Quality Assessment of Day-Old Chickens on the Broiler Farms of Hong Kong
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and Laboratory Testing
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van de Ven, L.; Van Wagenberg, A.; Uitdehaag, K.; Koerkamp, P.G.; Kemp, B.; Van den Brand, H. Significance of chick quality score in broiler production. Animal 2012, 6, 1677–1683. [Google Scholar] [CrossRef] [PubMed]
- Sozcu, A.; Ipek, A. Quality assessment chicks from different hatcher temperatures with different scoring methods and prediction of broiler growth performance. J. Appl. Anim. Res. 2015, 43, 409–416. [Google Scholar] [CrossRef] [Green Version]
- BMT Asia Pacific. Study on the way forward of live poultry trade in Hong Kong. Food Health Bur. 2017, 5, R9191. [Google Scholar]
- Agriculture, Fisheries & Conservation Department. Agriculture and Fisheries. 2020. Available online: https://www.gov.hk/en/about/abouthk/factsheets/docs/agriculture.pdf (accessed on 5 May 2022).
- Willemsen, H.; Everaert, N.; Witters, A.; De Smit, L.; Debonne, M.; Verschuere, F.; Garain, P.; Berckmans, D.; Decuypere, E.; Bruggeman, V. Critical assessment of chick quality measurements as an indicator of posthatch performance. Poult. Sci. 2008, 87, 2358–2366. [Google Scholar] [CrossRef]
- Foote, M. Chick Quality. 2019. Available online: https://www.cobb-vantress.com/assets/EMEA-Best-Management-Practices/4ba333d62d/Mark-Foote-Chick-Quality-Article-2019.pdf (accessed on 5 May 2022).
- Yeboah, P.; Konadu, L.; Hamidu, J.; Poku, E.; Wakpal, D.; Kudaya, P.; Dey, A.; Siddiq, S. Comparative analysis of hatcheries contribution to poor development of day-old chicks based on biological and immunological performance. Vet. World 2019, 12, 1849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, T.-S. Custom, taste and science: Raising chickens in the Pearl River Delta Region, South China. Anthropol. Med. 2008, 15, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Stadig, L.M.; Rodenburg, T.B.; Reubens, B.; Aerts, J.; Duquenne, B.; Tuyttens, F.A. Effects of free-range access on production parameters and meat quality, composition and taste in slow-growing broiler chickens. Poult. Sci. 2016, 95, 2971–2978. [Google Scholar] [CrossRef] [PubMed]
- Rayner, A.C.; Newberry, R.C.; Vas, J.; Mullan, S. Slow-growing broilers are healthier and express more behavioural indicators of positive welfare. Sci. Rep. 2020, 10, 15151. [Google Scholar] [CrossRef] [PubMed]
- Fasenko, G.; O’Dea, E. Evaluating broiler growth and mortality in chicks with minor navel conditions at hatching. Poult. Sci. 2008, 87, 594–597. [Google Scholar] [CrossRef] [PubMed]
- Grafl, B.; Gaußmann, B.; Sulejmanovic, T.; Hess, C.; Hess, M. Risks and disease aetiologies of compromised performance in commercial broilers kept at lower stocking density and limited antimicrobial use. Avian Pathol. 2020, 49, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Sander, J. Omphalitis in Poultry. In MSD Veterinary Manual; Merck & Co., Inc.: Rahway, NJ, USA, 2019; Available online: https://www.msdvetmanual.com/poultry/omphalitis/omphalitis-in-poultry (accessed on 5 May 2022).
- Powell, J.; Bowman, J. An estimate of maternal effects in early growth characteristics and their effects upon comparative tests of chicken varieties. Br. Poult. Sci. 1964, 5, 121–132. [Google Scholar] [CrossRef]
- McLoughlin, L.; Gous, R. The effect of egg size on pre-and post-natal growth of broiler chickens. Poult. Bull. 1999, 15, 104–107. [Google Scholar]
- Tona, K.; Onagbesan, O.; De Ketelaere, B.; Decuypere, E.; Bruggeman, V. Effects of age of broiler breeders and egg storage on egg quality, hatchability, chick quality, chick weight, and chick posthatch growth to forty-two days. J. Appl. Poult. Res. 2004, 13, 10–18. [Google Scholar] [CrossRef]
- Wolanski, N.; Luiten, E.; Meijerhof, R.; Vereijken, A. Yolk utilisation and chick length as parameters for embryo development. Avian Poult. Biol. Rev. 2004, 15, 233–234. [Google Scholar] [CrossRef]
- Hill, D. Chick length uniformity profiles as a field measurement of chick quality. Avian Poult. Biol. Rev 2001, 12, 188. [Google Scholar]
- Meijerhof, R. Chick size matters. World Poult 2006, 22, 30–31. [Google Scholar]
- Molenaar, R.; Reijrink, I.; Meijerhof, R.; Van den Brand, H. Relationship between chick length and chick weight at hatch and slaughter weight and breast meat yield in broilers. In Proceedings of the 3rd Combined Workshop on Fundamental Physiology and Perinatal Development in Poultry, Berlin, Germany, 5–17 October 2007. [Google Scholar]
- Wolanski, N.; Renema, R.; Robinson, F.; Carney, V.; Fancher, B. Relationship between chick conformation and quality measures with early growth traits in males of eight selected pure or commercial broiler breeder strains. Poult. Sci. 2006, 85, 1490–1497. [Google Scholar] [CrossRef] [PubMed]
- Van der Wagt, I.; de Jong, I.C.; Mitchell, M.A.; Molenaar, R.; van den Brand, H. A review on yolk sac utilization in poultry. Poult. Sci. 2020, 99, 2162–2175. [Google Scholar] [CrossRef] [PubMed]
- Gharaibeh, S.; Mahmoud, K. Decay of maternal antibodies in broiler chickens. Poult. Sci. 2013, 92, 2333–2336. [Google Scholar] [CrossRef] [PubMed]
- Shane, S. Health and performance of poultry in tropical climates. In ASA Handbook on Poultry Diseases, 2nd ed.; American Soybean Association: Singapore, 2005; pp. 13–20. [Google Scholar]
- Block, H.; Meyer-Block, K.; Rebeski, D.E.; Scharr, H.; de Wit, S.; Rohn, K.; Rautenschlein, S. A field study on the significance of vaccination against infectious bursal disease virus (IBDV) at the optimal time point in broiler flocks with maternally derived IBDV antibodies. Avian Pathol. 2007, 36, 401–409. [Google Scholar] [CrossRef]
- World Organisation for Animal Health. Newcastle Disease. 2013. Available online: https://www.oie.int/fileadmin/Home/eng/Animal_Health_in_the_World/docs/pdf/Disease_cards/NEWCASTLE_DISEASE.pdf (accessed on 5 May 2022).

| Parameter | Age (Day) 1 | Level | Source | Total | |
|---|---|---|---|---|---|
| Imported | Local | ||||
| Navel quality | 1 | 976 | |||
| Abnormal | 141 | 310 | |||
| Normal | 290 | 235 | |||
| 3 | 448 | ||||
| Abnormal | 81 | 112 | |||
| Normal | 183 | 72 | |||
| Dehydrated | 1 | 831 | |||
| No | 330 | 378 | |||
| Yes | 29 | 94 | |||
| 3 | 423 | ||||
| No | 240 | 125 | |||
| Yes | 0 | 58 | |||
| Crop-filled | 3 | 444 | |||
| No | 39 | 96 | |||
| Yes | 221 | 88 | |||
| Variable | Age (Day) 1 | Source | Number of Batches | Mean | SD | p-Value 2 |
|---|---|---|---|---|---|---|
| Length (cm) | 1 | I | 20 | 18.9 | 1.3 | 0.017 |
| L | 25 | 18.1 | 0.8 | |||
| Body weight (g) | 1 | I | 20 | 35.0 | 2.2 | 0.016 |
| L | 25 | 33.6 | 1.6 | |||
| Yolk sac residual (g) | 3 | I | 11 | 1.1 | 0.5 | 0.265 |
| L | 8 | 0.9 | 0.3 | |||
| Net weight (g) | 3 | I | 11 | 49.6 | 7.1 | <0.001 |
| L | 8 | 35.6 | 5.4 | |||
| CV of length (%) | 1 | I | 20 | 3.2 | 0.18 | 0.025 |
| L | 25 | 3.9 | 0.21 | |||
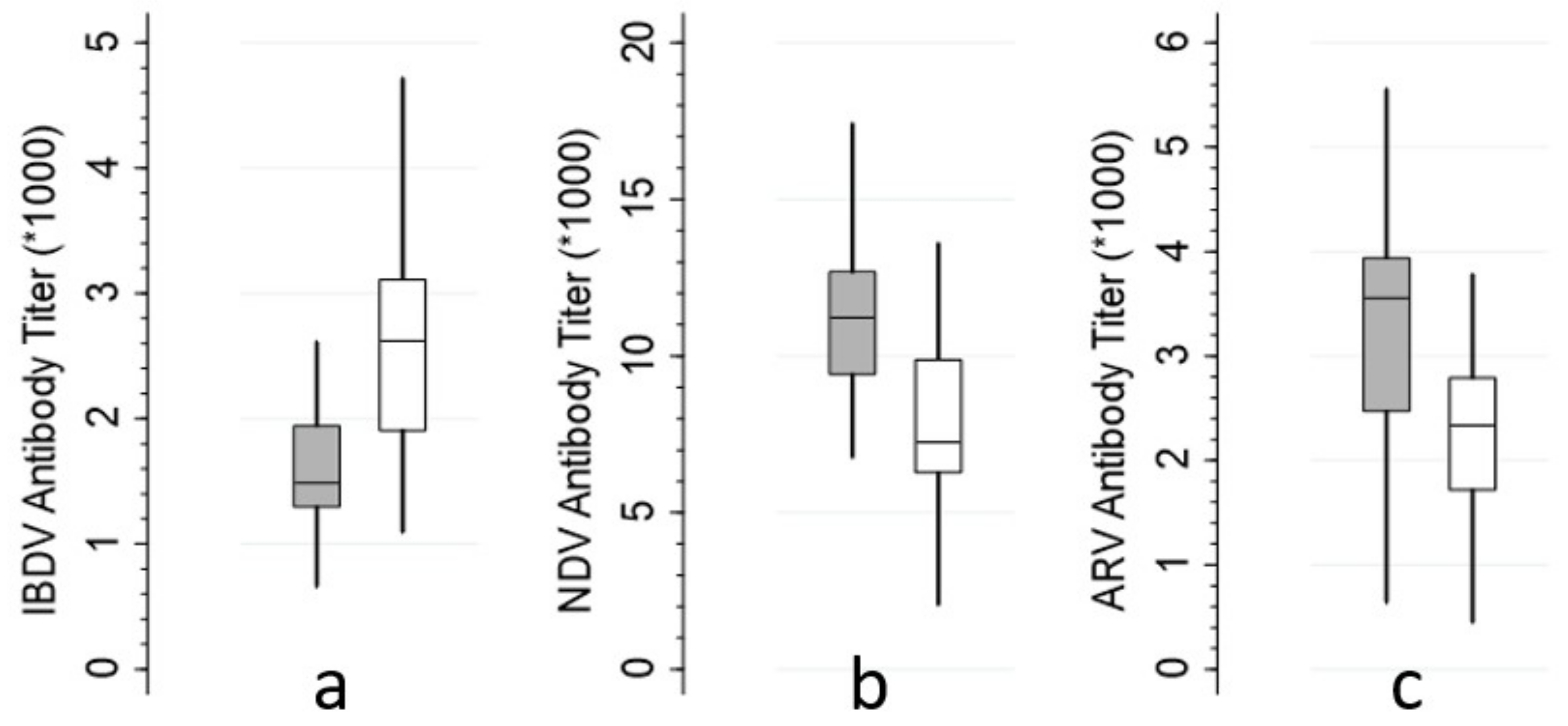
| IBDV titer | 1 & 3 | I | 33 | 1756.9 | 848.4 | <0.001 |
| L | 36 | 2680.1 | 891.3 | |||
| NDV titer | 1 & 3 | I | 33 | 11,255.6 | 3278.8 | <0.001 |
| L | 36 | 7664.3 | 2831.9 | |||
| ARV titer | 1 & 3 | I | 33 | 3781.2 | 2121.1 | 0.001 |
| L | 35 | 2341.5 | 1023.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nekouei, O.; Yau, D.; MacKinnon, B.; Magouras, I.; Conan, A.; Elsohaby, I.; Paudel, S.; Pfeiffer, D.U. Quality Assessment of Day-Old Chickens on the Broiler Farms of Hong Kong. Animals 2022, 12, 1520. https://doi.org/10.3390/ani12121520
Nekouei O, Yau D, MacKinnon B, Magouras I, Conan A, Elsohaby I, Paudel S, Pfeiffer DU. Quality Assessment of Day-Old Chickens on the Broiler Farms of Hong Kong. Animals. 2022; 12(12):1520. https://doi.org/10.3390/ani12121520
Chicago/Turabian StyleNekouei, Omid, Denis Yau, Brett MacKinnon, Ioannis Magouras, Anne Conan, Ibrahim Elsohaby, Surya Paudel, and Dirk U. Pfeiffer. 2022. "Quality Assessment of Day-Old Chickens on the Broiler Farms of Hong Kong" Animals 12, no. 12: 1520. https://doi.org/10.3390/ani12121520
APA StyleNekouei, O., Yau, D., MacKinnon, B., Magouras, I., Conan, A., Elsohaby, I., Paudel, S., & Pfeiffer, D. U. (2022). Quality Assessment of Day-Old Chickens on the Broiler Farms of Hong Kong. Animals, 12(12), 1520. https://doi.org/10.3390/ani12121520









