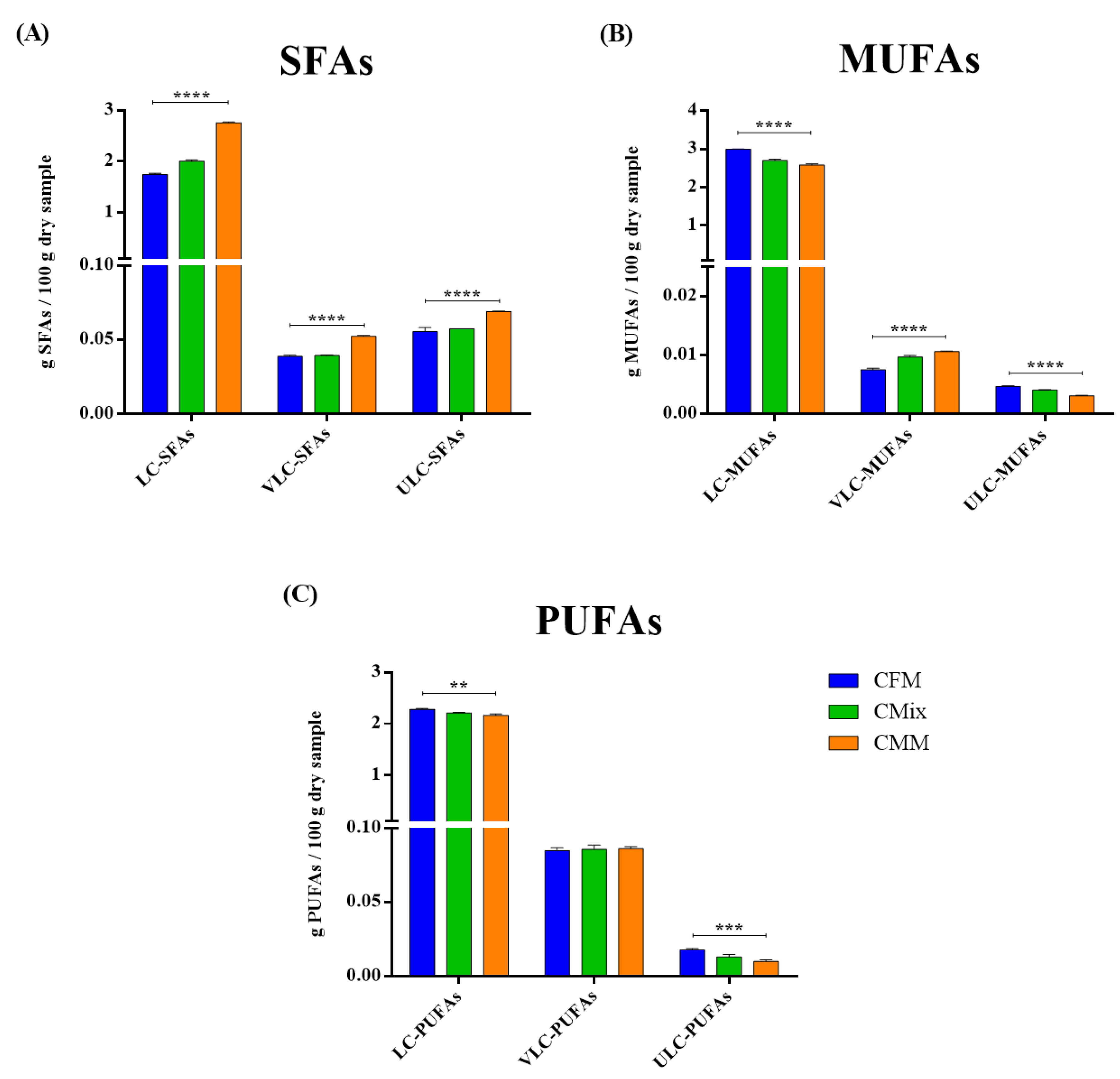
3.4. Lipid Profile
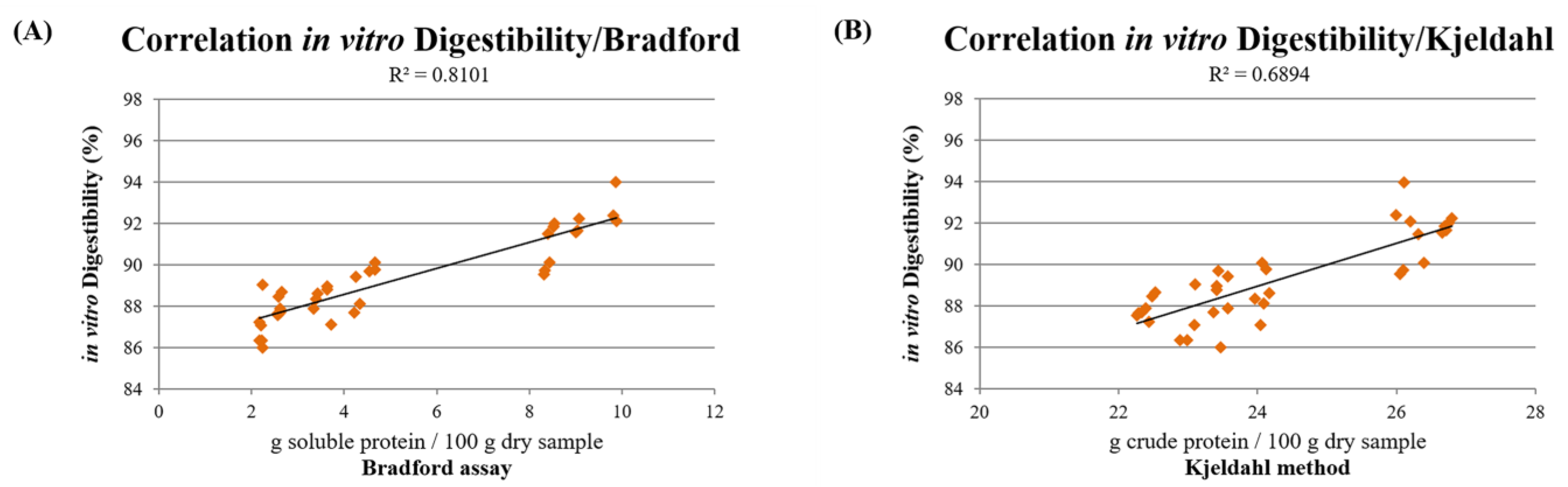
3.5. In Vitro Digestibility
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Aldrich, G. Rendered Products in Pet Food. Essent. Render. 2006, 159–178. [Google Scholar]
- Gibson, M.W.; Sajid, A. Pet Food Processing: Understanding Transformations in Starch during Extrusion and Baking. Cereal Foods World 2013, 58, 232–236. [Google Scholar] [CrossRef] [Green Version]
- Zicker, S.C. Evaluating Pet Foods: How Confident Are You When You Recommend a Commercial Pet Food? Top. Companion Anim. Med. 2008, 23, 121–126. [Google Scholar] [CrossRef]
- Remillard, R.L. Homemade Diets: Attributes, Pitfalls, and a Call for Action. Top. Companion Anim. Med. 2008, 23, 137–142. [Google Scholar] [CrossRef]
- Morelli, G.; Stefanutti, D.; Ricci, R. A Survey among Dog and Cat Owners on Pet Food Storage and Preservation in the Households. Animals 2021, 11, 273. [Google Scholar] [CrossRef]
- Di Donfrancesco, B.; Koppel, K.; Swaney-Stueve, M.; Chambers, E. Consumer Acceptance of Dry Dog Food Variations. Animals 2014, 4, 313–330. [Google Scholar] [CrossRef]
- Rombach, M.; Dean, D.L. It Keeps the Good Boy Healthy from Nose to Tail: Understanding Pet Food Attribute Preferences of US Consumers. Animals 2021, 11, 3301. [Google Scholar] [CrossRef]
- Vinassa, M.; Vergnano, D.; Valle, E.; Giribaldi, M.; Nery, J.; Prola, L.; Bergero, D.; Schiavone, A. Profiling Italian Cat and Dog Owners’ Perceptions of Pet Food Quality Traits. BMC Vet. Res. 2020, 16, 131. [Google Scholar] [CrossRef]
- Thompson, A. Ingredients: Where Pet Food Starts. Top. Companion Anim. Med. 2008, 23, 127–132. [Google Scholar] [CrossRef]
- Montegiove, N.; Pellegrino, R.M.; Emiliani, C.; Pellegrino, A.; Leonardi, L. An Alternative Approach to Evaluate the Quality of Protein-Based Raw Materials for Dry Pet Food. Animals 2021, 11, 458. [Google Scholar] [CrossRef]
- Montegiove, N.; Calzoni, E.; Cesaretti, A.; Alabed, H.; Pellegrino, R.M.; Emiliani, C.; Pellegrino, A.; Leonardi, L. Comprehensive Evaluation of Lipidic Content in Dry Pet Food Raw Materials: Comparison between Fresh Meats and Meat Meals. Sci. Bull. Ser. F Biotechnol. 2020, 24, 122–129. [Google Scholar]
- Montegiove, N.; Calzoni, E.; Cesaretti, A.; Alabed, H.; Pellegrino, R.M.; Emiliani, C.; Pellegrino, A.; Leonardi, L. Biogenic Amine Analysis in Fresh Meats and Meat Meals Used as Raw Materials for Dry Pet Food Production. Sci. Bull. Ser. F Biotechnol. 2020, 24, 33–42. [Google Scholar]
- Montegiove, N.; Calzoni, E.; Cesaretti, A.; Pellegrino, R.M.; Emiliani, C.; Pellegrino, A.; Leonardi, L. Soluble Protein Content Assessment in Dry Pet Food Raw Materials: Comparison between Fresh Meat and Meat Meal Formulations. Sci. Bull. Ser. F Biotechnol. 2021, 25, 54–60. [Google Scholar]
- Hamper, B.A. Raw Meat-Based Diets: Current Evidence Regarding Benefits and Risks. A Healthy Begin. 2014, 99–107. [Google Scholar]
- Laflamme, D.; Izquierdo, O.; Eirmann, L.; Binder, S. Myths and Misperceptions about Ingredients Used in Commercial Pet Foods. Vet. Clin. Small Anim. Pract. 2014, 44, 689–698. [Google Scholar] [CrossRef]
- Murray, S.M.; Patil, A.R.; Fahey, G.C., Jr.; Merchen, N.R.; Hughes, D.M. Raw and Rendered Animal By-Products as Ingredients in Dog Diets. J. Anim. Sci. 1997, 75, 2497–2505. [Google Scholar] [CrossRef]
- Kies, C. Bioavailability: A Factor in Protein Quality. J. Agric. Food Chem. 1981, 29, 435–440. [Google Scholar] [CrossRef]
- Yamka, R.M.; Jamikorn, U.; True, A.D.; Harmon, D.L. Evaluation of Low-Ash Poultry Meal as a Protein Source in Canine Foods. J. Anim. Sci. 2003, 81, 2279–2284. [Google Scholar] [CrossRef]
- Chanadang, S.; Koppel, K.; Aldrich, G. The Impact of Rendered Protein Meal Oxidation Level on Shelf-Life, Sensory Characteristics, and Acceptability in Extruded Pet Food. Animals 2016, 6, 44. [Google Scholar] [CrossRef] [Green Version]
- Lankhorst, C.; Tran, Q.D.; Havenaar, R.; Hendriks, W.H.; van der Poel, A.F.B. The Effect of Extrusion on the Nutritional Value of Canine Diets as Assessed by in Vitro Indicators. Anim. Feed Sci. Technol. 2007, 138, 285–297. [Google Scholar] [CrossRef]
- Rokey, G.J.; Plattner, B.; De Souza, E.M. Feed Extrusion Process Description. Rev. Bras. De Zootec. 2010, 39, 510–518. [Google Scholar] [CrossRef] [Green Version]
- Tran, Q.D.; Hendriks, W.H.; van der Poel, A.F. Effects of Extrusion Processing on Nutrients in Dry Pet Food. J. Sci. Food Agric. 2008, 88, 1487–1493. [Google Scholar] [CrossRef]
- Williams, P.A.; Hodgkinson, S.; Rutherfurd, S.; Hendriks, W. Lysine Content in Canine Diets Can Be Severely Heat Damaged. J. Nutr. 2006, 136, 1998S–2000S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, M.L.; Parsons, C.M.; Fahey, G.C., Jr.; Merchen, N.R.; Aldrich, C.G. Effects of Species Raw Material Source, Ash Content, and Processing Temperature on Amino Acid Digestibility of Animal by-Product Meals by Cecectomized Roosters and Ileally Cannulated Dogs. J. Anim. Sci. 1998, 76, 1112–1122. [Google Scholar] [CrossRef]
- Shirley, R.B.; Parsons, C.M. Effect of Ash Content on Protein Quality of Meat and Bone Meal. Poult. Sci. 2001, 80, 626–632. [Google Scholar] [CrossRef]
- Wang, X.; Parsons, C. Effect of Raw Material Source, Processing Systems, and Processing Temperatures on Amino Acid Digestibility of Meat and Bone Meals. Poult. Sci. 1998, 77, 834–841. [Google Scholar] [CrossRef]
- Haines, J.; Patel, M.; Knight, A.I.; Corley, D.; Gibson, G.; Schaaf, J.; Moulin, J.; Zuber, S. Thermal Inactivation of Feline Calicivirus in Pet Food Processing. Food Environ. Virol. 2015, 7, 374–380. [Google Scholar] [CrossRef]
- Okelo, P.O.; Joseph, S.W.; Wagner, D.D.; Wheaton, F.W.; Douglass, L.W.; Carr, L.E. Improvements in Reduction of Feed Contamination: An Alternative Monitor of Bacterial Killing During Feed Extrusion1. J. Appl. Poult. Res. 2008, 17, 219–228. [Google Scholar] [CrossRef]
- Leiva, A.; Molina, A.; Redondo-Solano, M.; Artavia, G.; Rojas-Bogantes, L.; Granados-Chinchilla, F. Pet Food Quality Assurance and Safety and Quality Assurance Survey within the Costa Rican Pet Food Industry. Animals 2019, 9, 980. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Gamlath, S.; Wakeling, L. Nutritional Aspects of Food Extrusion: A Review. Int. J. Food Sci. Technol. 2007, 42, 916–929. [Google Scholar] [CrossRef]
- Altan, A.; McCarthy, K.L.; Maskan, M. Effect of Extrusion Cooking on Functional Properties and in Vitro Starch Digestibility of Barley-Based Extrudates from Fruit and Vegetable By-Products. J. Food Sci. 2009, 74, E77–E86. [Google Scholar] [CrossRef] [PubMed]
- Inal, F.; Alatas, M.S.; Kahraman, O.; Inal, S.; Uludag, M.; Gurbuz, E.; Polat, E.S. Using of Pelleted and Extruded Foods in Dog Feeding. Kafkas Univ. Vet. Fak. Derg. 2018, 24, 131–136. [Google Scholar] [CrossRef]
- Koppel, K.; Monti, M.; Gibson, M.; Alavi, S.; Donfrancesco, B.D.; Carciofi, A.C. The Effects of Fiber Inclusion on Pet Food Sensory Characteristics and Palatability. Animals 2015, 5, 110–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, S.; Hsieh, F.; Huff, H.E. Effects of Lipids and Processing Conditions on Lipid Oxidation of Extruded Dry Pet Food during Storage1Contribution from the Missouri Agricultural Experiment Station, Journal Series No. 12590.1. Anim. Feed Sci. Technol. 1998, 71, 283–294. [Google Scholar] [CrossRef]
- Hullar, I.; Fekete, S.; Szöcs, Z. Effect of Extrusion on the Quality of Soybean-Based Catfood. J. Anim. Physiol. Anim. Nutr. 1998, 80, 201–206. [Google Scholar] [CrossRef]
- Alonso, R.; Aguirre, A.; Marzo, F. Effects of Extrusion and Traditional Processing Methods on Antinutrients and in Vitro Digestibility of Protein and Starch in Faba and Kidney Beans. Food Chem. 2000, 68, 159–165. [Google Scholar] [CrossRef]
- Hendriks, W.H.; Sritharan, K. Apparent Ileal and Fecal Digestibility of Dietary Protein Is Different in Dogs. J. Nutr. 2002, 132, 1692S–1694S. [Google Scholar] [CrossRef] [Green Version]
- Rikken, G.L.J.A.; Raupach, E. Enantioselective Magnetochiral Photochemistry. Nature 2000, 405, 932–935. [Google Scholar] [CrossRef]
- Obrosova, I.G.; Fathallah, L.; Stevens, M.J. Taurine Counteracts Oxidative Stress and Nerve Growth Factor Deficit in Early Experimental Diabetic Neuropathy. Exp. Neurol. 2001, 172, 211–219. [Google Scholar] [CrossRef]
- Zhang, M.; Izumi, I.; Kagamimori, S.; Sokejima, S.; Yamagami, T.; Liu, Z.; Qi, B. Role of Taurine Supplementation to Prevent Exercise-Induced Oxidative Stress in Healthy Young Men. Amino Acids 2004, 26, 203–207. [Google Scholar] [CrossRef]
- Pion, P.D.; Kittleson, M.D.; Rogers, Q.R.; Morris, J.G. Myocardial Failure in Cats Associated with Low Plasma Taurine: A Reversible Cardiomyopathy. Science 1987, 237, 764–768. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.G.; Rogers, Q.R.; Pacioretty, L.M. Taurine: An Essential Nutrient for Cats. J. Small Anim. Pract. 1990, 31, 502–509. [Google Scholar] [CrossRef]
- Hayes, K.C.; Trautwein, E.A. Taurine Deficiency Syndrome in Cats. Vet. Clin. N. Am. Small Anim. Pract. 1989, 19, 403–413. [Google Scholar] [CrossRef]
- Knopf, K.; Sturman, J.A.; Armstrong, M.; Hayes, K.C. Taurine: An Essential Nutrient for the Cat. J. Nutr. 1978, 108, 773–778. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Latimer, G.W., Jr. Official Methods of Analysis of AOAC International, 20th ed.; AOAC International: Rockville, MD, USA, 2016. [Google Scholar]
- Food Analysis. Food Science Text Series, 5th ed.; Nielsen, S., Ed.; Springer International Publishing: Cham, Switzerland, 2017; ISBN 978-3-319-45774-1. [Google Scholar]
- Bradstreet, R.B. Kjeldahl Method for Organic Nitrogen. Anal. Chem. 1954, 26, 185–187. [Google Scholar] [CrossRef]
- Jiang, B.; Tsao, R.; Li, Y.; Miao, M. Food Safety: Food Analysis Technologies/Techniques. In Encyclopedia of Agriculture and Food Systems; Van Alfen, N.K., Ed.; Academic Press: Oxford, UK, 2014; pp. 273–288. ISBN 978-0-08-093139-5. [Google Scholar]
- Otter, D.E. Standardised Methods for Amino Acid Analysis of Food. Br. J. Nutr. 2012, 108, S230–S237. [Google Scholar] [CrossRef] [Green Version]
- Adler, S.A.; Slizyte, R.; Honkapää, K.; Løes, A.-K. In Vitro Pepsin Digestibility and Amino Acid Composition in Soluble and Residual Fractions of Hydrolyzed Chicken Feathers. Poult. Sci. 2018, 97, 3343–3357. [Google Scholar] [CrossRef]
- Gehrke, C.W.; Wall, L.L., Sr.; Absheer, J.S.; Kaiser, F.E.; Zumwalt, R.W. Sample Preparation for Chromatography of Amino Acids: Acid Hydrolysis of Proteins. J. Assoc. Off. Anal. Chem. 1985, 68, 811–821. [Google Scholar] [CrossRef]
- Pellegrino, R.M.; Di Veroli, A.; Valeri, A.; Goracci, L.; Cruciani, G. LC/MS Lipid Profiling from Human Serum: A New Method for Global Lipid Extraction. Anal. Bioanal. Chem. 2014, 406, 7937–7948. [Google Scholar] [CrossRef]
- Sud, M.; Fahy, E.; Cotter, D.; Brown, A.; Dennis, E.A.; Glass, C.K.; Merrill, A.H., Jr.; Murphy, R.C.; Raetz, C.R.H.; Russell, D.W.; et al. LMSD: LIPID MAPS Structure Database. Nucleic Acids Res. 2007, 35, D527–D532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biagi, G.; Piva, A. In Vitro Effects of Some Organic Acids on Swine Cecal Microflora. Ital. J. Anim. Sci. 2007, 6, 361–374. [Google Scholar] [CrossRef] [Green Version]
- Baker, D.H.; Czarnecki-Maulden, G.L. Comparative Nutrition of Cats and Dogs. Annu. Rev. Nutr. 1991, 11, 239–263. [Google Scholar] [CrossRef] [PubMed]
- Legrand-Defretin, V. Differences between Cats and Dogs: A Nutritional View. Proc. Nutr. Soc. 1994, 53, 15–24. [Google Scholar] [CrossRef] [Green Version]
- Massey, K.A.; Blakeslee, C.H.; Pitkow, H.S. A Review of Physiological and Metabolic Effects of Essential Amino Acids. Amino Acids 1998, 14, 271–300. [Google Scholar] [CrossRef]
- Morris, J.G.; Rogers, Q.R. Assessment of the Nutritional Adequacy of Pet Foods through the Life Cycle. J. Nutr. 1994, 124, 2520S–2534S. [Google Scholar] [CrossRef]
- Gross, K.L.; Becvarova, I.; Debraekeleer, J. Feeding Nursing and Orphaned Kittens from Birth to Weaning. In Small Animal Clinical Nutrition, 5th ed.; Mark Morris Institute: Topeka, KS, USA, 2010; pp. 415–427. [Google Scholar]
- Nie, C.; He, T.; Zhang, W.; Zhang, G.; Ma, X. Branched Chain Amino Acids: Beyond Nutrition Metabolism. Int. J. Mol. Sci. 2018, 19, 954. [Google Scholar] [CrossRef] [Green Version]
- Shimomura, Y.; Murakami, T.; Nakai, N.; Nagasaki, M.; Harris, R.A. Exercise Promotes BCAA Catabolism: Effects of BCAA Supplementation on Skeletal Muscle during Exercise. J. Nutr. 2004, 134, 1583S–1587S. [Google Scholar] [CrossRef] [Green Version]
- Calders, P.; Matthys, D.; Derave, W.; Pannier, J.L. Effect of Branched-Chain Amino Acids (BCAA), Glucose, and Glucose plus BCAA on Endurance Performance in Rats. Med. Sci. Sports Exerc. 1999, 31, 583–587. [Google Scholar] [CrossRef]
- Rieu, I.; Sornet, C.; Bayle, G.; Prugnaud, J.; Pouyet, C.; Balage, M.; Papet, I.; Grizard, J.; Dardevet, D. Leucine-Supplemented Meal Feeding for Ten Days Beneficially Affects Postprandial Muscle Protein Synthesis in Old Rats. J. Nutr. 2003, 133, 1198–1205. [Google Scholar] [CrossRef] [Green Version]
- Proud, C.G. Regulation of Mammalian Translation Factors by Nutrients. Eur. J. Biochem. 2002, 269, 5338–5349. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.G. Protein in Dog Food. Can. Vet. J. 1989, 30, 528–531. [Google Scholar] [PubMed]
- Case, L.; Daristotle, L.; Hayek, M.; Raasch, M. Canine and Feline Nutrition, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Milner, J.A. Lysine Requirements of the Immature Dog. J. Nutr. 1981, 111, 40–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rutherfurd-Markwick, K.J.; McGrath, M.C.; Weidgraaf, K.; Hendriks, W.H. γ-Glutamylfelinylglycine Metabolite Excretion in the Urine of the Domestic Cat (Felis Catus). J. Nutr. 2006, 136, 2075S–2077S. [Google Scholar] [CrossRef] [Green Version]
- Burns, R.A.; Milner, J.A. Sulfur Amino Acid Requirements of Immature Beagle Dogs. J. Nutr. 1981, 111, 2117–2124. [Google Scholar] [CrossRef]
- Teeter, R.G.; Baker, D.H.; Corbin, J.E. Methionine and Cystine Requirements of the Cat. J. Nutr. 1978, 108, 291–295. [Google Scholar] [CrossRef]
- Hendriks, W.H.; Rutherfurd, S.M.; Rutherfurd, K.J. Importance of Sulfate, Cysteine and Methionine as Precursors to Felinine Synthesis by Domestic Cats (Felis Catus). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2001, 129, 211–216. [Google Scholar] [CrossRef]
- EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) Scientific Opinion on the Safety and Efficacy of L-Tyrosine for All Animal Species. EFSA J. 2013, 11, 3310. [CrossRef]
- Morris, J.G.; Yu, S.; Rogers, Q.R. Red Hair in Black Cats Is Reversed by Addition of Tyrosine to the Diet. J. Nutr. 2002, 132, 1646S–1648S. [Google Scholar] [CrossRef] [Green Version]
- Anderson, P.J.B.; Rogers, Q.R.; Morris, J.G. Cats Require More Dietary Phenylalanine or Tyrosine for Melanin Deposition in Hair than for Maximal Growth. J. Nutr. 2002, 132, 2037–2042. [Google Scholar] [CrossRef] [Green Version]
- Burns, R.A.; Milner, J.A. Threonine, Tryptophan and Histidine Requirements of Immature Beagle Dogs. J. Nutr. 1982, 112, 447–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Titchenal, C.A.; Rogers, Q.R.; Indrieri, R.J.; Morris, J.G. Threonine Imbalance, Deficiency and Neurologic Dysfunction in the Kitten. J. Nutr. 1980, 110, 2444–2459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwazaki, E.; Nade, T.; Kimura, N. Effects of Overfeeding on the Fatty Acid Profile and Stearoyl-CoA Desaturase-1 Indices in the Liver and Subcutaneous Adipose Tissue in Cats. J. Vet. Med. Sci. 2019, 81, 1080–1085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahlstrøm, Ø.; Krogdahl, Å.; Vhile, S.G.; Skrede, A. Fatty Acid Composition in Commercial Dog Foods. J. Nutr. 2004, 134, 2145S–2147S. [Google Scholar] [CrossRef]
- Butterwick, R.F.; Salt, C.; Watson, T.D.G. Effects of Increases in Dietary Fat Intake on Plasma Lipid and Lipoprotein Cholesterol Concentrations and Associated Enzyme Activities in Cats. Am. J. Vet. Res. 2012, 73, 62–67. [Google Scholar] [CrossRef]
- Grundy, S.M. Influence of Stearic Acid on Cholesterol Metabolism Relative to Other Long-Chain Fatty Acids. Am. J. Clin. Nutr. 1994, 60, 986S–990S. [Google Scholar] [CrossRef]
- Sassa, T.; Kihara, A. Metabolism of Very Long-Chain Fatty Acids: Genes and Pathophysiology. Biomol. Ther. 2014, 22, 83–92. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Zhang, Y.; Su, D.; Lv, X.; Wang, M.; Ding, D.; Ma, J.; Xia, M.; Wang, D.; Yang, Y.; et al. The Opposite Associations of Long-Chain versus Very Long-Chain Monounsaturated Fatty Acids with Mortality among Patients with Coronary Artery Disease. Heart 2014, 100, 1597–1605. [Google Scholar] [CrossRef]
- Yaqoob, P. Monounsaturated Fatty Acids and Immune Function. Eur. J. Clin. Nutr. 2002, 56 (Suppl. 3), S9–S13. [Google Scholar] [CrossRef] [Green Version]
- Palmquist, D.L. Omega-3 Fatty Acids in Metabolism, Health, and Nutrition and for Modified Animal Product Foods. Prof. Anim. Sci. 2009, 25, 207–249. [Google Scholar] [CrossRef]


| Dry Pet Food | Type of Sample | |||
|---|---|---|---|---|
| CFM | CMix | CMM | p-Value | |
| Moisture (%) | 8.42 ± 0.05 | 5.43 ± 0.08 | 8.6 ± 0.1 | **** |
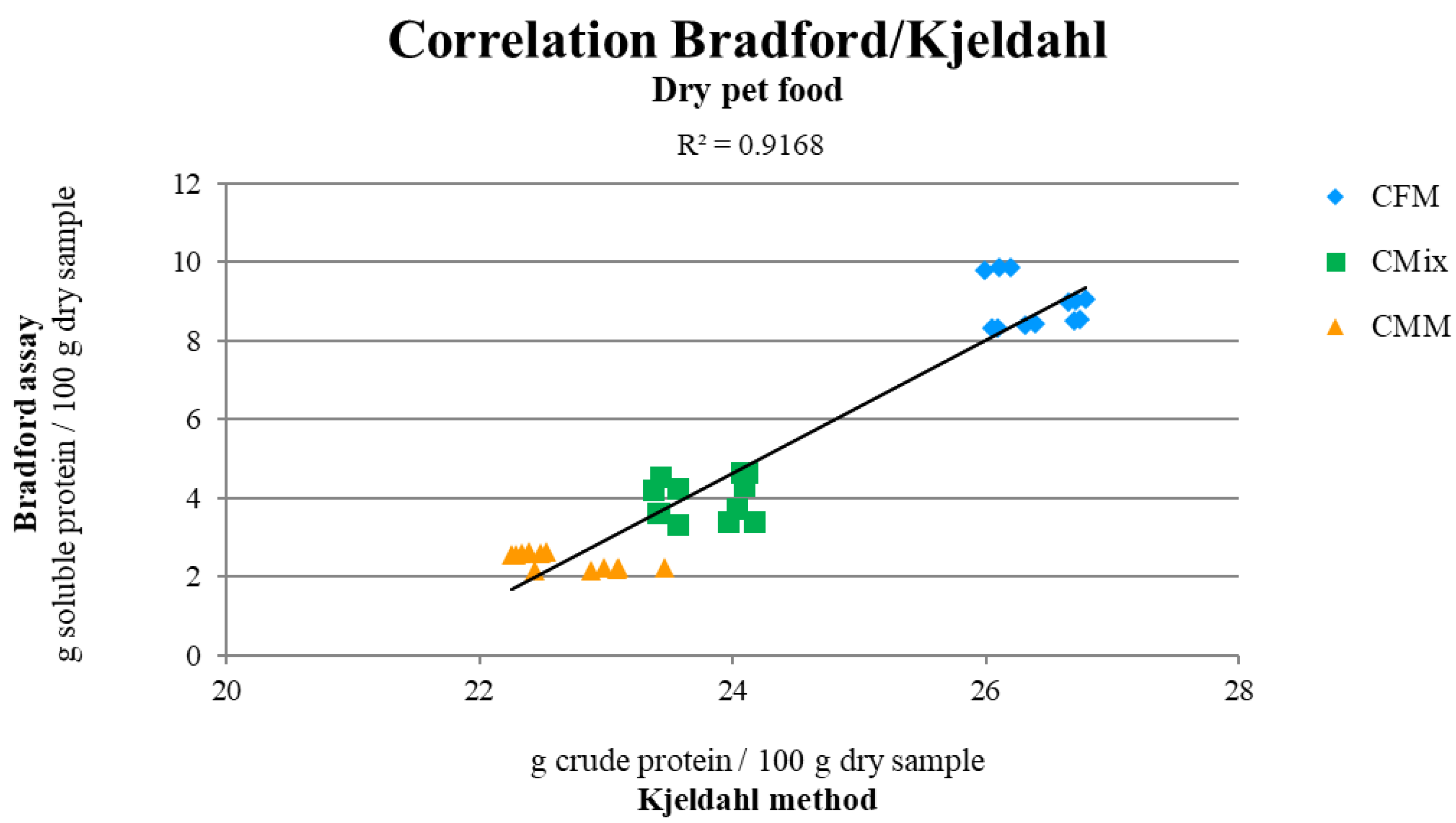
| Bradford Assay (g SP/100 g Dry Sample) | 8.9 ± 0.2 | 4.0 ± 0.1 | 2.4 ± 0.4 | **** |
| Kjeldahl Method (g CP/100 g Dry Sample) | 26.40 ± 0.09 | 23.77 ± 0.09 | 22.7 ± 0.1 | **** |
| AA Content (g AA/100 g Dry Sample) | Type of Sample | |||
|---|---|---|---|---|
| CFM | CMix | CMM | p-Value | |
| Arginine | 1.48 ± 0.02 | 1.15 ± 0.01 | 1.02 ± 0.01 | **** |
| Histidine | 0.536 ± 0.006 | 0.456 ± 0.005 | 0.425 ± 0.006 | **** |
| Isoleucine | 1.19 ± 0.02 | 1.09 ± 0.02 | 1.07 ± 0.02 | *** |
| Leucine | 2.22 ± 0.06 | 1.99 ± 0.01 | 1.94 ± 0.02 | **** |
| Lysine | 2.40 ± 0.02 | 1.98 ± 0.01 | 1.82 ± 0.01 | **** |
| Methionine | 0.320 ± 0.006 | 0.281 ± 0.003 | 0.212 ± 0.003 | **** |
| Phenylalanine | 0.812 ± 0.009 | 0.751 ± 0.004 | 0.74 ± 0.01 | **** |
| Taurine | 0.201 ± 0.009 | 0.162 ± 0.005 | 0.134 ± 0.004 | **** |
| Threonine | 0.314 ± 0.005 | 0.298 ± 0.006 | 0.296 ± 0.007 | ns |
| Tryptophan | 0.49 ± 0.01 | 0.32 ± 0.01 | 0.286 ± 0.008 | **** |
| Valine | 0.943 ± 0.005 | 0.935 ± 0.003 | 0.927 ± 0.003 | * |
| TOT EEAs | 10.9 ± 0.2 | 9.4 ± 0.1 | 8.9 ± 0.1 | **** |
| Alanine | 1.53 ± 0.07 | 1.66 ± 0.07 | 1.99 ± 0.09 | *** |
| Asparagine | 0.0221 ± 0.0004 | 0.0163 ± 0.0005 | 0.0140 ± 0.0003 | **** |
| Aspartic acid | 2.26 ± 0.06 | 1.80 ± 0.01 | 1.95 ± 0.01 | **** |
| Glutamic acid | 2.31 ± 0.06 | 1.78 ± 0.01 | 1.70 ± 0.03 | **** |
| Glycine | 0.72 ± 0.05 | 0.67 ± 0.03 | 0.60 ± 0.02 | ns |
| Hydroxyproline | 0.571 ± 0.005 | 0.399 ± 0.003 | 0.308 ± 0.003 | **** |
| Proline | 0.66 ± 0.01 | 0.614 ± 0.009 | 0.538 ± 0.008 | **** |
| Serine | 0.74 ± 0.07 | 1.03 ± 0.08 | 1.07 ± 0.06 | ** |
| Tyrosine | 0.91 ± 0.04 | 0.87 ± 0.01 | 0.84 ± 0.01 | ns |
| TOT non EAAs | 9.7 ± 0.4 | 8.8 ± 0.2 | 9.0 ± 0.2 | ns |
| TOT AAs | 20.6 ± 0.5 | 18.2 ± 0.3 | 17.9 ± 0.3 | **** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montegiove, N.; Calzoni, E.; Cesaretti, A.; Pellegrino, R.M.; Emiliani, C.; Pellegrino, A.; Leonardi, L. The Hard Choice about Dry Pet Food: Comparison of Protein and Lipid Nutritional Qualities and Digestibility of Three Different Chicken-Based Formulations. Animals 2022, 12, 1538. https://doi.org/10.3390/ani12121538
Montegiove N, Calzoni E, Cesaretti A, Pellegrino RM, Emiliani C, Pellegrino A, Leonardi L. The Hard Choice about Dry Pet Food: Comparison of Protein and Lipid Nutritional Qualities and Digestibility of Three Different Chicken-Based Formulations. Animals. 2022; 12(12):1538. https://doi.org/10.3390/ani12121538
Chicago/Turabian StyleMontegiove, Nicolò, Eleonora Calzoni, Alessio Cesaretti, Roberto Maria Pellegrino, Carla Emiliani, Alessia Pellegrino, and Leonardo Leonardi. 2022. "The Hard Choice about Dry Pet Food: Comparison of Protein and Lipid Nutritional Qualities and Digestibility of Three Different Chicken-Based Formulations" Animals 12, no. 12: 1538. https://doi.org/10.3390/ani12121538
APA StyleMontegiove, N., Calzoni, E., Cesaretti, A., Pellegrino, R. M., Emiliani, C., Pellegrino, A., & Leonardi, L. (2022). The Hard Choice about Dry Pet Food: Comparison of Protein and Lipid Nutritional Qualities and Digestibility of Three Different Chicken-Based Formulations. Animals, 12(12), 1538. https://doi.org/10.3390/ani12121538










