Molecular Epidemiology and Genetic Diversity of Enterocytozoon bieneusi in Cervids from Milu Park in Beijing, China
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Collection
2.2. DNA Extraction and Nested PCR Amplification
2.3. DNA Sequence Analysis
2.4. Statistical Analysis
3. Results
3.1. Occurrence of E. bieneusi
3.2. Distribution and Genetic Characterization of E. bieneusi
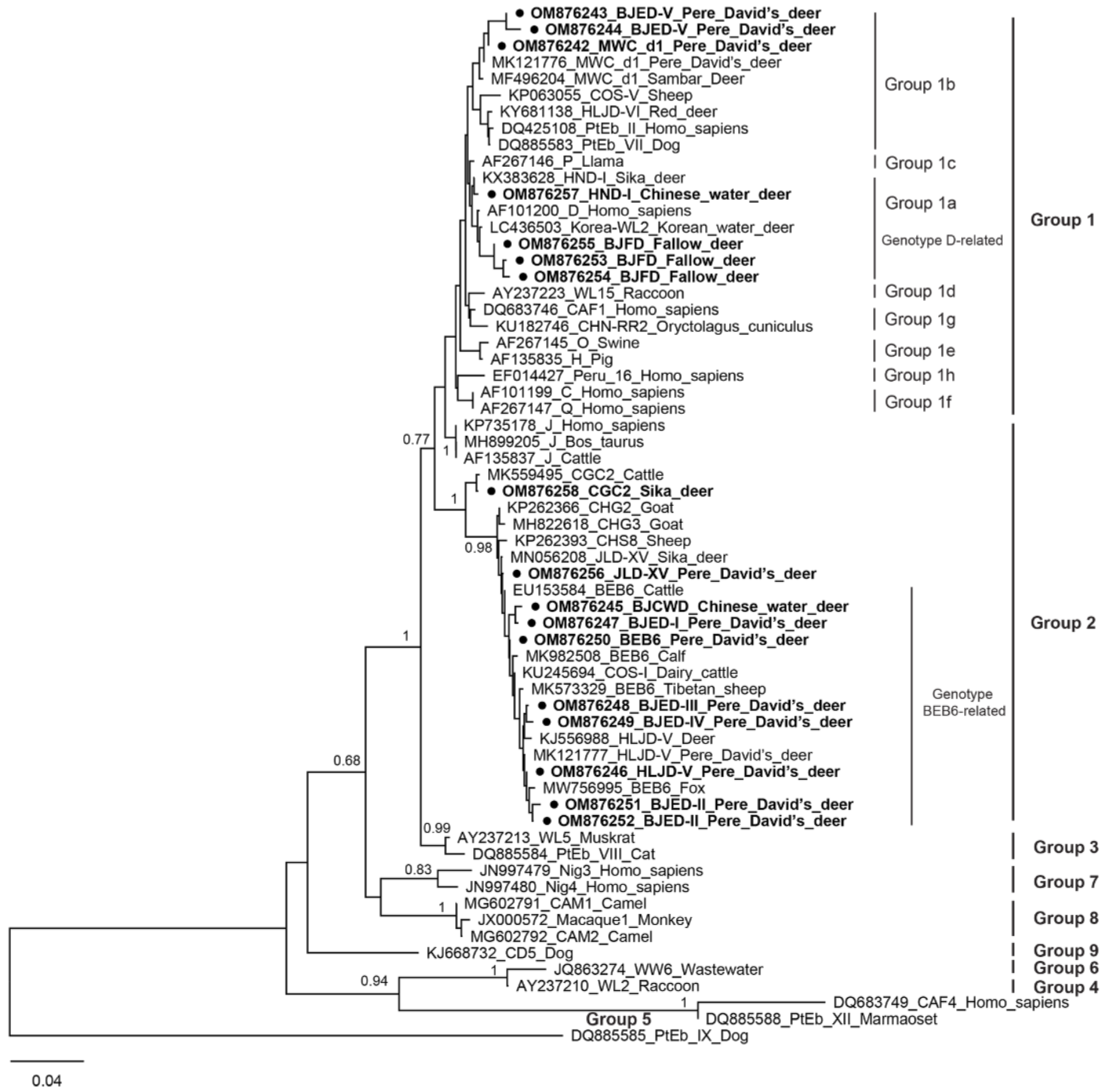
3.3. Phylogenetic Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, W.; Xiao, L. Ecological and public health significance of Enterocytozoon bieneusi. One Health 2020, 12, 100209. [Google Scholar] [CrossRef] [PubMed]
- Matos, O.; Lobo, M.L.; Xiao, L. Epidemiology of Enterocytozoon bieneusi infection in humans. J. Parasitol. Res. 2012, 2012, 981424. [Google Scholar] [CrossRef] [PubMed]
- Didier, E.S. Microsporidiosis: An emerging and opportunistic infection in humans and animals. Acta Tropica. 2005, 94, 61–76. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhong, Z.; Song, Y.; Gong, C.; Deng, L.; Cao, Y.; Tian, Y.; Li, H.; Feng, F.; Zhang, Y.; et al. Human-pathogenic Enterocytozoon bieneusi in captive giant pandas (Ailuropoda melanoleuca) in China. Sci. Rep. 2018, 8, 6590. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Ni, H.; Du, H.; Jiang, J.; Li, J.; Qiu, H.; Li, Y.; Zhang, X. Molecular detection of Cryptosporidium and Enterocytozoon bieneusi in dairy calves and sika deer in four provinces in Northern China. Parasitol. Res. 2020, 119, 105–114. [Google Scholar] [CrossRef]
- Han, B.; Weiss, L.M. Therapeutic targets for the treatment of microsporidiosis in humans. Expert Opin. Ther. Targets 2018, 22, 903–915. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Jia, T.; Huang, J.; Fan, Y.; Chang, H.; Han, S.; Luo, J.; He, H. Identification of Enterocytozoon bieneusi and Cryptosporidium spp. in farmed wild boars (Sus scrofa) in Beijing, China. Infect. Genet. Evol. 2020, 80, 104231. [Google Scholar] [CrossRef] [PubMed]
- Santin, M.; Fayer, R. Microsporidiosis: Enterocytozoon bieneusi in domesticated and wild animals. Res. Vet. Sci. 2011, 90, 363–371. [Google Scholar] [CrossRef]
- Li, W.; Feng, Y.; Santin, M. Host specificity of Enterocytozoon bieneusi and public health implications. Trends Parasitol. 2019, 35, 436–451. [Google Scholar] [CrossRef]
- Wang, L.; Xiao, L.; Duan, L.; Ye, J.; Guo, Y.; Guo, M.; Liu, L.; Feng, Y. Concurrent infections of Giardia duodenalis, Enterocytozoon bieneusi, and Clostridium difficile in children during a cryptosporidiosis outbreak in a pediatric hospital in China. PLoS Negl. Trop. Dis. 2013, 7, e2437. [Google Scholar] [CrossRef]
- Li, W.; Feng, Y.; Xiao, L. Diagnosis and molecular typing of Enterocytozoon bieneusi: The significant role of domestic animals in transmission of human microsporidiosis. Res. Vet. Sci. 2020, 133, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Amer, S.; Kim, S.; Han, J.; Na, K. Prevalence and genotypes of Enterocytozoon bieneusi in wildlife in Korea: A public health concern. Parasites Vectors 2019, 12, 160. [Google Scholar] [CrossRef]
- Cong, W.; Qin, S.Y.; Meng, Q.F. Molecular characterization and new genotypes of Enterocytozoon bieneusi in minks (Neovison vison) in China. Parasite 2018, 25, 34. [Google Scholar] [CrossRef] [PubMed]
- Perec-Matysiak, A.; Bunkowska-Gawlik, K.; Kvac, M.; Sak, B.; Hildebrand, J.; Lesnianska, K. Diversity of Enterocytozoon bieneusi genotypes among small rodents in southwestern Poland. Vet. Parasitol. 2015, 214, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Koehler, A.V.; Wang, T.; Haydon, S.R.; Gasser, R.B. First detection and genetic characterisation of Enterocytozoon bieneusi in wild deer in Melbourne’s water catchments in Australia. Parasites Vectors 2018, 11, 2. [Google Scholar] [CrossRef]
- Yamazaki, S.; Motoi, Y.; Nagai, K.; Ishinazaka, T.; Asano, M.; Suzuki, M. Sex determination of sika deer (Cervus nippon yesoensis) using nested PCR from feces collected in the field. J. Vet. Med. Sci. 2011, 73, 1611–1616. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Z.; Ai, S.; Wang, X.; Zhang, R.; Duan, Z. Cryptosporidium spp., Enterocytozoon bieneusi, and Giardia duodenalis from animal sources in the Qinghai-Tibetan Plateau Area (QTPA) in China. Comp. Immunol. Microbiol. Infect. Dis. 2019, 67, 101346. [Google Scholar] [CrossRef]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547. [Google Scholar] [CrossRef]
- Cama, V.A.; Pearson, J.; Cabrera, L.; Pacheco, L.; Gilman, R.; Meyer, S.; Ortega, Y.; Xiao, L. Transmission of Enterocytozoon bieneusi between a child and guinea pigs. J. Clin. Microbiol. 2007, 45, 2708–2710. [Google Scholar] [CrossRef]
- Liu, W.; Nie, C.; Zhang, L.; Wang, R.; Liu, A.; Zhao, W.; Li, H. First detection and genotyping of Enterocytozoon bieneusi in reindeers (Rangifer tarandus): A zoonotic potential of ITS genotypes. Parasites Vectors 2015, 8, 526. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Santin, M.; Fayer, R. Enterocytozoon bieneusi, Giardia, and Cryptosporidium infecting white-tailed deer. J. Eukaryot. Microbiol. 2015, 62, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Zhang, Z.; Zhao, A.; Jing, B.; Qi, M.; Wang, R. Molecular characterization of Cryptosporidium and Enterocytozoon bieneusi in Père David’s deer (Elaphurus davidianus) from Shishou, China. Int. J. Parasitol. Parasites Wildl. 2019, 10, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Huang, J.; Karim, M.R.; Zhao, J.; Dong, H.; Ai, W.; Li, F.; Zhang, L.; Wang, R. Zoonotic Enterocytozoon bieneusi genotypes in Père David’s deer (Elaphurus davidianus) in Henan, China. Exp. Parasitol. 2015, 155, 46–48. [Google Scholar] [CrossRef]
- Li, W.; Deng, L.; Yu, X.; Zhong, Z.; Wang, Q.; Liu, X.; Niu, L.; Xie, N.; Deng, J.; Lei, S.; et al. Multilocus genotypes and broad host-range of Enterocytozoon bieneusi in captive wildlife at zoological gardens in China. Parasites Vectors 2016, 9, 395. [Google Scholar] [CrossRef]
- Song, Y.; Li, W.; Liu, H.; Zhong, Z.; Luo, Y.; Wei, Y.; Fu, W.; Ren, Z.; Zhou, Z.; Deng, L.; et al. First report of Giardia duodenalis and Enterocytozoon bieneusi in forest musk deer (Moschus berezovskii) in China. Parasites Vectors 2018, 11, 204. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, J.; Yang, Z.; Liu, A. Dominance of the Enterocytozoon bieneusi genotype BEB6 in red deer (Cervus elaphus) and Siberian roe deer (Capreolus pygargus) in China and a brief literature review. Parasite 2017, 24, 54. [Google Scholar] [CrossRef]
- Zhang, X.; Cong, W.; Liu, G.; Ni, X.; Ma, J.; Zheng, W.; Zhao, Q.; Zhu, X. Prevalence and genotypes of Enterocytozoon bieneusi in sika deer in Jilin province, Northeastern China. Acta Parasitol. 2016, 61, 382–388. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, W.; Wang, R.; Liu, W.; Liu, A.; Yang, D.; Yang, F.; Karim, M.R.; Zhang, L. Enterocytozoon bieneusi in sika deer (Cervus nippon) and red deer (Cervus elaphus): Deer specificity and zoonotic potential of ITS genotypes. Parasitol. Res. 2014, 113, 4243–4250. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, Z.; Yang, Y.; Wang, R.; Zhao, J.; Jian, F.; Ning, C.; Zhang, L. New genotypes of Enterocytozoon bieneusi isolated from sika deer and red deer in China. Front. Microbiol. 2017, 8, 879. [Google Scholar] [CrossRef]
- Guo, Y.; Alderisio, K.A.; Yang, W.; Cama, V.; Feng, Y.; Xiao, L. Host specificity and source of Enterocytozoon bieneusi genotypes in a drinking source watershed. Appl. Environ. Microbiol. 2014, 80, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Yue, D.; Ma, J.; Li, F.; Hou, J.; Zheng, W.; Zhao, Q.; Zhang, X.; Zhu, X. Occurrence of Enterocytozoon bieneusi in Donkeys (Equus asinus) in China: A Public Health Concern. Front. Microbiol. 2017, 8, 565. [Google Scholar] [CrossRef] [PubMed]
| Species | No. Tested | No. Positive (%) | Genotype (No.) | Group |
|---|---|---|---|---|
| Pere David’s deer | 80 | 24 (30.0) | HLJD-V (n = 12), MWC_d1 (n = 4), BEB6 (n = 1), BJED-I to BJED-V (n = 7) | Group 1 (n = 6), Group 2 (n = 18) |
| Fallow deer | 55 | 15 (27.3) | HLJD-V (n = 2), BEB6 (n = 2), MWC_d1 (n = 1), BJFD (n = 10) | Group 1 (n = 11), Group 2 (n = 4) |
| Sika deer | 40 | 5 (12.5) | CGC2 (n = 3), JLD-XV (n = 2) | Group 2 (n = 5) |
| Chinese water deer | 40 | 3 (7.5) | HLJD-V (n = 1), HND-I (n = 1), BJCWD (n = 1) | Group 1 (n = 1), Group 2 (n = 2) |
| Total | 215 | 47 (21.9) | HLJD-V (n = 15), MWC_d1 (n = 5), BEB6 (n = 3), CGC2 (n = 3), JLD-XV (n = 2), HND-I (n = 1), BJED-I to BJED-V (n = 7), BJFD (n = 10), BJCWD (n = 1) | Group 1 (n = 18), Group 2 (n = 29) |
| Factor | Category | No. Positive/No. Tested | Prevalence % (95% CI) | p-Value |
|---|---|---|---|---|
| Species | Pere David’s deer | 24/80 | 30.0 (19.7–40.3) | 0.012 |
| Fallow deer | 15/55 | 27.3 (15.1–39.4) | ||
| Sika deer | 5/40 | 12.5 (1.8–23.2) | ||
| Chinese water deer | 3/40 | 7.5 (−1.0–16.0) | ||
| Gender | Male | 18/92 | 19.6 (11.3–27.8) | 0.670 |
| Female | 27/123 | 22.0 (14.5–29.4) | ||
| Living condition | Captive | 18/95 | 18.9 (10.9–27.0) | 0.525 |
| Free-ranging | 27/120 | 22.5 (14.9–30.1) |
| Genotype | 12 | 376 | 378 | 380 | 381 | 382 | 383 | 386 | 390 | Accession No. | Reference Genotypes |
|---|---|---|---|---|---|---|---|---|---|---|---|
| genotype D | C | G | T | C | G | T | C | C | G | AF101200 | Reference |
| Korea-WL2 | C | G | T | C | G | T | C | C | G | LC436503 | Reference |
| BJFD | - | G | T | G | T | T | C | C | A | OM876253 | Novel |
| HLJD-V | - | G | T | C | G | T | C | C | A | OM876246 | Known |
| BJCWD | T | - | T | G | G | T | G | - | - | OM876245 | Novel |
| BJED-I | C | G | A | - | - | - | - | - | - | OM876247 | Novel |
| BJED-II | C | G | T | G | G | T | C | G | T | OM876251 | Novel |
| BJED-III | C | G | T | G | G | T | T | G | T | OM876248 | Novel |
| BJED-IV | T | G | T | G | G | T | C | C | A | OM876249 | Novel |
| MWC_d1 | C | G | T | C | G | T | C | C | G | MF496204 | Reference |
| MWC_d1 | - | G | T | C | G | T | C | C | A | OM876242 | Known |
| BJED-V | - | G | C | G | T | T | C | C | A | OM876243 | Novel |
| Country/Location | Source | Infection Rate | Genotype (No.) | Reference |
|---|---|---|---|---|
| China/Northern | Sika deer | 13.57% (111/818) | BEB6 (84), EbpC (3), I (1), JLD-III (1), JLD-VIII (3), JLD-IX (1), JLD-XV to JLD-XXIII (17), LND-I (1) | [5] |
| China/Northeast | Reindeer | 16.8% (21/125) | Peru6 (6), CHN-RD1 (12), CHN-RD2 to CHN-RD4 (1) | [21] |
| China/Hubei | Pere David’s deer | 35.2% (45/128) | HLJD-V (42), MWC_d1 (3) | [23] |
| China/Henan | Pere David’s deer | 34.0% (16/47) | Type IV (4), EbpC (4), EbpA (4), BEB6 (2), COS-I (1), COS-II (1) | [24] |
| China/Sichuan | Hog deer, Sambar deer, Fallow deer, Red deer, Pere David’s deer, Sika deer | 24.0% (6/25) | BEB6 (4), CHS9 (1), SC03 (1) | [25] |
| China/Sichuan | Forest musk deer | 17.04% (38/223) | SOC3 (38) | [26] |
| China/Northern | Red deer, Siberian roe deer | 8.2% (10/122) | BEB6 (9), HLJD-VI (1) | [27] |
| China/Jilin | Sika deer | 7.1% (23/326) | J (11), BEB6 (4), EbpC (1), CHN-DC1 (1), KIN-1 (1), JLD-1 (2), JLD-2 (2), JLD-3 (1) | [28] |
| China/Northeast | Sika deer, Red deer | 31.9% (29/91) | BEB6 (20), HLJD-I to HLJD-IV (1), HLJD-V (5) | [29] |
| China/Henan and Jilin | Sika deer, Red deer | 35.9% (221/615) | BEB6 (131), HLJD-I (18), EbpC (3), HLJD-IV (2), COS-I (1), EbpA (1), D (1), JLD-I (7), JLD-II (5), HND-I (4), JLD-III (2), HND-II (1), JLD-IV (6), JLD-V (2), JLD-VI (5), HND-III (1), JLD-VII (1), JLD-VIII (16), JLD-IX (1), JLD-X (1), HND-IV (1), JLD-XI (2), JLD-XII (1), JLD-XIII (1), JLD-XIV (7) | [30] |
| China/Beijing | Pere David’s deer, Fallow deer, Sika deer, Chinese water deer | 21.9% (47/215) | HLJD-V (n = 15), MWC_d1 (n = 5), BEB6 (n = 3), CGC2 (n = 3), JLD-XV (n = 2), HND-I (n = 1), BJED-I to BJED-V (n = 7), BJFD (n = 10), BJCWD (n = 1) | This study |
| USA/Maryland | White-tailed deer | 32.5% (26/80) | I (7), J (1), WL4 (11), LW1 (1), DeerEb1-DeerEb13 (13) | [22] |
| USA/New York | White-tailed deer | 12.2% (6/49) | WL18 (2), WL19 (2), WL4 (2) | [31] |
| Korean/Wildlife centers | Korean water deer | 53.6% (52/97) | D (29), Korea-WL1-WL6 (23) | [12] |
| Australia | Sambar deer *, Red deer, Fallow deer | 4.1% (25/610) | D (3), J (1), Type IV (1), MWC_d1 (19), MWC_d2 (1) | [16] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Zhong, Z.; Xia, Z.; Meng, Q.; Shan, Y.; Guo, Q.; Cheng, Z.; Zhang, P.; He, H.; Bai, J. Molecular Epidemiology and Genetic Diversity of Enterocytozoon bieneusi in Cervids from Milu Park in Beijing, China. Animals 2022, 12, 1539. https://doi.org/10.3390/ani12121539
Zhang Q, Zhong Z, Xia Z, Meng Q, Shan Y, Guo Q, Cheng Z, Zhang P, He H, Bai J. Molecular Epidemiology and Genetic Diversity of Enterocytozoon bieneusi in Cervids from Milu Park in Beijing, China. Animals. 2022; 12(12):1539. https://doi.org/10.3390/ani12121539
Chicago/Turabian StyleZhang, Qingxun, Zhenyu Zhong, Zhiqiang Xia, Qinghui Meng, Yunfang Shan, Qingyun Guo, Zhibin Cheng, Peiyang Zhang, Hongxuan He, and Jiade Bai. 2022. "Molecular Epidemiology and Genetic Diversity of Enterocytozoon bieneusi in Cervids from Milu Park in Beijing, China" Animals 12, no. 12: 1539. https://doi.org/10.3390/ani12121539
APA StyleZhang, Q., Zhong, Z., Xia, Z., Meng, Q., Shan, Y., Guo, Q., Cheng, Z., Zhang, P., He, H., & Bai, J. (2022). Molecular Epidemiology and Genetic Diversity of Enterocytozoon bieneusi in Cervids from Milu Park in Beijing, China. Animals, 12(12), 1539. https://doi.org/10.3390/ani12121539






