Small Animals, Big Impact? Early Farmers and Pre- and Post-Harvest Pests from the Middle Neolithic Site of Les Bagnoles in the South-East of France (L’Isle-sur-la-Sorgue, Vaucluse, Provence-Alpes-Côte-d’Azur)
Abstract
Simple Summary
Abstract
1. Introduction
1.1. Prehistoric Crop Pests: An Overview
1.1.1. Rodents
1.1.2. Invertebrates
2. Materials and Methods
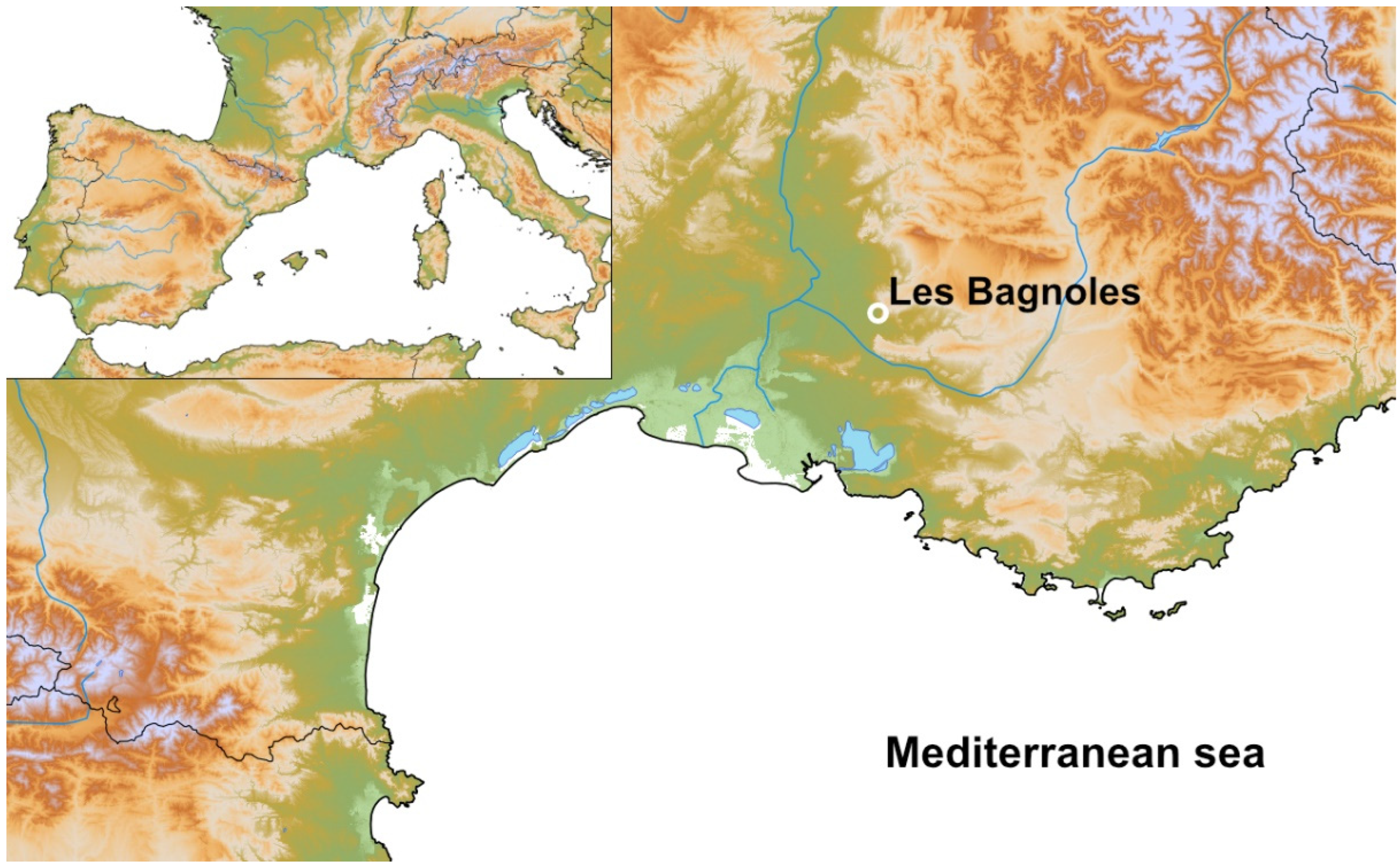
2.1. The Archaeological Site of Les Bagnoles, Its Three Middle Neolithic Wells (Structures 250, 990, 994), and Their Contexts
2.2. The Fill of the Wells, Preservation, and Taphonomical Aspects
2.3. Archaeozoological Material and Analysis
3. Results
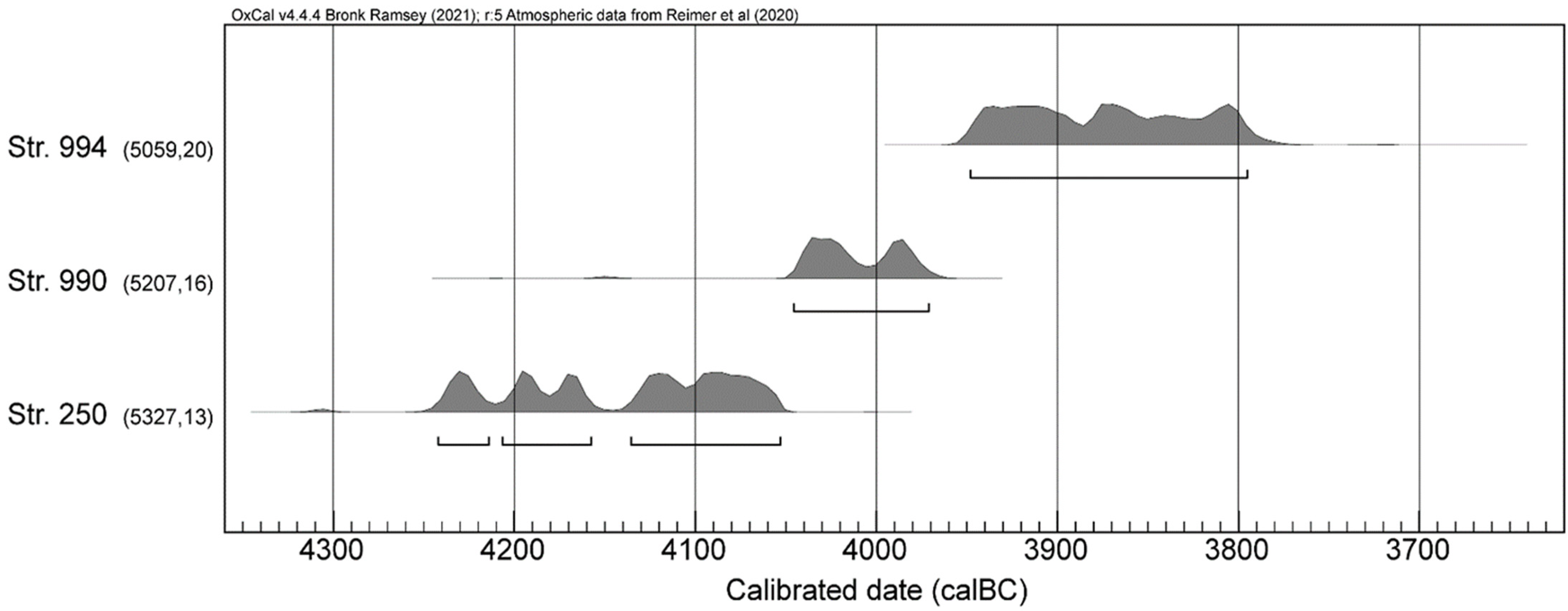
3.1. Radiocarbon Dating
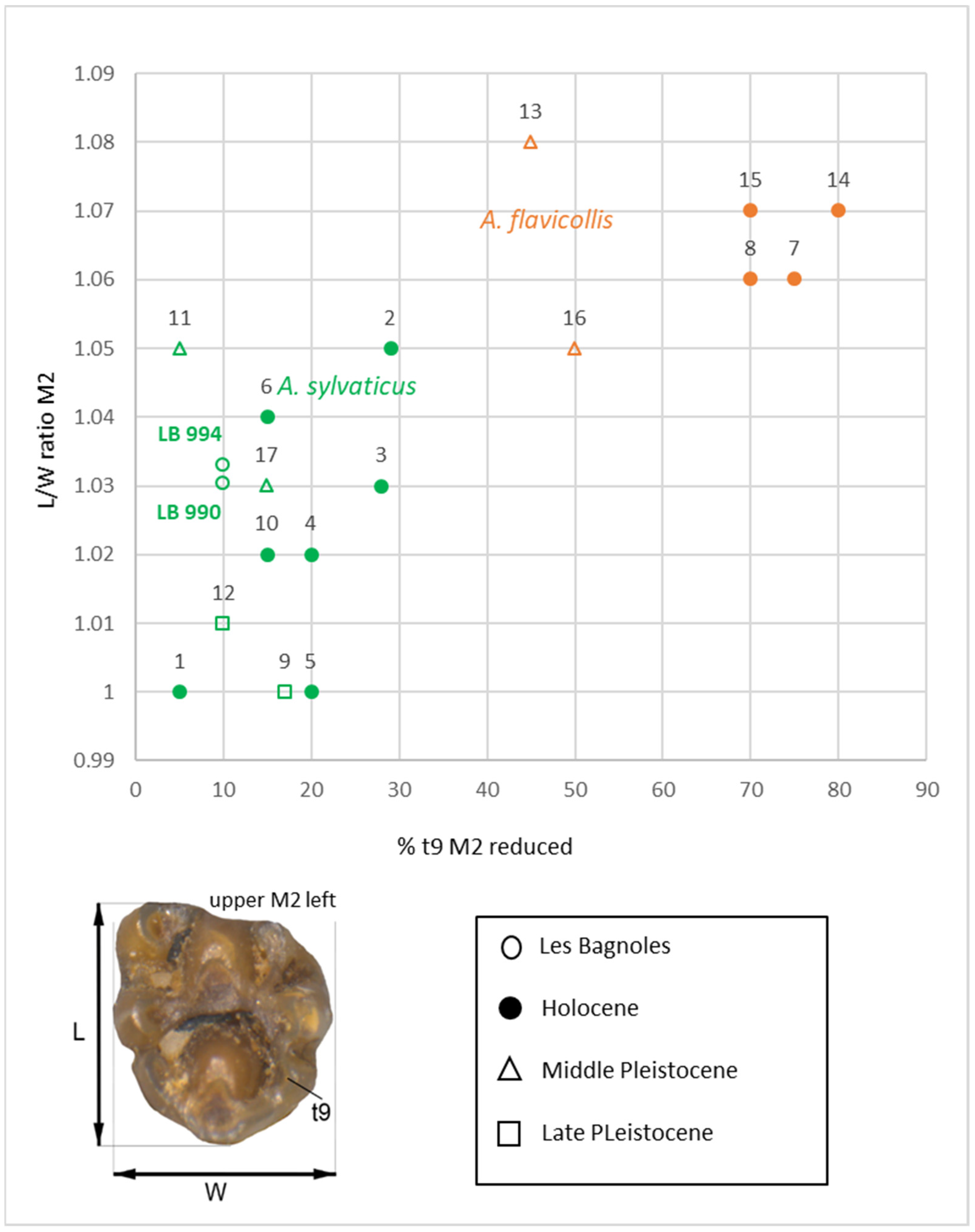
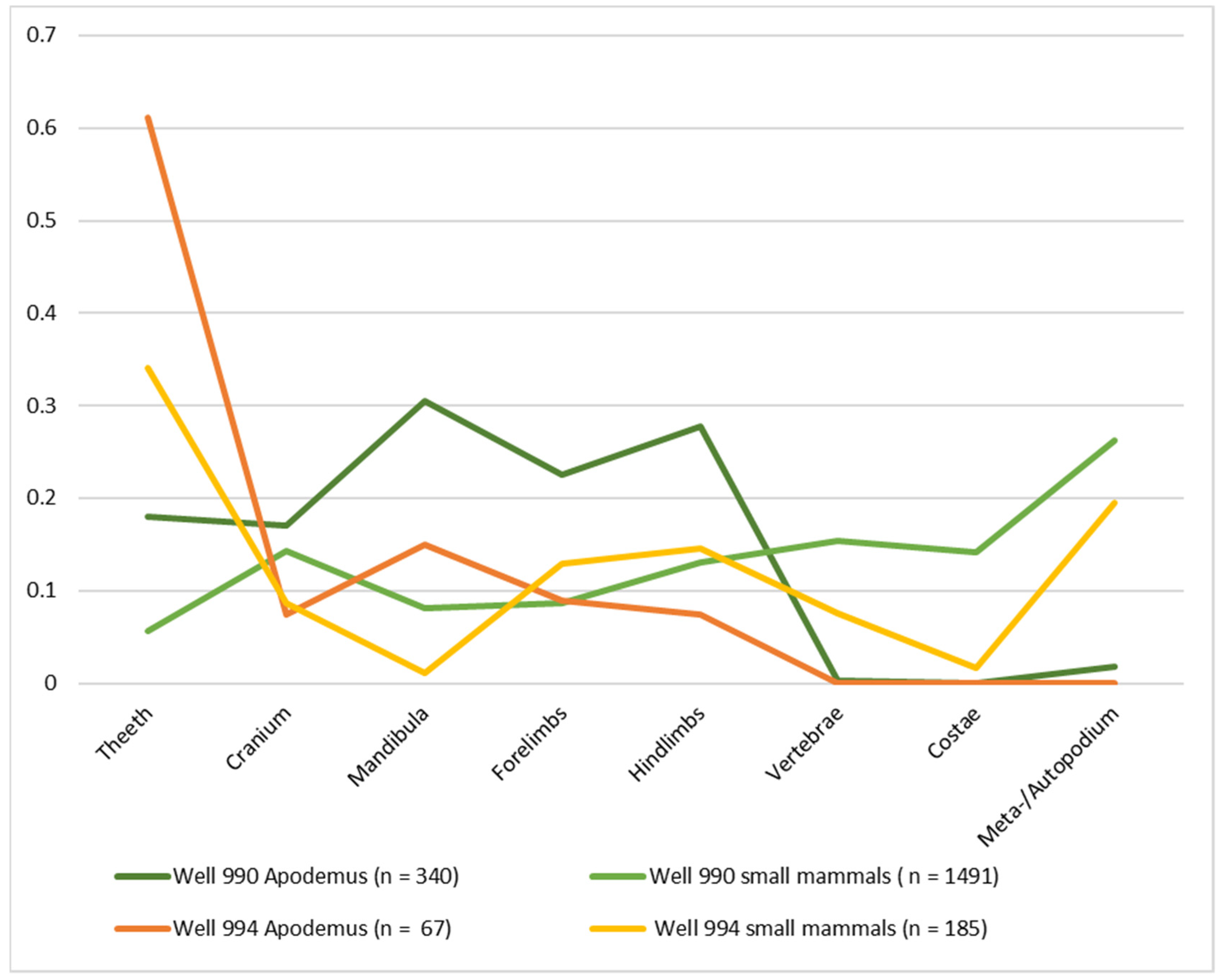
3.2. Microvertebrates
3.3. Invertebrates
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gronenborn, D. Climate fluctuations and trajectories to complexity in the Neolithic: Towards a theory. Doc. Praehist. 2009, 36, 97–110. [Google Scholar] [CrossRef]
- Antolín, F.; Häberle, S.; Jesus, A.; Martinez-Grau, H.; Prats, G.; Schäfer, M.; Steiner, B. The AgriChange project: An integrated on-site approach to agricultural and land-use change during the Neolithic in Western Europe. PAGES News-Past Glob. Chang. Mag. 2018, 26, 26–27. [Google Scholar]
- Martin, L.; Bouby, L.; Marinval, P.; Dietsch-Sellami, M.-F.; Rousselet, O.; Cabanis, M.; Durand, F.; Figueiral, I. L’exploitation des ressources végétales durant le Chasséen: Un bilan des données carpologiques en France entre 4400 et 3500 avant notre ère. In Le Chasséen, des Chasséens… Retour sur une Culture Nationale et ses Parallèles: Sepulcres de fossa, Proceedings of the Cortaillod, Lagozza Colloque International, Paris, France, 18–20 Novembre 2014; Perrin, T., Chambon, P., Gibaja, J.F., Goude, G., Eds.; Archives d’Écologie Préhistorique: Toulouse, France, 2016; pp. 259–272. [Google Scholar]
- Marston, J.M. Archaeological markers of agricultural risk management. J. Anthr. Archaeol. 2011, 30, 190–205. [Google Scholar] [CrossRef]
- Reichmuth, C.H. Vorratschädlinge im Getreide Aussehen-Biologie-Schadbild-Bekämpfung; Mann Verlag: Gelsenkirchen, Germany, 1997. [Google Scholar]
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef]
- Belmaker, M.; Brown, B. Chapter 2. A new look at «on mice and men»: Should commensal species be used as a universal indicator of sedentism? In Bones and Identity. Zooarchaeological Approaches to Reconstructing Social and Cultural Landscapes in Southwest Asia; Marom, N., Yeshuran, R., Weissbro, L., Bar-Oz, G., Eds.; Oxbow Books: Oxford, UK, 2016. [Google Scholar]
- Panagiotakopulu, E.; Buckland, P.C. A thousand bites—Insect introductions and late Holocene environments. Quat. Sci. Rev. 2017, 156, 23–35. [Google Scholar] [CrossRef]
- Cucchi, T.; Papayianni, K.; Cersoy, S.; Aznar-Cormano, L.; Zazzo, A.; Debruyne, R.; Berthon, R.; Bălășescu, A.; Simmons, A.; Valla, F.; et al. Tracking the Near Eastern origins and European dispersal of the western house mouse. Sci. Rep. 2020, 10, 8276. [Google Scholar] [CrossRef]
- Preece, C.; Livarda, A.; Christin, P.-A.; Wallace, M.; Martin, G.; Charles, M.; Jones, G.; Rees, M.; Osborne, C.P. How did the domestication of Fertile Crescent grain crops increase their yields? Funct. Ecol. 2017, 31, 387–397. [Google Scholar] [CrossRef]
- de Vareilles, A.; Bouby, L.; Jesus, A.; Martin, L.; Rottoli, M.; Linden, M.V.; Antolín, F. One sea but many routes to Sail. The early maritime dispersal of Neolithic crops from the Aegean to the western Mediterranean. J. Archaeol. Sci. Rep. 2019, 29, 102140. [Google Scholar] [CrossRef]
- Jesus, A.; Prats, G.; Follmann, F.; Jacomet, S.; Antolín, F. Middle Neolithic farming of open-air sites in SE France: New insights from archaeobotanical investigations of three wells found at Les Bagnoles (L’Isle-sur-la-Sorgue, Dépt. Vaucluse, France). Veg. Hist. Archaeobotany 2020, 30, 445–461. [Google Scholar] [CrossRef]
- Magny, M.; Haas, J.N. A major widespread climatic change around 5300 cal. yr BP at the time of the Alpine Iceman. J. Quat. Sci. 2004, 19, 423–430. [Google Scholar] [CrossRef]
- Delhon, C.; Thiébault, S.; Berger, J.-F. Environment and landscape management during the Middle Neolithic in Southern France: Evidence for agro-sylvo-pastoral systems in the Middle Rhone Valley. Quat. Int. 2009, 200, 50–65. [Google Scholar] [CrossRef]
- André, G.; Antolín, F.; Bader, M.; Bailly, M.; Denaire, A.; Follmann, F.; Jacomet, S.; Jesus, A.; Quesnel, Y.; Reggio, A.; et al. Déroulement des opérations et moyens mis en œuvre. In Les Bagnoles à L’Isle-sur-la-Sorgue—Un Site Majeur du Néolithique Moyen en Vaucluse; van Willigen, S., Bailly, M., Röder, B., Schibler, J., Schmitt, A., Eds.; Presses Universitaires de Provence: Aix-en-Provence, France, 2020; pp. 87–100. [Google Scholar]
- Brochier, J.E.; D’Anna, A.; Guendon, J.-L.; Hélard, B.; Jacomet, S.; Rentzel, P.H.; Reggio, A.; Röder, B.; van Willigen, S. Environment et formation du site. In Les Bagnoles à L’Isle-sur-la-Sorgue (Vaucluse)—Un Site Majeur du Néolithique Moyen en Vaucluse; van Willigen, S., Bailly, M., Röder, B., Schibler, J., Schmitt, A., Eds.; Presses Universitaires de Provence: Aix-en-Provence, France, 2020; pp. 37–86. [Google Scholar]
- Beeching, A. La fin du Chasséen et le Néolithique final dans le bassin du Rhône moyen. In Il Declino del Mondo Neolitico. Ricerche in Italia Centro-Settentrionale fra Aspetti Peninsulari, Occidentali e Nord-Alpini; Quaderni del Museo Archeologico del Friuli Occidentale, 4; Ferrari, A., Visentini, P., Eds.; Museo delle Scienze: Pordenone, Italy, 2002; pp. 67–83. [Google Scholar]
- Berger, J.-F.; Shennan, S.; Woodbridge, J.; Palmisano, A.; Mazier, F.; Nuninger, L.; Guillon, S.; Doyen, E.; Begeot, C.; Andrieu-Ponel, V.; et al. Holocene land cover and population dynamics in Southern France. Holocene 2019, 29, 776–798. [Google Scholar] [CrossRef]
- Thirault, É.; Remicourt, M.; Vannieuwenhuyse, D. Les puits à eau néolithiques dans le Sud de la France: Une Question à Creuser. In Méthodologie des Recherches de Terrain sur la Préhistoire Récente en France. Nouveaux Acquis, Nouveaux Outils (1987–2012). Actes des Premières Rencontres Nord-Sud de Préhistoire Récente, Marseille, Mai 2012; Sénépart, I., Billard, C., Bostyn, F., Praud, I., Thirault, E., Eds.; Archives d’Écologie Préhistorique: Toulouse, France, 2014; pp. 231–250. [Google Scholar]
- Remicourt, M.; Andrieu-Ponel, V.; Audibert, C.; Baradat, A.; Battentier, J.; Blaise, É.; Bonnardin, S.; Caverne, J.-B.; Fernandes, P.; Furestier, R.; et al. Les occupations pré-et protohisoriques du Clos de Roque à Saint-Maximin-la-Sainte-Baume dans le Var. In Chronologie de la Préhistoire Récente dans le Sud de la France. Acquis 1992–2012. Actualité de la Recherche. Actes des 10e Rencontres Méridionales de Préhistoire Récente; Sénépart, I., Leandri, F., Cauliez, J., Perrin, T., Thirault, É., Eds.; Archives d’Écologie Préhistorique: Toulouse, France, 2014; pp. 523–548. [Google Scholar]
- Chaline, J.M.; Baudvin, H.; Jammot, D.; Saint Girons, M.S. Les Proies des Rapaces, Petits Mammifères et Leur Environnement; Doin: Paris, France, 1974. [Google Scholar]
- Andrew, P. Owls, Caves and Fossils. Predation, Preservation and Accumulation of Small Mammal Bones in Caves, with an Analysis of the Pleistocene Cave Faunas from Westbury-Sub-Mendip, Somerset, U.K.; University of Chicago Press: Chicago, IL, USA, 1990. [Google Scholar]
- Van Willigen, S.; Bailly, M.; Röder, B.; Schibler, J.; Schmitt, A. (Eds.) Les Bagnoles à L’Isle-sur-la-Sorgue (Vaucluse)—Un Site Majeur du Néolithique Moyen en Vaucluse; Presses Universitaires de Provence: Aix-en-Provence, France, 2020. [Google Scholar]
- Antolin, F.; Schmitt, A.; Bader, M.; d’Anna, A.; Delefosse, C.; Errera, M.; Follmann, F.; Jacomet, S.; Jesus, A.; Kühn, M.; et al. Les structures attribuables au Néolithique moyen de type Chassey (dernier tiers du Ve millénaire avant notre ère). In Les Bagnoles à L’Isle-sur-la-Sorgue (Vaucluse)—Un Site Majeur du Néolithique Moyen en Vaucluse; Van Willigen, S., Bailly, M., Röder, B., Schibler, J., Schmitt, A., Eds.; Presses Universitaires de Provence: Aix-en-Provence, France, 2020; pp. 117–228. [Google Scholar]
- Antolin, F.; Delefosse, C.; Denaire, A.; Errera, M.; Follmann, F.; Jacomet, S.; Jesus, A.; Kühn, M.; Martínez-Grau, H.; Pétrequin, P.; et al. Les structures attribuables au Néolithique moyen de type La Roberte (fin du Ve millénaire-début du l’IVe millénaire avant notre ère). In Les Bagnoles à L’Isle-sur-la-Sorgue (Vaucluse)—Un Site Majeur du Néolithique Moyen en Vaucluse; Van Willigen, S., Bailly, M., Röder, B., Schibler, J., Schmitt, A., Eds.; Presses Universitaires de Provence: Aix-en-Provence, France, 2020; pp. 229–280. [Google Scholar]
- Steiner, B.L.; Schäfer, M.; Follmann, F.; Jacomet, S.; Jésus, A.; Soteras, R.; Martínez-Grau, H.; Röder, B.; van Willigen, S.; Antolín, F.; et al. Palaeoecological dynamic of the local environment of the Middle Neolithic site of Les Bagnoles; Department of Environmental Sciences, Integrative Prehistory and Archaeological Science (IPAS), Basel University: Basel, Switzerland, 2022; manuscript in preparation. [Google Scholar]
- Hill, D.S. The Economic Importance of Insects; Chapmann & Hall: London, UK, 1997. [Google Scholar]
- Reichmuth, C.H. Vorratsschädlinge und Vorratsschutz im Wandel der Zeit. In Beiträge zum Göttinger Umwelthistorischen Kolloquium 2008–2009; Herman, B., Ed.; Universitätsverlag Göttingen: Göttingen, Germany, 2009; pp. 17–50. [Google Scholar]
- Jansen, S. “Schädlinge”: Geschichte eines Wissenschaftlichen und Politischen Konstrukts 1840–1920; Campus Verlag (Campus Historische Studien): Frankfurt, Germany; New York, NY, USA, 2003; Volume 25. [Google Scholar]
- IPPC Secretariat. Scientific Review of the Impact of Climate Change on Plant Pests—A Global Challenge to Prevent and Mitigate Plant Pest Risks in Agriculture, Forestry and Ecosystems; FAO on behalf of the IPPC Secretariat: Rome, Italy, 2021. [Google Scholar] [CrossRef]
- Dark, P.; Gent, H. Pests and Diseases of prehistoric Crops a yield “Honeymoon” for early Crops in Europe? Oxf. J. Archaeol. 2001, 20, 59–78. [Google Scholar] [CrossRef]
- Wood, B.J.; Singleton, G.R. Rodents in agriculture and forestry. In Rodent Pests and Their Control, 2nd ed.; Buckle, A.P., Smith, R.H., Eds.; School of Biological Sciences, University of Reading: Gelang Patah, Malaysia, 2014; pp. 33–80. [Google Scholar]
- Tobin, M.E.; Fall, M.W. Pest Control: Rodents. In Agricultural Science; Lal, R., Ed.; EOLSS: Oxford, UK, 2009; Volume II, pp. 352–371. [Google Scholar]
- Hansen, S.C.; Stolter, C.; Imholt, C.; Jacob, J. Like or dislike: Response of rodents to the odor of plant secondary metabolites. Integr. Zool. 2017, 12, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Battersyby, S.A. Chapter 4: Rodents as carries of disease. In Rodent Pests and Their Control, 2nd ed.; Buckle, A.P., Smith, R.H., Eds.; CAB Internationalc: London, UK, 2015; pp. 81–100. [Google Scholar]
- Antolín, F.; Caruso, L.; Mensua, C.; Olària, C.; Piqué, R.; Alonso, N. Forest resources exploitation in the Late Mesolithic and Early Neolithic site of Cova Fosca (Ares del Maestre, Castelló, Spain). In Des Hommes et des Plantes. Exploitation du Milieu et Gestion des Ressources Végétales de la Préhistoire á nos Jours, Proceedings of the XXXe Rencontres Internationales d’Archéologie et d’Histoire d’Antibes; Delhon, C., Théry-Parisot, I., Thiébault, S., Eds.; Éditions APDCA: Antibes, France, 2010; pp. 223–234. [Google Scholar]
- Hüster Plogmann, H. Von Leckerbissen und Schädlingen—Die Untersuchung der Kleintierreste. In Zur Frühzeit von Vindonissa. Auswertung der Holzbauten der Grabung Windisch-Breite 1996–1998; Veröffentlichungen der Gesellschaft pro Vindonissa XVIII/1; Hagendorn, A., Doppler, H.W., Huber, A., Hüster Plogmann, H., Jacomet, S., Meyer-Freuler, C., Pfäffli, B., Schibler, J., Eds.; Gesellschaft pro Vindonissa: Brugg, Switzerland, 2003; pp. 231–243. [Google Scholar]
- Costamagno, S.; Laroulandie, V. L’exploitation des petits vertébrés dans les Pyrénées françaises du Paléolithique au Mésolithique: Un inventaire taphonomique et archéozoologique. In Petits Animaux et Sociétés Humaines. Du Complément Alimentaire aux Ressources Utilitaires, Proceedings of the Actes des XXIVe Rencontres Internationales D’archéologie et D’histoire, Antibes, France, 23–25 October 2003; Brugal, J.-P., Desse, J., Eds.; APDCA: Antibes, France, 2004; pp. 369–382. [Google Scholar]
- Rofes, J.; Zuluaga, M.C.; Murelaga, X.; Fernández-Eraso, J.; Bailon, S.; Iriarte, M.J.; Ortega, L.Á.; Alonso-Olazabal, A. Palaeoenvironmental reconstruction of the Early Neolithic to Middle Bronze Age Peña Larga rock shelter (Álava, Spain) from the small mammal record. Quat. Res. 2013, 79, 158–167. [Google Scholar] [CrossRef]
- Hüster Plogmann, H.; Häberle, S. Archäozoologische Schlämmreste aus den Schichten 13 und 14. In Zürich-Parkhaus Opéra. Eine Neolithische Feuchtbodenfundstelle. Bd. 3: Naturwissenschaftliche Analysen und Synthese (Monographien der Kantonsarchäologie Zürich 50); Bleicher, N., Harb, C., Eds.; Baudirektion Kanton Zürich, Amt für Raumentwicklung, Kantonsarchäologie: Zürich, Switzerland, 2017; pp. 127–144. [Google Scholar]
- Fiedler, L.A. Rodents as a food source. In Proceedings of the 14th Vertebrate Pest Conference, Sacramento, CA, USA, 6–8 March 1990; Davis, C.A., Ed.; University of California: Berkeley, CA, USA, 1990; pp. 149–155. [Google Scholar]
- Romaniuk, A.A.; Shepherd, A.N.; Clarke, D.V.; Sheridan, A.J.; Fraser, S.; Bartosiewicz, L. Rodents: Food or pests in Neolithic Orkney. R. Soc. Open Sci. 2016, 3, 160514. [Google Scholar] [CrossRef]
- Romaniuk, A.A.; Panciroli, E.; Buckley, M.; Chowdhury, M.P.; Willars, C.; Herman, J.S.; Troalen, L.G.; Shepherd, A.N.; Clarke, D.V.; Sheridan, A.; et al. Combined visual and biochemical analyses confirm depositor and diet for Neolithic coprolites from Skara Brae. Archaeol. Anthropol. Sci. 2020, 12, 274. [Google Scholar] [CrossRef]
- Cucchi, T.; Vigne, J.-D.; Auffray, J.-C. First occurrence of the house mouse (Mus musculus domesticus Schwarz & Schwarz, 1943) in the Western Mediterranean: A zooarchaeological revision of subfossil occurrences. Biol. J. Linn. Soc. 2005, 84, 429–445. [Google Scholar]
- Cucchi, T.; Auffray, J.; Vigne, J. On the origin of the house mouse synanthropy and dispersal in the Near East and Europe: Zooarchaeological review and perspectives. In Evolution of the House Mouse (Cambridge Studies in Morphology and Molecules: New Paradigms in Evolutionary Bio); Macholán, M., Baird, S., Munclinger, P., Piálek, J., Eds.; Cambridge University Press: Cambridge, UK, 2012; pp. 65–93. [Google Scholar] [CrossRef]
- Puckett, E.; Orton, D.; Munshi-South, J. Commensal Rats and Humans: Integrating Rodent Phylogeography and Zooarchaeology to Highlight Connections between Human Societies. Bioessays 2020, 42, 1900160. [Google Scholar] [CrossRef]
- Hüster Plogmann, H.; Kühn, M. Fisch, Lamm und Pflaumen aus Latrinen und Gruben—Einblicke in Ernährung und Pflanzenwelt im mittelalterlichen Winterthur. Archäologie Kanton Zürich 2013, 2, 75–118. [Google Scholar]
- Vigne, J.-D. Les micromammifères au Néolithique final à Clairvaux-MM et Chalain 3. Contribution à l’histoire du commensalisme. In Les Sites Littoraux Néolithiques de Clairvaux-les-lacs et de Chalain (Jura), Chalain Station 3 3200-2900; Avant, J.-C., Pétrequin, P., Eds.; Éditions de la Maison des Sciences de l’Homme: Paris, France, 1997; Volume 2, pp. 717–722. [Google Scholar]
- Ronniger, M. Ein Kleinsäuger-Fundkomplex aus Troia VIII; Magisterarbeit, Geowissenschaftliche Fakultät der Eberhard-Karls-Universität: Tübingen, Germany, 2002. [Google Scholar]
- Zwahlen, R.; Frey-Kupper, S.; Grundbacher, B.; Hüster Plogmann, H.; Klee, M.; Schlumbaum, A.; Stopp, B.; Wick, L.; Zwahlen, R. Vicus Petinesca-Vorderberg: Die Ziehbrunnen; Verlag Rub Media: Bern, Switzerland, 2007; Volume 4. [Google Scholar]
- Chaline, J. Les Rongeurs du Pléistocène Moyen et Supérieur de France. Cahiers de Paléontologie, C.N.R.S. 13ème Monographie; Éditions du Centre National de la Recherche Scientifique: Paris, France, 1972. [Google Scholar]
- Vigne, J.-D. Le Mulot sylvestre: Apodemus sylvaticus (Linné, 1758). In Évolution Holocène de la Faune de Vertébrés de France: Invasions et Disparitions; Rapport au Ministère de l’Écologie et du Développement Durable (Direction de la Nature et des, Paysages); Pascal, M., Lorvelec, O., Vigne, J.-D., Keith, P., Clergeau, P., Eds.; Institut National de la Recherche Agronomique, Centre National de la Recherche Scientifique, Muséum National d’Histoire Naturelle: Paris, France, 2003; pp. 313–314. [Google Scholar]
- Herman, J.S.; Jóhannesdóttir, F.E.P.; McDevitt, A.D.; Michaux, J.R.; White, T.A.; Wójcik, J.M.; Searle, J.B. Post-glacial colonization of Europe by the wood mouse, Apodemus sylvaticus: Evidence of a northern refugium and dispersal with humans. Biol. J. Linn. Soc. 2017, 120, 313–332. [Google Scholar] [CrossRef]
- Görner, M.; Hackethal, H. Säugetiere Europas; Ferdinand Enke Verlag: Stuttgart, Germany, 1988; pp. 177–179. [Google Scholar]
- Halle, S. Wood Mice (Apodemus sylvaticus L.) as pioneers of recolonization in a reclaimed area. Oecologia 1993, 94, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Ehrmann, O.; Rösch, M.; Schier, W. Experimentelle Rekonstruktion eines jungneolithischen Wald-Feldbaus mit Feuereinsatz—Ein multidisziplinäres Forschungsprojekt zur Wirtschaftsarchäologie und Landschaftsökologie. Praehist. Z. 2009, 84, 44–72. [Google Scholar] [CrossRef]
- King, G. Exaptation and synanthropic insects: A diachronic interplay between Biology and culture. Environ. Archaeol. 2014, 19, 12–22. [Google Scholar] [CrossRef]
- King, G.; Kenward, H.; Schmidt, E.; Smith, D. Six-legged Hitchhikers: An Archaeobiogeographical Account of the Early Dispersal of Grain Beetles. J. North Atl. 2014, 2014, 1–18. [Google Scholar] [CrossRef]
- Obata, H.; Manabe, A.; Nakamuna, N.; Onishi, T.; Senba, T. A New Light on the Evolution and Propagation of Prehistoric Grain Pests: The World’s Oldest Maize Weevils Found in Jomon Potteries, Japan. PLoS ONE 2011, 6, e14785. [Google Scholar] [CrossRef] [PubMed]
- Plarre, R. An attempt to reconstruct the natural and cultural history of the granary weevil, Sitophilus granarius (Coleoptera: Curculionidae). Eur. J. Entomol. 2010, 107, 1–11. [Google Scholar] [CrossRef]
- Kislev, M.; Anat, H.; Galili, E. Archaeobotanical and archaeoentomological evidence from a well at Atlit-Yam indicates colder, more humid climate on the Israeli coast during the PPNC period. J. Archaeol. Sci. 2004, 31, 1301–1310. [Google Scholar] [CrossRef]
- Panagiotakopulu, E.; Buckland, P.C. Early invaders: Farmers, the granary weevil and other uninvited guests in the Neolithic. Biol. Invasions 2018, 20, 219–233. [Google Scholar] [CrossRef]
- Schmidt, E. Vorratsschädlinge im Mitteleuropa des 5. Jahrtausends. In Mitteleuropa im 5. Jahrtausend vor Christus, Beiträge zur Internationalen Konferenz in Münster 2010; Gleser, R., Becker, V., Eds.; Literatur-Verlag: Berlin/Münster, Germany, 2012; pp. 321–329. [Google Scholar]
- Schmidt, E. Die Insektenreste aus der linienbandkeramischen Brunnenanlage Leipzig-Plaussig. In Der linienbandkeramischen Brunnen von Leipzig-Plaussig; Veröffentlichungen des Landesamtes für Archäologie, Sachsen; Friederich, S., Ed.; Lfa Sachsen: Dresden, Germany, 2017; Volume 62, pp. 285–346. [Google Scholar]
- Arobba, D.; Giacobini, G.; Castelletti, L.; Gardini, G.; Meriggi, A.; Ottoboni, F. Analisi di un coprolite rinvenuto nei livelli del Neolitico Medio. In Il Neolitico Nella Caverna Delle Arene Candide (Scavi 1972–1977); Tiné, S., Ed.; Istituto Internazionale Studi Liguri: Bordighera, Italy, 1999; pp. 25–35. [Google Scholar]
- Schmidt, E. Der Kornkäfer Sitophilus granarius Schoen. Curculionidae aus der Schuttschicht des bandkeramischen Brunnens von Erkelenz-Kückhoven. In Brunnen der Jungsteinzeit, Proceedings of the Internationales Symposium, Erkelenz, Germany, 27–29 October 1997; Rheinisches Amt für Bodendenkmalpflege (eds) Materialien zur Bodendenkmalpflege; Rheinland-Verlag: Köln, Germany, 1998; Volume 11, pp. 261–269. [Google Scholar]
- Huchet, J.-B. Le Coléoptère, la Graine et l’Archéologue: Approche archéoentomologique de quelques ravageurs des denrées stockées. In Plantes, Produits Végétaux et Ravageurs, Proceedings of the Actes des Xe Rencontres d’Archéobotanique, Les Eyzies-de-Tayac, France, 24–27 September 2014; Dietsch-Sellami, M.-F., Bouby, L., Hallavant, C., Pradat, B., Eds.; Aquitania: Bordeaux, France, 2016; pp. 17–42. [Google Scholar]
- Weidner, H. Vorratsschädlinge und Hausungeziefer, Bestimmungstabellen für Mitteleuropa; Springer Spektrum: Wiesbaden, Germany, 2010. [Google Scholar]
- Schmidt, E. Viele Käfer, aber keine Vorratsschädlinge Fehlen Vorratsschädlinge in Feuchtbodensiedlungen wirklich? Denkmalpfl. Baden-Württemberg 2016, 45, 208–212. [Google Scholar]
- Pécréaux, D. Potentialités de l’entomologie appliquée aux sites archéologiques subaquatiques. L’exemple du Bronze final du Lac du Bourget (Savoie, France). Bull. De La Société Préhistorique Française 2010, 107, 156–157. [Google Scholar]
- Antolín, F.; Schäfer, M. Insect Pests of Pulse Crops and their Management in Neolithic Europe. Environ. Archaeol. 2019, 1–14. [Google Scholar] [CrossRef]
- Sargiano, J.-P.; Van Willigen, S.; d’Anna, A.; Renault, S.; Hunger, K.; Woerle-Soares, M.; Gaday, R. Les Bagnoles a l’Isle-sur-la-Sorgue (Vaucluse). Aspects nouveaux dans le Néolithique moyen du midi de la France. Gall. Préhistoire 2010, 52, 193–239. [Google Scholar] [CrossRef][Green Version]
- Martínez-Grau, H.; Denaire, A.; Antolín, F.; van Willigen, S. Relations spatiales et chronologiques entre les occupations du Néolithique moyen de type La Roberte et Néolithique moyen de type Chassey. In Les Bagnoles à L’Isle-sur-la-Sorgue (Vaucluse)—Un Site Majeur du Néolithique Moyen en Vaucluse; van Willigen, S., Bailly, M., Röder, B., Schibler, J., Schmitt, A., Eds.; Presses Universitaires de Provence: Aix-en-Provence, France, 2020; pp. 270–278. [Google Scholar]
- Röder, B.; Schmitt, A.; van Willigen, S.; Antolín, F.; Bader, M.; D’Anna, A.; Delefosse, C.; Errera, M.; Follmann, F.; Jacomet, S.; et al. Le Néolithique moyen de type Chassey: Conclusions. In Les Bagnoles à L’Isle-sur-la-Sorgue—Un Site Majeur du Néolithique Moyen en Vaucluse; van Willigen, S., Bailly, M., Röder, B., Schibler, J., Schmitt, A., Eds.; Presses Universitaires de Provence: Aix-en-Provence, France, 2020; pp. 225–228. [Google Scholar]
- Jacomet, S. Environnement végétal naturel et paysage. In Les Bagnoles a L’Isle-sur-la-Sorgue (Vaucluse)—Un Site Majeur du Néolithique Moyen en Vaucluse; van Willigen, S., Bailly, M., Röder, B., Schibler, J., Schmitt, A., Eds.; Presses Universitaires de Provence: Aix-en-Provence, France, 2020; pp. 60–77. [Google Scholar]
- van Willigen, S.; Antolin, F.; Brochier, J.É.; d’Anna, A.; Delefosse, C.; Errera, M.; Follmann, F.; Jacomet, S.; Jesus, A.; Kühn, M.; et al. Les apports du site pour la connaissance du Néolithique moyen. In Les Bagnoles à L’Isle-sur-la-Sorgue (Vaucluse)—Un Site Majeur du Néolithique Moyen en Vaucluse; van Willigen, S., Bailly, M., Röder, B., Schibler, J., Schmitt, A., Eds.; Presses Universitaires de Provence: Aix-en-Provence, France, 2020; pp. 281–352. [Google Scholar]
- Herbig, C.; Maier, U.; Stäuble, H.; Elburg, R. «Neolithische Füllhörner»—Archäobotanische Untersuchungen in fünf Linienbandkeramischen Brunnen in Westsachsen. Offa 2013, 69, 265–293. [Google Scholar]
- Tegel, W.; Elburg, R.; Hakelberg, D.; Stäuble, H.; Büntgen, U. Early Neolithic Water Wells Reveal the World’s Oldest Wood Architecture. PLoS ONE 2012, 7, e51374. [Google Scholar] [CrossRef]
- Antolín, F.; Steiner, B.; Akeret, Ö.; Brombacher, C.; Kühn, M.; Vandorpe, P.; Bleicher, N.; Gross, E.; Schaeren, G.; Jacomet, S. Studying the preservation of plant macroremains from waterlogged archaeological deposits for an assessment of layer taphonomy. Rev. Palaeobatany Palynol. 2017, 246, 120–145. [Google Scholar] [CrossRef]
- Bronk Ramsey, C. Bayesian Analysis of Radiocarbon Dates. Radiocarbon 2009, 51, 337–360. [Google Scholar] [CrossRef]
- Reimer, P.J.; Austin, W.E.N.; Bard, E.; Bayliss, A.; Blackwell, P.G.; Bronk Ramsey, C.; Butzin, M.; Cheng, H.; Edwards, R.L.; Friedrich, M.; et al. The IntCal20 Northern Hemisphere Radiocarbon Age Calibration Curve (0–55 cal kBP). Radiocarbon 2020, 62, 725–757. [Google Scholar] [CrossRef]
- Steiner, B.; Antolín, F.; Jacomet, S. Testing of the consistency of the sieving (wash-over) process of waterlogged sediments by multiple operators. J. Archaeol. Sci. Rep. 2015, 2, 310–320. [Google Scholar] [CrossRef]
- André, G.; Antolín, F.; Bader, M.; Bailly, M.; Brochier, J.E.; D’Anna, A.; Delefosse, C.; Denaire, A.; Errera, M.; Follmann, F.; et al. Résumé. In Les Bagnoles à L’Isle-sur-la-Sorgue—Un Site Majeur du Néolithique Moyen en Vaucluse; van Willigen, S., Bailly, M., Röder, B., Schibler, J., Schmitt, A., Eds.; Presses Universitaires de Provence: Aix-en-Provence, France, 2020; pp. 387–390. [Google Scholar]
- Pasquier, L. Dynamique Évolutive d’un Sous-Genre de Muridae, Apodemus (Sylveamus): Étude Biométrique des Caractères Dentaires de Populations Fossiles et Actuelles d’Europe Occidentale. Ph.D. Dissertation, Université des Sciences et Techniques du Languedoc, Montpellier, France, 1974. [Google Scholar]
- Kaltenthaler, D.; Lohrer, J.; Kröger, P.; van der Meijden, C.; Granado, E.; Lamprecht, J.; Nücke, F.; Obermaier, H.; Stopp, B.; Baly, I.; et al. OssoBook v5.6.2. SAPM & IPNA: Munich & Basel 2018. Available online: http://xbook.vetmed.uni-muenchen.de/ (accessed on 11 March 2022).
- Van der Veen, M.; Fjeller, N.R.J. Sampling seeds. J. Archaeol. Sci. 1982, 9, 287–298. [Google Scholar] [CrossRef]
- Jacomet, S.; Kreuz, A.; Rösch, M. Archäobotanik: Aufgaben, Methoden und Ergebnisse Vegetations-und Agratgeschichtlicher Forschung; Eugen Ulmer: Stuttgart, Germany, 1999. [Google Scholar]
- Bronk Ramsey, C. Probability and Dating. Radiocarbon 1997, 40, 461–474. [Google Scholar] [CrossRef]
- Domínguez García, Á.C.; Laplana, C.; Sevilla, P.; Blain, H.-A.; Zumajo, N.P.; Luis Benítez, L.; Enrich, L. New data on the introduction and dispersal process of small mammals in southwestern Europe during the Holocene: Castillejo del Bonete site (southeastern Spain). Quat. Sci. Rev. 2019, 225, 106008. [Google Scholar] [CrossRef]
- Bayle, P. Les mulots—Apodemus ssp. In Les Mammifères de Provences-Alpes-Côte d’Azur; Ligue de Protection des Oiseaux (LPO) PACA, le Groupe d’étude des Cétacés de Méditerranée (GECEM), le Groupe Chiroptères de Provence (GCP), Eds.; Biotope: Mèze, France, 2016; pp. 267–268. [Google Scholar]
- Hecht, P. Vergleichende anatomische und biometrische Untersuchungen an Beckenknochen, Scapula, Femur und Humerus bei Waldmaus (Apodemus sylvaticus), Gelbhalsmaus (Apodemus flavicollis), Feldmaus (Microtus arvalis) und Rötelmaus (Cleithrionomys glareolus). Säugetierkundliche Mitt. 1971, 19, 132–157. [Google Scholar]
- Rigaux, P. Le Campagnol provençal. In Les mammifères de Provence-Alpes-Côte d’Azur; Ligue de protection des Oiseaux (LPO) PACA, le Groupe d’étude des Cétacés de Méditerranée (GECEM), le Groupe Chiroptères de Provence (GCP), Eds.; Biotope: Mèze, France, 2016; pp. 250–251. [Google Scholar]
- Rigaux, P. Le Campagnol agreste. In Les mammifères de Provence-Alpes-Côte d’Azur; Ligue de protection des Oiseaux (LPO) PACA, le Groupe d’étude des Cétacés de Méditerranée (GECEM), le Groupe Chiroptères de Provence (GCP), Eds.; Biotope: Mèze, France, 2016; pp. 256–257. [Google Scholar]
- Bayle, P. La taupe aveugle—Talpa caeca. In Les mammifères de Provence-Alpes-Côte d’Azur; Ligue de protection des Oiseaux (LPO) PACA, le Groupe d’étude des Cétacés de Méditerranée (GECEM), le Groupe Chiroptères de Provence (GCP), Eds.; Biotope: Mèze, France, 2016; pp. 178–179. [Google Scholar]
- Reichstein, H. Mustela erminea Linné 1758—Hermelin. In Handbuch der Säugetiere Europas, Band 5/I: Raubsäuger I Canidae, Ursidae, Procyonidae, Mustelidae 1; Niethammer, J., Krapp, F., Eds.; Aula-Verlag: Wiesbaden, Germany, 1993; pp. 533–570. [Google Scholar]
- Reichstein, H. Mustela nivalis Linné 1766—Mauswiesel. In Handbuch der Säugetiere Europas, Band 5/I: Raubsäuger I Canidae, Ursidae, Procyonidae, Mustelidae 1; Niethammer, J., Krapp, F., Eds.; Aula-Verlag: Wiesbaden, Germany, 1993; pp. 571–626. [Google Scholar]
- Marciszak, A.; Socha, P. Stoat Mustela erminea Linnaeus, 1758 and weasel Mustela nivalis Linnaeus, 1766 in palaeoecological analysis: A case study of Bisnik Cave. Quat. Int. 2014, 339–340, 258–265. [Google Scholar] [CrossRef]
- Hakbijl, T.; Pals, J.P.; Troostheide, C.D. Plant and Insect Remains from the Late Neolithic well at Kolhorn. Palaeohistoria 1989, 31, 157–163. [Google Scholar]
- Dettner, K. Zoologische Analyse des Schachtinhaltes einer keltischen Vierreckschanze auf der Basis von Arthropodenfragmente (Vorbericht). In Die keltischen Vierreckschanzen von Fellbach-Schmiden (Rems-Murr-Kreis) und Ehningen (Kreis Böblingen), Forschungen und Berichte zur Vor- und Frühgeschichte in Baden-Württemberg; Theiss Verlag Stuttgart; Landesamt für Denkmalpflege im Regierungspräsidium Stuttgart: Esslingen am Neckar, Germany, 1999; Volume 80, pp. 150–161. [Google Scholar]
- Ponel, P.; Yvinec, J.-H.; Andrieu-Ponel, V.; Marian, J. L’intérêt archéo-entomologique des anciens puits: Le paléoenvironnment du Clos-Paul à l’époque gallo-romaine, reconstruit par l’analyse des Coléoptères fossiles (Charleville-Mézières, Ardennes). Quat. Rev. L’association Française Pour L’étude Quat. 2018, 29, 347–361. [Google Scholar] [CrossRef]
- Lumaret, J.-P.; Kirk, A.A. South Temperate Dung Beetles. In Dung Beetle Ecology; Hanski, I., Cambefort, Y., Eds.; Princeton University Press: Princeton, NJ, USA, 1991; Chapter 6; pp. 97–115. [Google Scholar] [CrossRef]
- Koch, K. Die Käfer Mitteleuropas, Ökologie; Goecke & Evers: Krefeld, Germany, 1992; Volume 3. [Google Scholar]
- Buckland, P.C. Granaries Stores and Insects—The Archaeology of Insect Synanthropy. In La Préparation Alimentaire des Céréales. Rapports Presentés à la Table Ronde Ravello au Centre Universitaire pour les Biens Culturels, Avril 1988; Fournier, D., Sigaut, F., Eds.; PACT: Rixensart, Belgium, 1990; pp. 69–81. [Google Scholar]
- Zahradník, J. Käfer Mittel- und Nordwesteuropas; Paul Parey: Hamburg/Berlin, Germany, 1985. [Google Scholar]
- Buckland, P.C. An Insect Fauna from a Roman Well at Empingham, Rutland; Transactions Leicestershire Archaeological and Historical Society: Leicestershire, UK, 1986; Volume 60, pp. 1–6. [Google Scholar]
- Hughes, V.C.E. The Coleoptera from Well I. In Iron Age and Roman Settlement: Rescue Excavations at Lynch Farm 2, Orten Longueville: Peterborough; Upex, S.G., Ed.; Nene Valley Archaeological Trust: Manchester, England, 2018; pp. 152–158. [Google Scholar]
- Ponel, P.; Guiter, F.; Rocq, C.; Anrieu-Ponel, V. Le paléo-environnement du site de Lattes/Saint-Sauveur au Ier siècle de notre ère, reconstruit à partir de l’analyse des assemblages de coléoptères subfossiles. In Lattara 18: Onze Puits Gallo-Romains de Lattara (Ier s. av. n. è. IIe s. de n. è.); Py, M., Ed.; Association pour le Développement de l’Archéologie en Languedoc-Roussillon—ASM-UMR5140-390; Lattes: Lattara, France, 2005; pp. 319–326. [Google Scholar]
- Niethammer, J. Apodemus sylvaticus—Waldmaus. In Handbuch der Säugetiere Europas, Band 1/Nagetiere I; Niethammer, J., Krapp, F., Eds.; Aula-Verlag: Wiesbaden, Germany, 1978; pp. 337–358. [Google Scholar]
- Bäumler, W. Über einen Zusammenbruch der Gelbhalsmauspopulation im Nationalpark Bayerischer Wald. In Anzeiger für Schädlingskunde, Pflanzen- und Umweltschutz vereinigt mit Schädlingsbekämpfung, Heft 11; Verlag Paul Parey: Berlin/Hamburg, Germany, 1973; pp. 161–168. [Google Scholar]
- Compte, A.; Perales, J. Estudios de insectos coleópteros dotados en el inicio de la iberización y pertenecientes al poblado de Siriguarach (Alcañiz, Teruel). Kalathos Rev. Semin. Arquelogía Y Etnol. Turol. 1983, 3, 121–137. [Google Scholar]
- Hubbard, R.N.L. Appendix 2: Ancient Agriculture and Ecology at Servia. In Rescue Excavations at Servia 1971–1973: A preliminary Report, 185–230, The Annual of the British School of Athens; Wijnen, M., Ridley, C., Wardle, K.A., Hubbard, R.N.L., Watson, J.P.N., Jones, R.E., Eds.; British School at Athens: Athens, Greece, 1979; Volume 74, pp. 226–228. [Google Scholar]
- Henríquez-Valido, P.; Morales, J.; Vidal-Matutano, P.; Moreno-Benítez, M.; Marchante-Ortega, Á.; Rodríguez-Rodríguez, A.; Huchet, J.-B. Archaeoentomolgical indicators of long-term food plant storage at the Prehispanic granary of La Fortaleza (Gran Canaria, Spain). J. Archaeol. Sci. 2020, 120, 105179. [Google Scholar] [CrossRef]
- Osborne, P.J. An Insect Fauna from a Modern Cesspit and its comparion with probable cesspot Assemblages from Archaeologicial Sites. J. Archaeol. Sci. 1983, 10, 453–463. [Google Scholar] [CrossRef]
- Jones, G.; Wardle, K.; Halstead, P.; Wardle, D. Crop Storage at Assiros. At a site in northern Greece charred fragments of grain from crop storerooms that burned to the ground 3000 years ago are throwing new light on how the majestic Bronze Age Mycenaean palaces arose. Sci. Am. 1986, 254, 96–103. [Google Scholar] [CrossRef]
- Levinson, H.; Levinson, A. Die Ungezieferplagen und Anfänge der Schädlingsbekämpfung im Alten Orient. Anz. Für Schädlingskunde Pflanzenschutz Umw. 1990, 63, 81–96. [Google Scholar] [CrossRef]
- Prats, G.; Antolín, F.; Alonso, N. Household storage, surplus and supra-household storage in prehistoric and protohistoric societies of the Western Mediterranean. PLoS ONE 2020, 15, e0238237. [Google Scholar] [CrossRef] [PubMed]
- Prats, G.; Antolín, F.; Alonso, N. From the earliest farmers to the first urban centres: A socio-economic analysis of underground storage practices in north-eastern Iberia. Antiquity 2020, 94, 653–668. [Google Scholar] [CrossRef]
- Altieri, M.; Nicholls, C.I. Soil fertility management and insect pests; harmonizing soil and plant health in agroecosystems. Soil Tillage Res. 2003, 72, 203–211. [Google Scholar] [CrossRef]
- Abate, T.; van Huis, A.; Ampofo, J.K.O. Pest Management Strategies in Traditional Agriculture: An African Perspective. Annu. Rev. Entomol. 2000, 45, 631–659. [Google Scholar] [CrossRef] [PubMed]
- Brandes, D. Das Sambucetum ebuli Felf. 1942 im südlichen Mitteleuropa und seine geographische Gliederung. In Tuexenia- Mitteilung der Floristisch-Soziologischen Arbeitsgemeinschaft, Bd. 2; 1982; pp. 47–60. Available online: https://www.zobodat.at/pdf/Tuexenia_NS_2_0047-0060.pdf (accessed on 11 March 2022).
- Madaus, G. Lehrbuch der biologischen Heilmittel; Thieme: Leipzig, Germany, 1938; Volume 3. [Google Scholar]
- Hakbijl, T. The Traditional, Historical and Prehistoric Use of Ashes an Insecticide, with an Experimental Study on the Insecticidal Efficacy of Washed Ash. Environ. Archaeol. 2002, 7, 13–22. [Google Scholar] [CrossRef]
- Morales, J.; Herríquez-Valido, P.; Moreno-Benítez, M.; Naranjo-Mayo, Y.; Rodríguez-Rodríguez, A. Du laurier dans les greniers de Grande Canarie (Espagne). Tech. Cult. Rev. Semest. D’anthropologie Tech. 2018, pp. 1–20. Available online: http://journals.openedition.org/tc/8902 (accessed on 11 March 2022).
- Boeke, S.J.; van Loon, J.J.A.; van Huis, A.; Kossou, D.K.; Dicke, M. The Use of Plant Material to Protect Stored Leguminous Seeds against Seed Beetles: A Review; Wageningen University Papers 3; Backhuys Publishers: Leiden, The Netherlands, 2001. [Google Scholar]
- Panagiotakopupu, E.; Buckland, P.C.; Day, P.M. Natural Insecticides and Insect Repellents in Antiquity: A Review of the Evidence. J. Archaeol. Sci. 1995, 22, 705–710. [Google Scholar] [CrossRef]



| Structure | Sample | Specie | Lab Code | BP | ± | δ13C (‰) | C/N | Method | cal B.C. 2σ | Observations | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 250 | Grain | Cerealia | ETH-60867 | 5331 | 27 | −21.4 | nd | AMS | 4314−4051 | Martínez-Grau et al., 2020 [73] | |
| 250 | Fruit | Corylus avellana | ETH-60868 | 5306 | 27 | −24 | nd | AMS | 4241−4047 | Martínez-Grau et al., 2020 [73] | |
| 250 | Grain | Cerealia | ETH-60869 | 5334 | 27 | −20.5 | nd | AMS | 4316−4052 | Martínez-Grau et al., 2020 [73] | |
| 250 | Grain | Triticum sp. | ETH-60870 | 5302 | 31 | −22.4 | nd | AMS | 4242−4001 | Martínez-Grau et al., 2020 [73] | |
| 250 | Grain | Cerealia | ETH-60871 | 5323 | 31 | −24.2 | nd | AMS | 4312−4049 | Martínez-Grau et al., 2020 [73] | |
| 250 | Grain | Triticum sp. | POZ-64775 | 5400 | 40 | nd | nd | AMS | 4344−4061 | Martínez-Grau et al., 2020 [73] | |
| 990 | Grain | Triticum aestivum/durum/turgidum | ETH-88901 | 5226 | 25 | −24.6 | 18.62 | AMS | 4216−3969 | Martínez-Grau et al., 2020 [73] | |
| 990 | Grain | Triticum aestivum/durum/turgidum | ETH-88904 | 5213 | 25 | −24.8 | 18.96 | AMS | 4157−3965 | Martínez-Grau et al., 2020 [73] | |
| 990 | Bone | Apodemus/Muridae | ETH-113018 | 5161 | 33 | −19.8 | 2.8 | GIS | 4046−3812 | this paper | |
| 994 | Grain | Triticum monococcum | ETH-88902 | 5027 | 26 | −16.3 | 36.72 | AMS | 3947−3712 | Martínez-Grau et al., 2020 [73] | |
| 994 | Grain | Triticum aestivum/durum/turgidum | ETH-88903 | 5874 | 25 | −23.7 | 40.75 | AMS | 4828−4689 | outlier | Martínez-Grau et al., 2020 [73] |
| 994 | Grain | Triticum monococcum | ETH-96173 | 5096 | 28 | −23.7 | 15.3 | AMS | 3967−3799 | Jesus et al., 2021 [12] | |
| 994 | Bone | Apodemus | ETH-113016 * | 5672 | 52 | − | − | GIS | 4673−4364 | outlier | this paper |
| 994 | Bone | Apodemus/Muridae | ETH-113017 | 5480 | 92 | −23.2 | 0 | GIS | 4531−4052 | outlier | this paper |
| Structure | Well 250 | Well 990 | Well 994 | Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of Samples | 5 | 9 | 4 | 18 | |||||||||
| Sample Volume | 78 L | 346.8 L | 163.5 L | 588.3 L | |||||||||
| NISP | NISP | MNI | NISP | NISP | MNI | NISP | NISP | MNI | NISP | NISP | MNI | ||
| n | % | n | % | n | % | n | % | ||||||
| Small mammals indet. | 26 | 816 | 77 | 919 | |||||||||
| Carnivora | |||||||||||||
| Least weasel | Mustela nivalis | 2 | 0.2 | 1 | 2 | 0.2 | 1 | ||||||
| Least weasel/Stoat | Mustela niv./ereminea | 58 | 7 | 1 | 58 | 5.6 | 1 | ||||||
| Rodentia | |||||||||||||
| Murid/Vole | Muridae/Arvicolinae | 14 | 54 | 401 | 48.2 | 51 | 40 | 466 | 45.1 | ||||
| Woodmouse | Apodemus cf. sylvaticus | 3 | 12 | 1 | 340 | 40.9 | 42 | 67 | 53 | 4 | 410 | 39.7 | 47 |
| Vole | Arvicolinae | 2 | 2 | 6 | 0.7 | 5 | 4 | 13 | 1.3 | ||||
| Fieldmice | Microtus sp. | 9 | 1.1 | 1 | 1 | 10 | 1.4 | ||||||
| Mediterranean pine vole | Microtus duodecimcostatus | 6 | 0.7 | 2 | 6 | 0.6 | 2 | ||||||
| Field vole | Microtus agrestis | 2 | 0.2 | 1 | 2 | 0.2 | 1 | ||||||
| Eulipotyphla | |||||||||||||
| Shrews | Soricidae | 3 | 12 | 4 | 0.5 | 7 | 0.7 | ||||||
| Red-toothed shrews | Soricinae | 1 | 4 | 1 | 0.1 | ||||||||
| White-toothed shrews | Crocidurinae | 2 | 8 | 1 | 0.1 | 1 | 1 | 4 | 0.4 | ||||
| Greater white-toothed shrew | Crocidura russula | 1 | 0.1 | 1 | 1 | 1 | 1 | 2 | 0.2 | 1 | |||
| Mole | Talpa spec. | 1 | 4 | 2 | 0.2 | 1 | 3 | 0.3 | 1 | ||||
| total identified | 26 | 100 | 832 | 100 | 126 | 100 | 984 | 100 | |||||
| n remains/liter sample vol. | 0.7 | 4.7 | 1.2 | 1.7 | |||||||||
| Structure | Well 250 | Well 990 | Well 994 | ||||
|---|---|---|---|---|---|---|---|
| Number of Samples | 11 | 9 | 6 | ||||
| Sample Volume | 148.5 | 57.8 | 84.2 | ||||
| NISP | high | NISP | high | NISP | high | ||
| Pest insects | |||||||
| Carabidae/ground beetles | Zabrus tenebrioides (Goeze 1777) | 4 | 4 | ||||
| Chrysomelidae/leaf beetles | Bruchus sp. (Linnaeus 1767) | 1 | 1 | ||||
| Curculionidae/weevils | Sitophilus granarius (Linnaeus 1758) | 8 | 30 | 29 | 42 | 15 | 21 |
| Insecta | |||||||
| Lepidoptera/butterflies/moths | 1 | 2 | 2 | 6 | |||
| Hymnoptera/sawflies/waspe/bees/ants | 25 | 197 | 21 | 70 | 186 | 427 | |
| Coleoptera/beetles | 1080 | 4442 | 1880 | 3442 | 3792 | 5885 | |
| Trichoptera/caddisflies | 6 | 29 | |||||
| Diptera/true flies/mosquitoes | 30 | 149 | 46 | 70 | 6 | 11 | |
| Archanida | |||||||
| Araneae/spiders | 3 | 32 | 1 | 1 | 8 | 13 | |
| Acari/mites | 1 | 1 | |||||
| Oribatida/moss mites | 3 | 20 | 6 | 15 | 23 | 64 | |
| Invertebrates indet | 358 | 2796 | 402 | 527 | 2841 | 5095 | |
| total | 1515 | 7698 | 2386 | 4168 | 6877 | 11,526 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Häberle, S.; Schäfer, M.; Soteras, R.; Martínez-Grau, H.; Hajdas, I.; Jacomet, S.; Röder, B.; Schibler, J.; van Willigen, S.; Antolín, F. Small Animals, Big Impact? Early Farmers and Pre- and Post-Harvest Pests from the Middle Neolithic Site of Les Bagnoles in the South-East of France (L’Isle-sur-la-Sorgue, Vaucluse, Provence-Alpes-Côte-d’Azur). Animals 2022, 12, 1511. https://doi.org/10.3390/ani12121511
Häberle S, Schäfer M, Soteras R, Martínez-Grau H, Hajdas I, Jacomet S, Röder B, Schibler J, van Willigen S, Antolín F. Small Animals, Big Impact? Early Farmers and Pre- and Post-Harvest Pests from the Middle Neolithic Site of Les Bagnoles in the South-East of France (L’Isle-sur-la-Sorgue, Vaucluse, Provence-Alpes-Côte-d’Azur). Animals. 2022; 12(12):1511. https://doi.org/10.3390/ani12121511
Chicago/Turabian StyleHäberle, Simone, Marguerita Schäfer, Raül Soteras, Héctor Martínez-Grau, Irka Hajdas, Stefanie Jacomet, Brigitte Röder, Jörg Schibler, Samuel van Willigen, and Ferran Antolín. 2022. "Small Animals, Big Impact? Early Farmers and Pre- and Post-Harvest Pests from the Middle Neolithic Site of Les Bagnoles in the South-East of France (L’Isle-sur-la-Sorgue, Vaucluse, Provence-Alpes-Côte-d’Azur)" Animals 12, no. 12: 1511. https://doi.org/10.3390/ani12121511
APA StyleHäberle, S., Schäfer, M., Soteras, R., Martínez-Grau, H., Hajdas, I., Jacomet, S., Röder, B., Schibler, J., van Willigen, S., & Antolín, F. (2022). Small Animals, Big Impact? Early Farmers and Pre- and Post-Harvest Pests from the Middle Neolithic Site of Les Bagnoles in the South-East of France (L’Isle-sur-la-Sorgue, Vaucluse, Provence-Alpes-Côte-d’Azur). Animals, 12(12), 1511. https://doi.org/10.3390/ani12121511








