Niacin Status Indicators and Their Relationship with Metabolic Parameters in Dairy Cows during Early Lactation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Study Protocol
2.2. Blood Collection and Analysis
2.3. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cincović, M.; Hristovska, T.; Belić, B. Niacin, Metabolic Stress and Insulin Resistance in Dairy Cows. In B Group Vitamins-Current Uses and Perspectives; LeBlanc, G., de Gior, G.S., Eds.; IntechOpen: London, UK, 2018; pp. 107–125. [Google Scholar]
- Carloson, L.A. Nicotinic acid: The broad-spectrum lipid drug. A 50th anniversary review. J. Intern. Med. 2005, 258, 94–114. [Google Scholar] [CrossRef] [PubMed]
- Henderson, L.M. Niacin. Annu. Rev. Nutr. 1983, 3, 289–307. [Google Scholar] [CrossRef] [PubMed]
- Weiss, B. Update on vitamin nutrition of dairy cows. In Proceedings of the Ruminant Health and Nutrition Conference, Syracuse, NY and New England Dairy Feed Conference, New York, NY, USA, 11–15 March 2005; pp. 1–12. [Google Scholar]
- Keshri, A.; Bashir, Z.; Kumari, V.; Prasad, K.; Joysowal, M.; Singh, M.; Singh, D.; Tarun, A.; Shukla, S. Role of micronutrients during peri-parturient period of dairy animals—A review. Biol. Rhythm Res. 2021, 52, 1018–1030. [Google Scholar] [CrossRef]
- Sundrum, A. Metabolic disorders in the transition period indicate that the dairy cows’ ability to adapt is overstressed. Animals 2015, 5, 978–1020. [Google Scholar] [CrossRef]
- Cincović, M.R.; Đoković, R.; Belić, B.; Lakić, I.; Stojanac, N.; Stevančević, O.; Staničkov, N. Insulin resistance in cows during the periparturient period. Acta Agric. Serb. 2018, 23, 233–245. [Google Scholar] [CrossRef]
- Bender, D.A. Niacin. In Nutritional Biochemistry of the Vitamins; Cambridge University Press: Cambridge, UK, 2003. [Google Scholar]
- Hankes, L.V. Nicotinic acid and nicotinamide. In Handbook of Vitamines, Nutritional, Biochemical and Clinical Aspects; Machined, L.J., Ed.; Marcel Dekker: New York, NY, USA, 1984; pp. 329–377. [Google Scholar]
- Tunaru, S.; Kero, J.; Schaub, A.; Wufka, C.; Blaukat, A.; Pfeffer, K.; Offermanns, S. PUMA-G and HM74 are receptors for nicotinic acid and mediate its anti-lipolytic effect. Nat. Med. 2003, 9, 352–355. [Google Scholar] [CrossRef]
- Pires, A.A.; Grummer, R.R. The use of nicotinic acid to induce sustanined low plasma nonesterified fatty acids in feed-restricted Holstein cows. J. Dairy Sci. 2007, 90, 3725–3732. [Google Scholar] [CrossRef] [Green Version]
- Hristovska, T.; Cincović, M.; Stojanović, D.; Belić, B.; Kovačević, Z.; Jezdimirović, M. Influence of niacin supplementation on the metabolic parameters and lipolysis in dairy cows during early lactation. Kafkas Univ. Vet Fak Derg. 2017, 23, 773–778. [Google Scholar]
- Hristovska, T.; Cincović, M.; Belić, B.; Stojanović, D.; Jezdimirović, M.; Djoković, R.; Toholj, B. Effects of niacin supplementation on the insulin resistance in Holstein cows during early lactation. Acta Vet Brno 2017, 86, 231–238. [Google Scholar] [CrossRef] [Green Version]
- Pires, J.A.A.; Stumpf, L.F.; Soutullo, I.D.; Pescara, J.B.; Stocks, S.E.; Grummer, R.R. Effects of abomasal infusion of nicotinic acid on responses to glucose and β-agonist challenges in underfed lactating cows. J. Dairy Sci. 2016, 99, 2297–2307. [Google Scholar] [CrossRef]
- Jaster, E.H.; Ward, N.E. Supplemental nicotinic acid or nicotinamide for lactating dairy cows. J. Dairy Sci. 1990, 73, 2880–2887. [Google Scholar] [CrossRef]
- Luo, D.; Gao, Y.; Lu, Y.; Zhang, Q.; Qu, M.; Xiong, X.; Xu, L.; Zhao, X.; Pan, K.; Ouyang, K. Niacin supplementation improves growth performance and nutrient utilisation in Chinese Jinjiang cattle. Ital. J. Anim. Sci. 2018, 18, 57–62. [Google Scholar] [CrossRef]
- Al-Abbasy, E.G. Effect of adding two levels of niacin in milk production and controlling indicators of ketosis in Friesian cows postpartum. Br. J. Dairy Sci. 2013, 3, 1–4. [Google Scholar]
- Cervantes, A.; Smith, T.R.; Young, J.W. Effects of nicotinamide on milk composition and production in dairy cows fed supplemental fat. J. Dairy Sci. 1996, 79, 105–115. [Google Scholar] [CrossRef]
- Driver, L.S.; Grummer, R.R.; Schultz, L.H. Effects of feeding heat-treated soybeans and niacin to high producing cows in early lactation. J. Dairy Sci. 1990, 73, 463–469. [Google Scholar] [CrossRef]
- Ottou, J.F.; Doreau, M.; Chilliard, Y. Duodenal rapeseed oil infusion in midlactation cows. 6. Interaction with niacin on dairy performance and nutritional balances. J. Dairy Sci. 1995, 78, 1345–1352. [Google Scholar] [CrossRef]
- Martinez, N.; Depeters, E.J.; Bath, D.L. Supplemental niacin and fat effects on milk-composition of lactating Holstein cows. J. Dairy Sci. 1991, 74, 202–210. [Google Scholar] [CrossRef]
- Lanham, J.K.; Coppock, C.E.; Brooks, K.N.; Wilks, D.L.; Horner, J.L. Effects of whole cottonseed or niacin or both on casein synthesis by lactating Holstein cows. J. Dairy Sci. 1992, 75, 184–192. [Google Scholar] [CrossRef]
- Campbell, J.M.; Murphy, M.R.; Christensen, R.A.; Overton, T.R. Kinetics of niacin supplements in lactating dairy cows. J. Dairy Sci. 1994, 77, 566–575. [Google Scholar] [CrossRef]
- Tienken, R.; Kersten, S.; Frahm, J.; Hüther, L.; Meyer, U.; Huber, K.; Rehage, J.; Dänicke, S. Effects of Prepartum Dietary Energy Level and Nicotinic Acid Supplementation on Immunological, Hematological and Biochemical Parameters of Periparturient Dairy Cows Differing in Parity. Animals 2015, 5, 910–933. [Google Scholar] [CrossRef]
- Kollenkirchen, U.; Kuhnigk, C.; Breves, G.; Harmeyer, J. Effect of niacin supplementation on the concentration of niacin in rumen and duodenal digesta and in whole blood of sheep. J. Vet. Med. A 1992, 39, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Niehoff, I.D.; Hüther, L.; Lebzien, P. Niacin for dairy cattle: A review. Br. J. Nutr. 2008, 101, 5–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaowa, N.; Zhang, X.; Li, H.; Wang, Y.; Zhang, J.; Hao, Y.; Cao, Z.; Li, S. Effects of Rumen-Protected Niacin on Dry Matter Intake, Milk Production, Apparent Total Tract Digestibility, and Faecal Bacterial Community in Multiparous Holstein Dairy Cow during the Postpartum Period. Animals 2021, 11, 617. [Google Scholar] [CrossRef] [PubMed]
- Okabe, K.; Yaku, K.; Tobe, K.; Nakagawa, T. Implications of altered NAD metabolism in metabolic disorders. J. Biomed. Sci. 2019, 26, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, C.S.; Swendseid, M.E.; Jacob, R.A.; McKee, R.W. Biochemical markers forassessment of niacin status in young men: Levels of erythrocyte niacin coenzymes and plasma tryptophan. J. Nutr. 1989, 119, 1949–1955. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, E.L.; Jacobson, M.K. Tissue NAD as a biochemical measure of niacin status in humans. Methods Enzymol. 1997, 280, 221–230. [Google Scholar]
- Rungruang, S.; Collier, J.L.; Rhoads, R.P.; Baumgard, L.H.; De Veth, M.J.; Collier, R.J. A dose-response evaluation of rumen-protected niacin in thermoneutral or heat-stressed lactating Holstein cows. J. Dairy Sci. 2014, 97, 5023–5034. [Google Scholar] [CrossRef] [Green Version]
- NRC. Nutrient Requirements of Dairy Cattle, 7th ed.; National Academy Press: Washington, DC, USA, 2001. [Google Scholar]
- Petrović, M.Ž.; Cincović, M.; Starič, J.; Djoković, R.; Belić, B.; Radinović, M.; Majkić, M.; Ilić, Z.Ž. The Correlation between Extracellular Heat Shock Protein 70 and Lipid Metabolism in a Ruminant Model. Metabolites 2022, 12, 19. [Google Scholar] [CrossRef]
- DiPalma, J.R.; Thayer, W.S. Use of niacin as a drug. Annu. Rev. Nutr. 1991, 11, 169. [Google Scholar] [CrossRef]
- Santschi, D.E.; Chiquette, J.; Berthiaume, R.; Martineau, R.; Matte, J.J.; Mustafa, A.F.; Giard, C.L. Effects of the forage to concentrate ratio on B-vitamin concentrations in different ruminal fractiones of dairy cows. Can. J. Anim. Sci. 2005, 85, 385–399. [Google Scholar]
- Schwab, E.C.; Schwab, C.G.; Shaver, R.D.; Girard, C.L.; Putnam, D.E.; Whitehouse, N.L. Dietary Forage and Nonfiber Carbohydrate Contents Influence B-Vitamin Intake, Duodenal Flow, and Apparent Ruminal Synthesis in Lactating Dairy Cows. J. Dairy Sci. 2006, 89, 174–187. [Google Scholar] [CrossRef]
- Seyedsadjadi, N.; Berg, J.; Bilgin, A.A.; Braidy, N.; Salonikas, C.; Grant, R. High protein intake is associated with low plasma NAD+ levels in a healthy human cohort. PLoS ONE 2018, 13, e0201968. [Google Scholar] [CrossRef] [PubMed]
- Santschi, D.E.; Barthiaume, R.; Matte, J.J.; Mustafa, A.F.; Girard, C.L. Fate of supplementary B-vitamins in gastrointestinal tract of dairy cows. J. Dairy Sci. 2005, 88, 2043–2054. [Google Scholar] [CrossRef]
- Porter, J.W.G. Vitamin synthesis in the rumen. In Digestive Physiology and Nutrition of Theruminant; Lewis, D., Ed.; Butterworths: London, UK, 1961. [Google Scholar]
- Erickson, P.S.; Murphy, M.R.; McSweeney, C.S.; Trusk, A.M. Niacin absorption from the rumen. J. Dairy Sci. 1991, 74, 3492–3495. [Google Scholar] [CrossRef]
- Harmeyer, J.; Kollenkirchen, U. Thiamin and niacin in ruminant nutrition. Nutr. Res. Rev. 1989, 2, 201–205. [Google Scholar] [CrossRef] [Green Version]
- Miller, B.L.; Meiske, J.C.; Goodrich, R.D. Effects of grain source and concentrate level on B-vitamin production an absorption in steers. J. Anim. Sci. 1986, 62, 473–483. [Google Scholar] [CrossRef]
- Erickson, P.S.; Murphy, M.; Clark, J.H. Supplementation of dairy cow diets with calcium salts of long-chain fatty acids and nicotinic acid in early lactation. J. Dairy Sci. 1992, 75, 1078–1089. [Google Scholar] [CrossRef]
- Kenéz, A.; Locher, L.; Rehage, J.; Dänicke, S.; Huber, K. Agonists of the G protein-coupled receptor 109A-mediated pathway promote antilipolysis by reducing serine residue 563 phosphorylation of hormone-sensitive lipase in bovine adipose tissue explants. J. Dairy Sci. 2014, 97, 3626–3634. [Google Scholar] [CrossRef] [Green Version]
- Morey, S.D.; Mamedova, L.K.; Anderson, D.E.; Armendariz, C.K.; Titgemeyer, E.C.; Bradford, B.J. Effects of encapsulated niacin on metabolism and production of periparturient dairy cows. J. Dairy Sci. 2011, 94, 5090–5104. [Google Scholar] [CrossRef] [Green Version]
- Yuan, K.; Shaver, R.D.; Bertics, S.J.; Espineira, M.; Grummer, R.R. Effect of rumen-protected niacin on lipid metabolism, oxidative stress, and performance of transition dairy cows. J. Dairy Sci. 2012, 95, 2673–2679. [Google Scholar] [CrossRef] [Green Version]
- Dufva, G.S.; Bartley, E.E.; Dayton, A.D.; Riddell, D.O. Effect of niacin supplementation on milkproduction and ketosis of dairy cattle. J. Dairy Sci. 1983, 66, 2329–2336. [Google Scholar] [CrossRef]
- Pires, J.A.A.; Souza, A.H.; Grummer, R.R. Induction of hyperlipidemia by intravenous infusion of tallow emulsion causes insulin resistance in Holstein cows. J. Dairy Sci. 2007, 90, 2735–2744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thornton, J.H.; Schultz, L.H. Effects of administration ofnicotinic acid on glucose, insulin, and glucose tolerance in ruminants. J. Dairy Sci. 1980, 63, 262–268. [Google Scholar] [CrossRef]
- Pescara, J.B.; Pires, A.A.; Grummer, R.R. Antilipolytic and lipolytic effects of administering free or ruminally protected nicotinic acid to feed-restricted Holstein cows. J. Dairy Sci. 2010, 93, 5385–5396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Titgemeyer, E.; Spivey, K.; Mamedova, L.; Bradford, B. Effects of pharmacological amounts of nicotinic acid on lipolysis and feed intake in cattle. Int. J. Dairy Sci. 2011, 6, 134–141. [Google Scholar] [CrossRef] [Green Version]
- Ghorbani, B.; Vahdani, N.; Zerehdaren, S. Effects of niacin on milk production and blood parameters in early lactation of dairy cows. Pak. J. Biol Sci 2008, 11, 1582–1587. [Google Scholar] [CrossRef] [Green Version]
- Niehoff, I.D.; Hüther, L.; Lebzien, P.; Flachowsk, G. The effect of a Niacin supplementation to different diets on ruminal fermentation and flow of nutrients to the duodenum of dairy cows. Appl. Agric. For. Res. 2013, 63, 143–154. [Google Scholar]
- Samanta, A.K.; Kewalramani, N.; Kaur, H. Effect of niacin supplementationon VFA production and microbial protein synthesis in cattle. Indian J. Dairy Sci. 2000, 53, 150–153. [Google Scholar]
- Erickson, P.S.; Trusk, A.M.; Murphy, M.R. Effects of niacin source on epi-nephrine stimulation of plasma nonesterified Fatty acids and glucose concentrations, on diet digestibility and on rumen protozoal numbers in lactating dairy cows. J. Nutr. 1990, 120, 1648–1653. [Google Scholar] [CrossRef]
- Newbold, C.J.; McEwan, N.R.; Calza, R.E. An NAD+ dependent glutamate dehydrogenase cloned from the ruminal ciliate protozoan, Entodinium caudatum. FEMS Microbiol. Lett. 2005, 247, 113–121. [Google Scholar] [CrossRef] [Green Version]
- Jackson, T.M.; Rawling, J.M.; Roebuck, B.D.; Kirkland, J.B. Large supplements of nicotinic acid and nicotinamide increase tissue NAD+ and poly(ADP-ribose) levels but do not affect diethylnitrosamine-induced altered hepatic foci in Fischer-344 rats. J. Nutr. 1995, 125, 1455–1461. [Google Scholar]
- Sauve, A.A. NAD+ and Vitamin B3: From metabolism to therapies. J. Pharmacol. Exp. Ther. 2008, 324, 883–893. [Google Scholar] [CrossRef] [Green Version]
- Hara, N.; Yamada, K.; Shibata, T.; Osago, H.; Hashimoto, T.; Tsuchiya, M. Elevation of Cellular NAD Levels by Nicotinic Acid and Involvement of Nicotinic Acid Phosphoribosyltransferase in Human Cells. J. Biol. Chem. 2007, 28, 24574–24582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srikantia, S.G.; Narasinga Rao, B.S.; Raghuramulu, N.; Gopala, C. Pattern of nicotinamide nucleotides in the erythrocytes of pellagrins. Am. J. Clin. Nutr. 1968, 21, 1306–1309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amjad, S.; Nisar, S.; Bhat, A.A.; Frenneaux, M.P.; Fakhro, K.; Haris, M.; Reddy, R.; Patay, Z.; Baur, J.; Bagga, P. Role of NAD+ in regulating cellular and metabolic signaling pathways. Mol. Metab. 2021, 49, 101195. [Google Scholar] [CrossRef]
- Pollak, N.; Niere, M.; Ziegler, M. NAD kinase levels control the NADPH concentration in human cells. J. Biol Chem. 2007, 282, 33562–33571. [Google Scholar] [CrossRef] [Green Version]
- Gille, A.; Bodor, E.T.; Ahmed, K.; Offermanns, S. Nicotic acid: Pharmacological effects and mechanisms of action. Ann. Rev. Pharmacol. Toxicol. 2008, 48, 79–106. [Google Scholar] [CrossRef]
- Titgemeyer, E.C.; Mamedova, L.K.; Spivey, K.S.; Farney, J.K.; Bradford, B.J. An unusual distribution of the niacin receptor in cattle. J. Dairy Sci. 2011, 94, 4962–4967. [Google Scholar] [CrossRef] [Green Version]
- Jokinen, R.; Pirnes-Karhu, S.; Pietiläinen, K.H.; Pirinen, E. Adipose tissue NAD+-homeostasis, sirtuins and poly (ADP-ribose) polymerases-important players in mitochondrial metabolism and metabolic health. Redox Biol. 2017, 12, 246–263. [Google Scholar] [CrossRef]
- Vázquez-Meza, H.; de Piña, M.Z.; Pardo, J.P.; Riveros-Rosas, H.; Villalobos-Molina, R.; Piña, E. Non-steroidal anti-inflammatory drugs activate NADPH oxidase in adipocytes and raise the H2O2 pool to prevent cAMP-stimulated protein kinase a activation and inhibit lipolysis. BMC Biochem. 2013, 14, 13. [Google Scholar] [CrossRef] [Green Version]
- Baldwin, R.L.; Yang, Y.T.; Crist, K.; Grichting, G. Theoretical model of ruminant adipose tissue metabolism in relation to the whole animal. Fed. Proc. 1976, 35, 2314–2318. [Google Scholar] [PubMed]
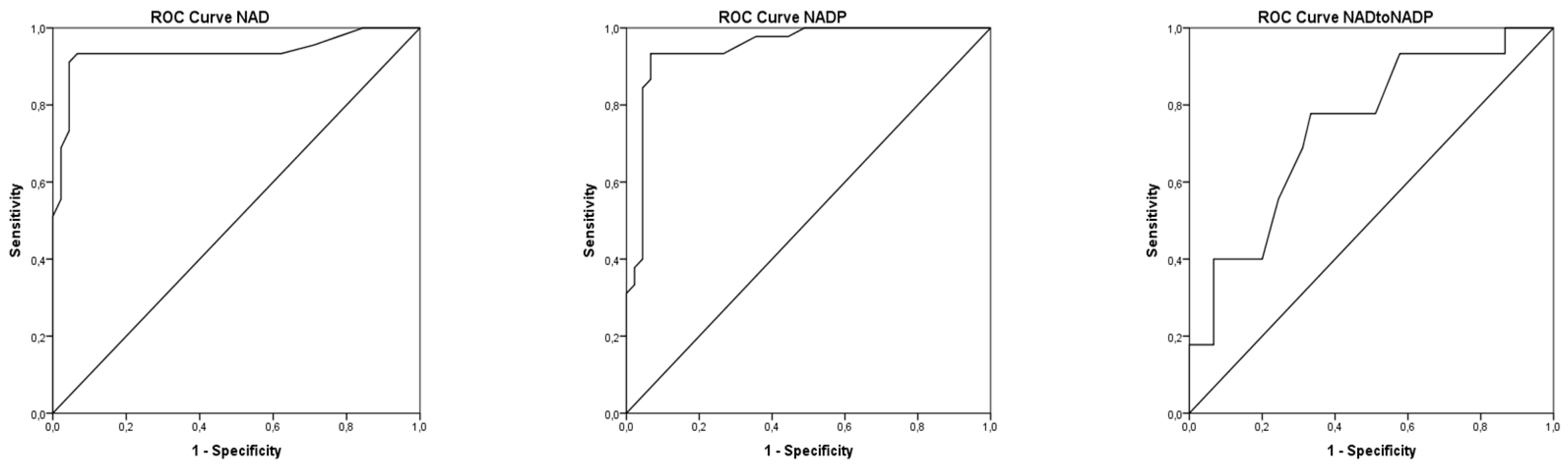
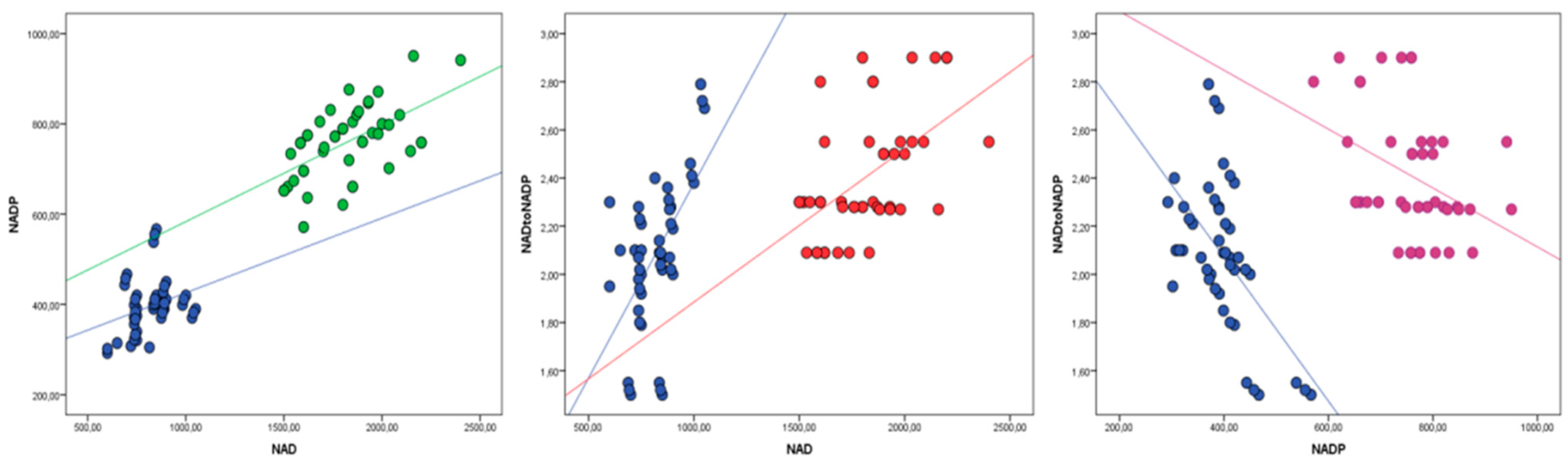
| Active Form of Niacin | Niacin | Control | p | ||
|---|---|---|---|---|---|
| Pretreatment | After Treatment | Pretreatment | After Treatment | ||
| NAD (nmol/L) | 809.1 ± 210.1 a | 1761.9 ± 344.8 b | 815.8 ± 199.1 a | 863.6 ± 217.8 a | ˂0.01 |
| NADP (nmol/L) | 399.4 ± 106.4 a | 737.8 ± 118.2 b | 400.3 ± 99.6 a | 412.5 ± 101.8 a | ˂0.01 |
| NAD:NADP | 2.02 ± 0.35 a | 2.38 ± 0.29 a | 2.03 ± 0.33 a | 2.11 ± 0.31 a | NS |
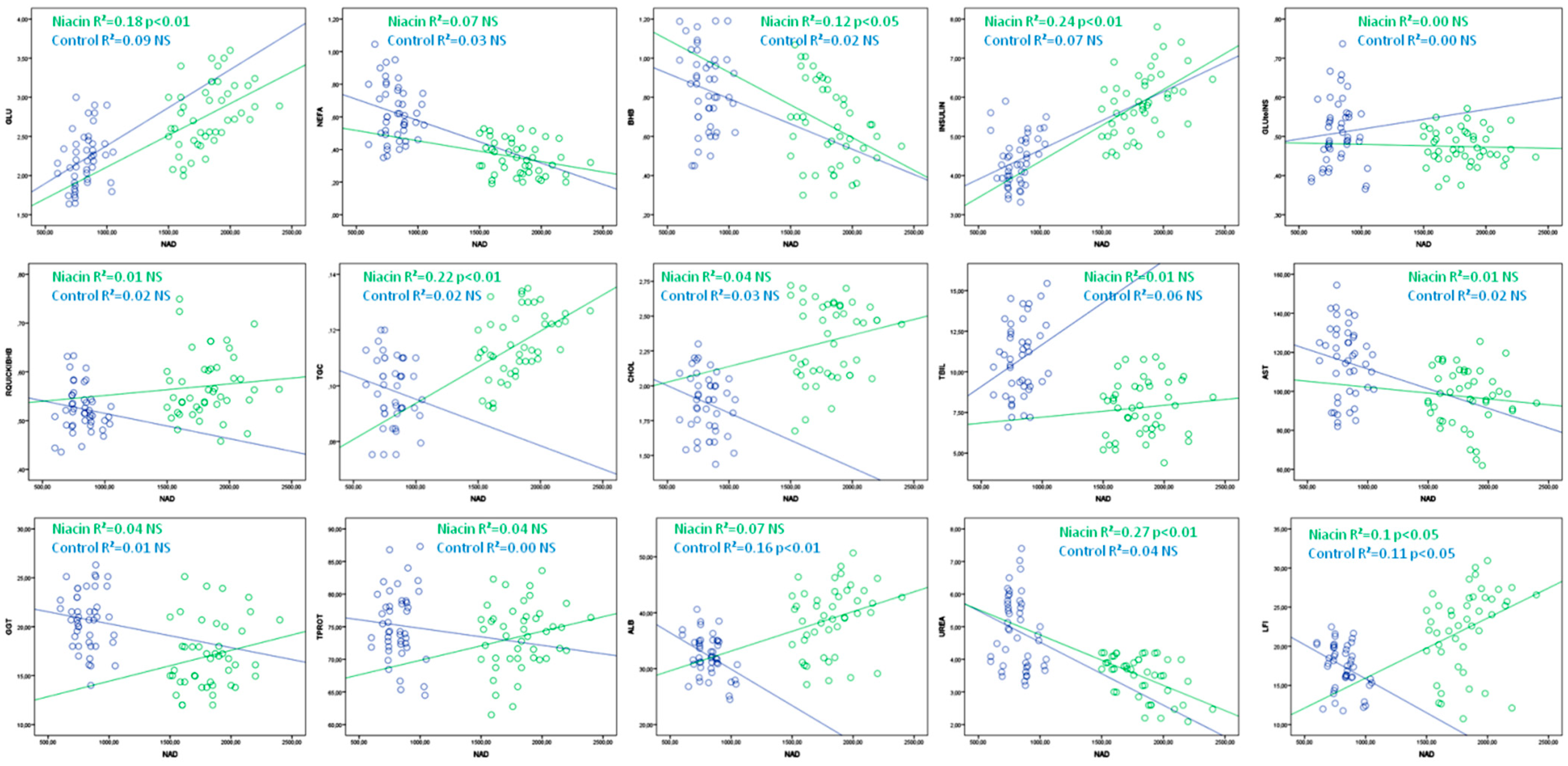
| Niacin | R2 | p | Control | R2 | p | |
|---|---|---|---|---|---|---|
| GLU | =1.3 + 0.0008 × NAD | 0.18 | ˂0.01 | =1.41 + 0.005 × NAD | 0.09 | NS |
| NEFA | =0.58 − 0.00013 × NAD | 0.07 | NS | =0.84 − 0.00061 × NAD | 0.03 | NS |
| BHB | =1.26 − 0.0003 × NAD | 0.12 | ˂0.05 | =1.05 − 0.00026 × NAD | 0.02 | NS |
| INSULIN | =2.52 + 0.0018 × NAD | 0.24 | ˂0.01 | =3.16 + 0.0015 × NAD | 0.07 | NS |
| GLU:INS | =0.49 − 0.00006 × NAD | 0.00 | NS | =0.47 + 0.00005 × NAD | 0.00 | NS |
| RQUICKIBHB | =0.53 + 0.00002 × NAD | 0.01 | NS | =0.57 − 0.00005 × NAD | 0.02 | NS |
| TGC | =0.07 + 0.00003 × NAD | 0.22 | ˂0.01 | =0.11 − 0.000017 × NAD | 0.02 | NS |
| CHOL | =1.91 + 0.00022 × NAD | 0.04 | NS | =2.2 − 0.0004 × NAD | 0.03 | NS |
| TBIL | =6.49 + 0.00073 × NAD | 0.01 | NS | =6.51 + 0.0052 × NAD | 0.06 | NS |
| AST | =108 − 0.006 × NAD | 0.01 | NS | =132 − 0.02 × NAD | 0.02 | NS |
| GGT | =11.28 + 0.0032 × NAD | 0.04 | NS | =22.74 − 0.0024 × NAD | 0.01 | NS |
| TPROT | =65.4 + 0.0045 × NAD | 0.04 | NS | =77.4 − 0.0022 × NAD | 0.00 | NS |
| ALB | =26.09 + 0.0071 × NAD | 0.07 | NS | =42.91 − 0.01 × NAD | 0.16 | ˂0.01 |
| UREA | =6.3 − 0.0016 × NAD | 0.27 | ˂0.01 | =6.46 – 0.0019 × NAD | 0.04 | NS |
| LFI | =8.28 + 0.0077 × NAD | 0.1 | ˂0.05 | =24.53 − 0.0087 × NAD | 0.11 | ˂0.05 |
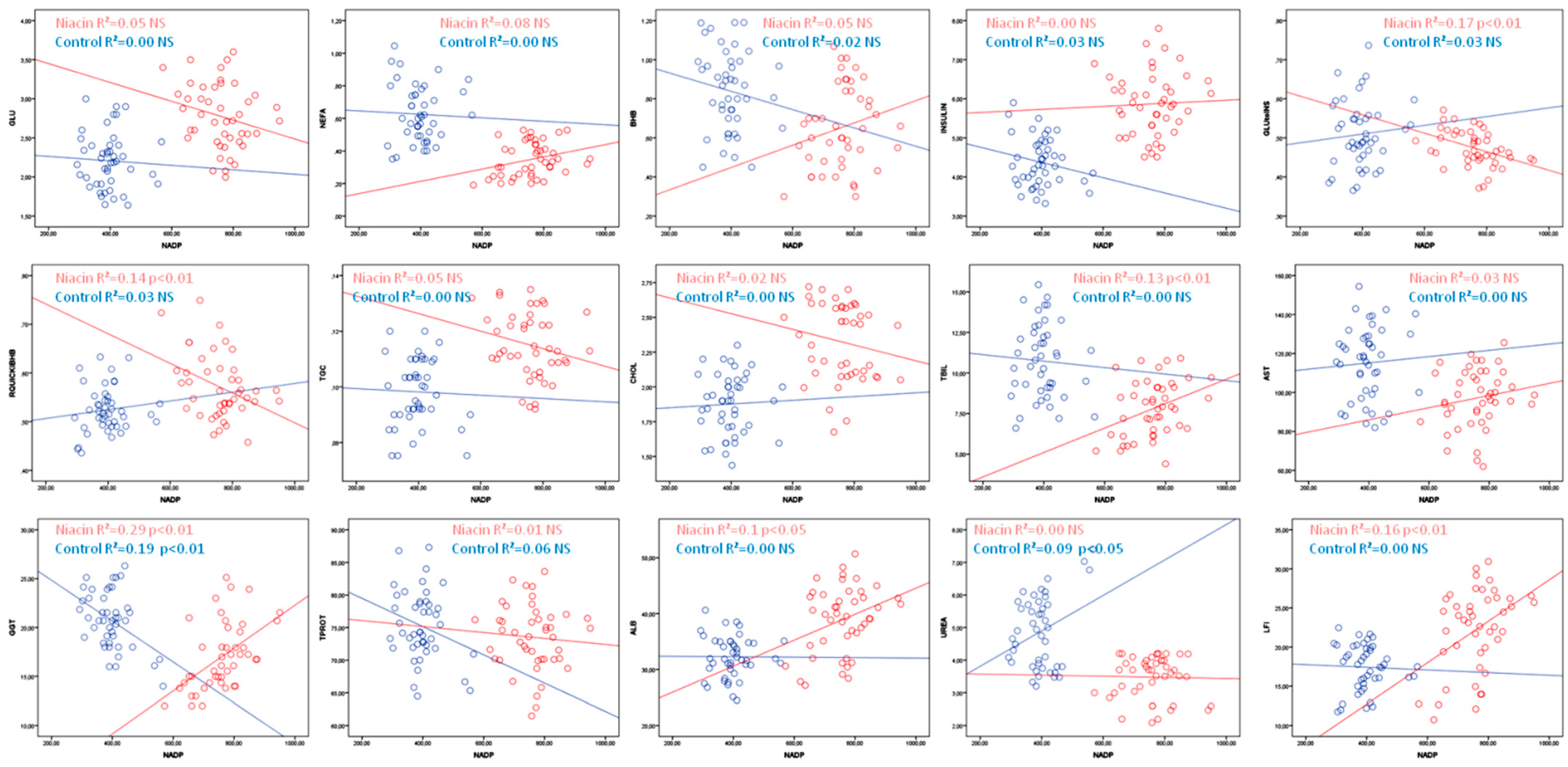
| NIACIN | R2 | p | CONTROL | R2 | p | |
|---|---|---|---|---|---|---|
| GLU | =3.69 − 0.0012 × NADP | 0.05 | NS | =2.32 − 0.000265 × NADP | 0.00 | NS |
| NEFA | =0.06 + 0.00038 × NADP | 0.08 | NS | =0.67 − 0.00016 × NADP | 0.00 | NS |
| BHB | =0.22 + 0.00057 × NADP | 0.05 | NS | =1.03 − 0.00047 × NADP | 0.02 | NS |
| INSULIN | =5.57 + 0.00039 × NADP | 0.00 | NS | =5.15 − 0.0019 × NADP | 0.03 | NS |
| GLU:INS | =0.65 − 0.00024 × NADP | 0.17 | ˂0.01 | =0.47 + 0.00011 × NADP | 0.01 | NS |
| RQUICKIBHB | =0.8 − 0.00031 × NADP | 0.14 | ˂0.01 | =0.49 + 0.00009 × NADP | 0.01 | NS |
| TGC | =0.14 − 0.000032 × NADP | 0.05 | NS | =0.1 − 0.000059 × NADP | 0.00 | NS |
| CHOL | =2.75 − 0.00057 × NADP | 0.02 | NS | =1.82 + 0.00034 × NADP | 0.00 | NS |
| TBIL | =2.09 + 0.0075 × NADP | 0.13 | ˂0.05 | =11.53 − 0.002 × NADP | 0.00 | NS |
| AST | =73.48 + 0.03 × NADP | 0.03 | NS | =109 + 0.02 × NADP | 0.00 | NS |
| GGT | =0.4 + 0.02 × NADP | 0.29 | ˂0.01 | =29.02 − 0.02 × NADP | 0.19 | ˂0.01 |
| TPROT | =76.99 − 0.0046 × NADP | 0.01 | NS | =83.81 − 0.02 × NADP | 0.06 | NS |
| ALB | =21.28 + 0.02 × NADP | 0.1 | ˂0.05 | =32.42 − 0.0004 × NADP | 0.00 | NS |
| UREA | =3.61 − 0.0002 × NADP | 0.00 | NS | =2.72 + 0.0054 × NADP | 0.09 | ˂0.05 |
| LFI | =2.14 + 0.03 × NADP | 0.16 | ˂0.01 | =18.1 − 0.0017 × NADP | 0.00 | NS |
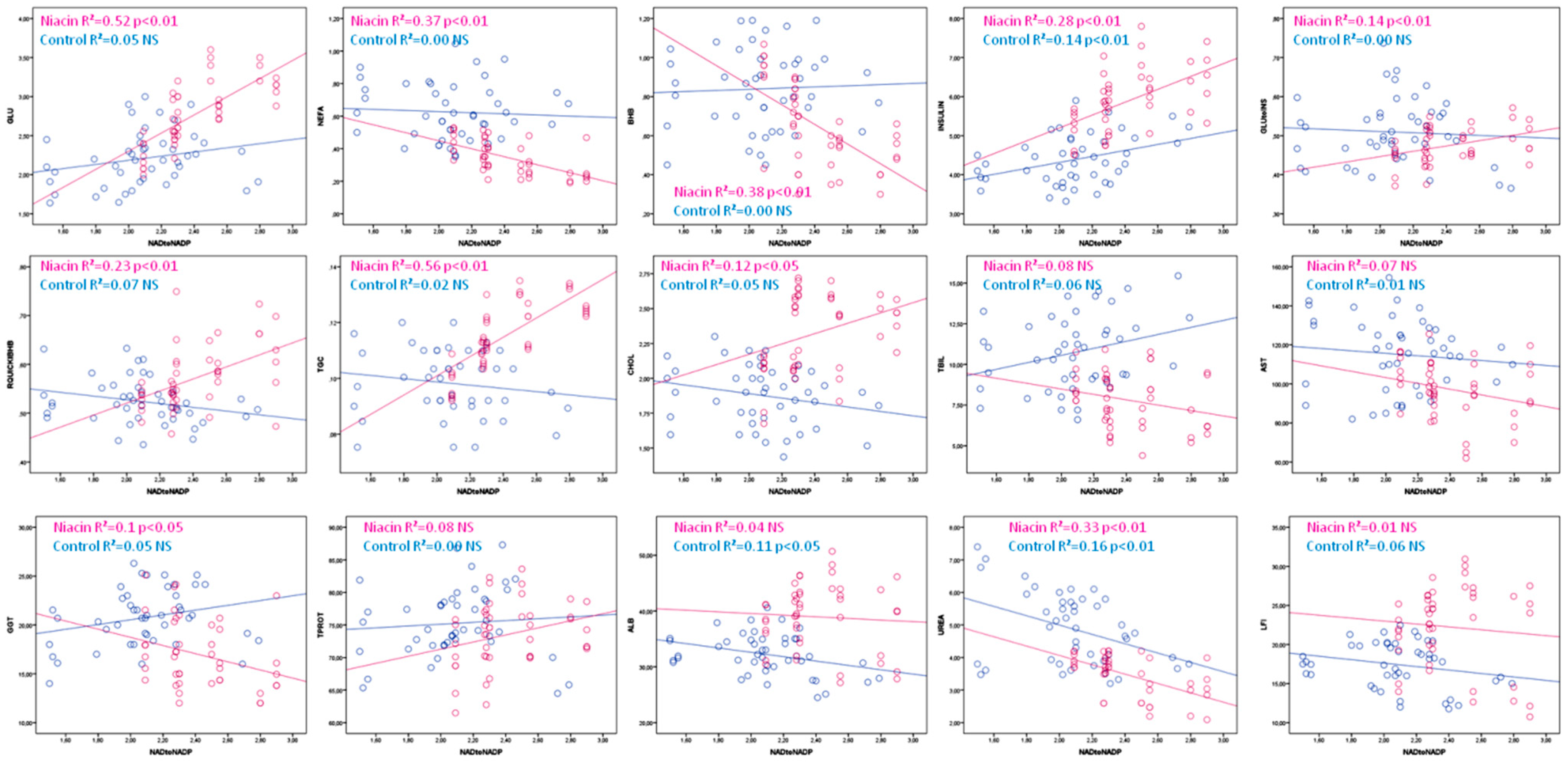
| Niacin | R2 | p | Control | R2 | p | |
|---|---|---|---|---|---|---|
| GLU | =−0.02 + 1.16 × NADtoNADP | 0.52 | ˂0.01 | =1.65 + 0.27 × NADtoNADP | 0.05 | NS |
| NEFA | =0.94 − 0.25 × NADtoNADP | 0.37 | ˂0.01 | =0.7 − 0.03 × NADtoNADP | 0.00 | NS |
| BHB | =1.86 − 0.5 × NADtoNADP | 0.38 | ˂0.01 | =0.78 + 0.03 × NADtoNADP | 0.00 | NS |
| INSULIN | =1.91 + 1.7 × NADtoNADP | 0.28 | ˂0.01 | =2.8 + 0.77 × NADtoNADP | 0.14 | ˂0.01 |
| GLU:INS | =0.3 + 0.07 × NADtoNADP | 0.14 | ˂0.01 | =0.54 − 0.02 × NADtoNADP | 0.00 | NS |
| RQUICKIBHB | =0.27 + 0.12 × NADtoNADP | 0.23 | ˂0.01 | =0.6 − 0.04 × NADtoNADP | 0.07 | NS |
| TGC | =0.03 + 0.03 × NADtoNADP | 0.56 | ˂0.01 | =0.11 − 0.005 × NADtoNADP | 0.02 | NS |
| CHOL | =1.43 + 0.37 × NADtoNADP | 0.12 | ˂0.05 | =2.21 − 0.16 × NADtoNADP | 0.05 | NS |
| TBIL | =11.8 − 1.7 × NADtoNADP | 0.08 | NS | =6.26 + 2.15 × NADtoNADP | 0.06 | NS |
| AST | =133 − 14.9 × NADtoNADP | 0.07 | NS | =128 − 6.18 × NADtoNADP | 0.01 | NS |
| GGT | =27.1 − 4.2 × NADtoNADP | 0.1 | ˂0.05 | =15.6 + 2.5 × NADtoNADP | 0.07 | NS |
| TPROT | =60.4 + 5.45 × NADtoNADP | 0.08 | NS | =72.34 + 1.4 × NADtoNADP | 0.00 | NS |
| ALB | =42.5 − 1.47 × NADtoNADP | 0.04 | NS | =40.4 − 3.9 × NADtoNADP | 0.11 | ˂0.05 |
| UREA | =6.93 − 1.44 × NADtoNADP | 0.33 | ˂0.01 | =7.86 − 1.43 × NADtoNADP | 0.16 | ˂0.01 |
| LFI | =26.7 − 1.9 × NADtoNADP | 0.01 | NS | =22.03 − 2.21 × NADtoNADP | 0.06 | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrović, K.; Djoković, R.; Cincović, M.; Hristovska, T.; Lalović, M.; Petrović, M.; Majkić, M.; Došenović Marinković, M.; Anđušić, L.; Devečerski, G.; et al. Niacin Status Indicators and Their Relationship with Metabolic Parameters in Dairy Cows during Early Lactation. Animals 2022, 12, 1524. https://doi.org/10.3390/ani12121524
Petrović K, Djoković R, Cincović M, Hristovska T, Lalović M, Petrović M, Majkić M, Došenović Marinković M, Anđušić L, Devečerski G, et al. Niacin Status Indicators and Their Relationship with Metabolic Parameters in Dairy Cows during Early Lactation. Animals. 2022; 12(12):1524. https://doi.org/10.3390/ani12121524
Chicago/Turabian StylePetrović, Kosta, Radojica Djoković, Marko Cincović, Talija Hristovska, Miroslav Lalović, Miloš Petrović, Mira Majkić, Maja Došenović Marinković, Ljiljana Anđušić, Gordana Devečerski, and et al. 2022. "Niacin Status Indicators and Their Relationship with Metabolic Parameters in Dairy Cows during Early Lactation" Animals 12, no. 12: 1524. https://doi.org/10.3390/ani12121524
APA StylePetrović, K., Djoković, R., Cincović, M., Hristovska, T., Lalović, M., Petrović, M., Majkić, M., Došenović Marinković, M., Anđušić, L., Devečerski, G., Stojanović, D., & Štrbac, F. (2022). Niacin Status Indicators and Their Relationship with Metabolic Parameters in Dairy Cows during Early Lactation. Animals, 12(12), 1524. https://doi.org/10.3390/ani12121524







