Multiple-Vessel-Based Blood Gas Profiles Analysis Revealed the Potential of Blood Oxygen in Mammary Vein as Indicator of Mammary Gland Health Risk of High-Yielding Dairy Cows
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Management
2.2. Sample Collection and Measurement
2.3. Measurement and Statistical Analysis
3. Results
3.1. Lactation Performance
3.2. Blood Gas Parameter
3.3. Oxidative-Stress-Related Variables and Mammary-Gland Permeability
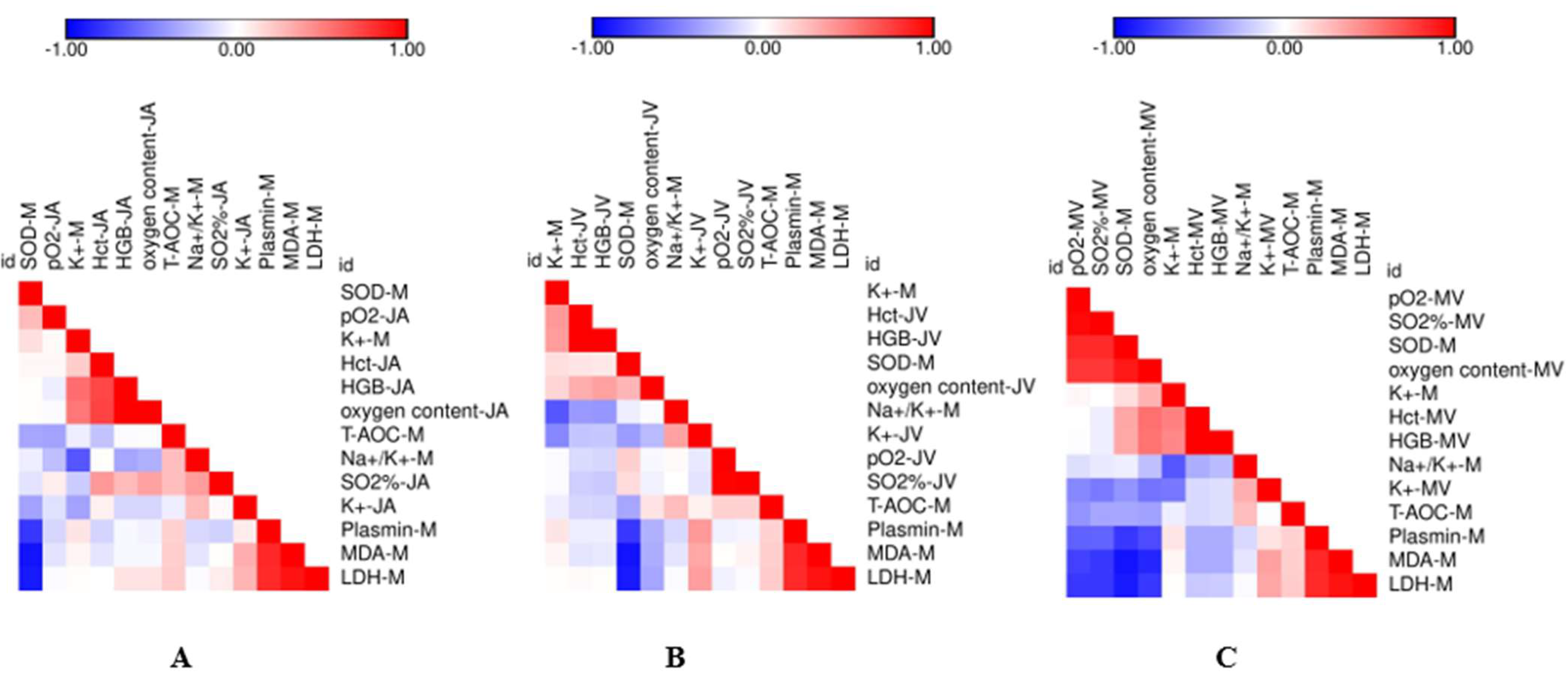
3.4. Correlation Analysis between the Measured Variables
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Knowles, T.G.; Edwards, J.E.; Bazeley, K.J.; Brown, S.N.; Butterworth, A.; Warriss, R.D. Changes in the blood biochemical and haematological profile of neonatal calves with age. Vet. Rec. 2000, 147, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Cornell University College of Veterinary Medicine. Venous Blood Gas and Electrolyte Reference Intervals. Available online: https://ahdc.vet.cornell.edu/sects/clinpath/reference/blood.cfm (accessed on 11 April 2017).
- American Association for Clinical Chemistry (AACC). Reference Ranges and What They Mean. Available online: https://labtestsonline.org/understanding/features/ref-ranges/start/1 (accessed on 29 March 2017).
- Russell, K.E.; Roussel, A.J. Evaluation of the ruminant serum chemistry profile. Vet. Clin. North Am. Food Anim. Pract. 2007, 23, 403–426. [Google Scholar] [CrossRef]
- Bleul, U.; Lejeune, B.; Schwantag, S.; Kahn, W. Blood gas and acid-base analysis of arterial blood in 57 newborn calves. Vet. Rec. 2007, 161, 688–691. [Google Scholar] [CrossRef]
- Smith, B.P. Clinical chemistry tests. In Large Animal Internal Medicine, 4th ed.; Mosby: Maryland Heights, MO, USA, 2009; pp. 375–398. [Google Scholar]
- Wood, D.; Quiroz-Rocha, G.F. Normal hematology of cattle. In Schalm’s Veterinary Hematology, 6th ed.; Wiley: Ames, IA, USA, 2010; pp. 829–835. [Google Scholar]
- Detry, B.; Cambier, C.; Frans, A.; Gustin, P.; Clerbaux, T. Calculation of bovine haemoglobin oxygen saturation by algorithms integrating age, haemoglobin content, blood pH, partial pressures of oxygen and carbon dioxide in the blood, and temperature. Vet. J. 2003, 165, 258–265. [Google Scholar] [CrossRef]
- Lorenz, I.; Gentile, A.; Klee, W. Investigations of d-lactate metabolism and the clinical signs of d-lactataemia in calves. Vet. Rec. 2005, 156, 412–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koch, A.; Kaske, M. Clinical efficacy of intravenous hypertonic saline solution or hypertonic bicarbonate solution in the treatment of inappetent calves with neonatal diarrhea. J. Vet. Intern. Med. 2008, 22, 202–211. [Google Scholar] [CrossRef]
- Lopera, C.; Zimpel, R.; Vieira-Neto, A.; Lopes, F.R.; Ortiz, W.; Faria, B.N.; Gambarini, M.L.; Poindexter, M.; Block, E.; Nelson, C.D.; et al. Effects of level of dietary cation-anion difference and duration of prepartum feeding on performance and metabolism of dairy cows. J. Dairy Sci. 2018, 101, 7907–7929. [Google Scholar] [CrossRef]
- Bauer, T.; Bürgers, H.F.; Rabie, T.; Marti, H.H. Matrix metalloproteinase-9 mediates hypoxia-induced vascular leakage in the brain via tight junction rearrangement. J. Cereb. Blood Flow Metab. 2009, 30, 837–848. [Google Scholar] [CrossRef] [Green Version]
- Sayers, R.G.; Kennedy, A.; Krump, L.; Sayers, G.P.; Kennedy, E. An observational study using blood gas analysis to assess neonatal calf diarrhea and subsequent recovery with a European Commission-compliant oral electrolyte solution. J. Dairy Sci. 2016, 99, 4647–4655. [Google Scholar] [CrossRef] [Green Version]
- Opsomer, G.; Wensing, T.; Laevens, H.; Coryn, M.; de Kruif, A. Insulin resistance: The link between metabolic disorders and cystic ovarian disease in high yielding dairy cows? Anim. Reprod. Sci. 1999, 56, 211–222. [Google Scholar] [CrossRef]
- Kessler, E.C.; Wall, S.K.; Hernandez, L.L.; Gross, J.J.; Bruckmaier, R.M. Short communication: Mammary gland tight junction permeability after parturition is greater in dairy cows with elevated circulating serotonin concentrations. J. Dairy Sci. 2019, 102, 1768–1774. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.; Liu, J.; Wang, K.; Wang, D. Effects of stocking density on oxidative stress status and mammary gland permeability in early lactating dairy cows. J. Anim. Sci. 2019, 90, 894–902. [Google Scholar] [CrossRef]
- Trevisi, E.; Amadori, M.; Cogrossi, S.; Razzuoli, E.; Bertoni, G. Metabolic stress and inflammatory response in high-yielding, periparturient dairy cows. Res. Vet. Sci. 2012, 93, 695–704. [Google Scholar] [CrossRef]
- Suriyasathaporn, W.; Vinitketkumnuen, U.; Chewonarin, T.; Boonyayatra, S.; Kreausukon, K.; Schukken, Y.H. Higher somatic cell counts resulted in higher malondialdehyde concentrations in raw cows’ milk. Int. Dairy J. 2006, 16, 1088–1091. [Google Scholar] [CrossRef]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar]
- Panchenko, L.F.; Brusov, O.S.; Gerasimov, A.M.; Loktaeva, T.D. Intramitochondrial localization and release of rat liver superoxide dismutase. FEBS Lett. 1975, 55, 84–87. [Google Scholar] [CrossRef] [Green Version]
- Roche, J.R.; Petch, S.; Kay, J.K. Manipulating the dietary cation-anion difference via drenching to early-lactation dairy cows grazing pasture. J. Dairy Sci. 2005, 88, 264–276. [Google Scholar] [CrossRef]
- Delamaire, E.; Guinard-Flament, J. Longer milking intervals alter mammary epithelial permeability and the udder’s ability to extract nutrients. J. Dairy Sci. 2006, 89, 2007–2016. [Google Scholar] [CrossRef]
- Silanikove, N.; Shamay, A.; Sinder, D.; Moran, A. Stress down regulates milk yield in cows by plasmin inducedβ-casein product that blocks K+ channels on the apical membranes. Life Sci. 2000, 67, 2201–2212. [Google Scholar] [CrossRef]
- Sakai, K. Coronary vasoconstriction by locally administered acetylcholine, carbachol and bethanechol in isolated, donor-perfused, rat hearts. Br. J. Pharmacol. 1980, 68, 625–632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gärtner, T.; Zoche-Golob, V.; Redlberger, S.; Reinhold, P.; Do-nat, K. Acid-base assessment of post-parturient German Holstein dairy cows from jugular venous blood and urine: A comparison of the strong ion approach and traditional blood gas analysis. PLoS ONE 2019, 14, e0210948. [Google Scholar] [CrossRef]
- Gianesella, M.; Morgante, M.; Cannizzo, C.; Stefani, A.; Dalvit, P.; Messina, V.; Giudice, E. Subacute ruminal acidosis and evaluation of blood gas analysis in dairy cow. Vet. Med. Int. 2010, 2010, 392371. [Google Scholar] [CrossRef] [Green Version]
- Dillane, P.; Krump, L.; Kennedy, A.; Sayers, R.G.; Sayers, G.P. Establishing blood gas ranges in healthy bovine neonates differentiated by age, sex, and breed type. J. Dairy Sci. 2018, 101, 3205–3212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Auvinen, J.; Tapio, J.; Karhunen, V.; Kettunen, J.; Serpi, R.; Dimova, E.Y.; Gill, D.; Soininen, P.; Tammelin, T.; Mykkänen, J.; et al. Systematic evaluation of the association between hemoglobin levels and metabolic profile implicates beneficial effects of hypoxia. Sci. Adv. 2021, 7, 29. [Google Scholar] [CrossRef]
- Erkkila, K.; Pentikainen, V.; Wikstrom, M.; Parvinen, M.; Dunkel, L. Partial oxygen pressure and mitochondrial permeability transition affect germ cell apoptosis in the human testis. J. Clin. Endocrinol. Metab. 1999, 84, 4253–4259. [Google Scholar] [CrossRef]
- Mecklenburgh, K.I.; Walmsley, S.R.; Cowburn, A.S.; Wiesener, M.; Reed, B.J.; Upton, P.D.; Deighton, J.; Greening, A.P.; Chilvers, E.R. Involvement of a ferroprotein sensor in hypoxia-mediated inhibition of neutrophil apoptosis. Blood 2002, 100, 3008–3016. [Google Scholar] [CrossRef]
- Blagitz, M.G.; Souza, F.N.; Gomes, V.; Della Libera, M.P. Apoptosis and necrosis of milk polymorphonuclear leukocytes with high and low somatic cell count. Small Ruminant Res. 2011, 100, 6. [Google Scholar] [CrossRef]
- Swain, D.K.; Kushwah, M.S.; Kaur, M.; Dang, A.K. Neutrophil dynamics in the blood and milk of crossbred cows naturally infected with Staphylococcus aureus. Vet. World 2015, 8, 336–345. [Google Scholar] [CrossRef]
- Shamay, A.; Shapiro, F.; Leitner, G.; Silanikove, N. Infusion of casein hydrolyzates into the mammary gland disrupt tight junction integrity and induce involution in cows. J. Dairy Sci. 2003, 86, 1250–1258. [Google Scholar] [CrossRef]
- Brown, R.C.; Mark, K.S.; Egleton, R.D.; Huber, J.D.; Burroughs, A.R.; Davis, T.P. Protection against hypoxia-induced increase in blood–brain barrier permeability: Role of tight junction proteins and NFκB. J. Cell. Sci. 2003, 116, 693–700. [Google Scholar] [CrossRef] [Green Version]
- Carvalho-Sombra, T.C.F.; Fernandes, D.D.; Bezerra, B.M.O.; Nunes-Pinheiroa, D.C.S. Systemic inflammatory biomarkers and somatic cell count in dairy cows with subclinical mastitis. Vet. Anim. Sci. 2021, 11, 100165. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Orellana, R.; Weng, X.; Marins, T.; Dahl, G.; Bernard, J. Symposium review: The influences of heat stress on bovine mammary gland function. J. Dairy Sci. 2018, 101, 5642–5654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Item 1 | L-SCC | H-SCC | SEM | p-Value |
|---|---|---|---|---|
| Milk yield, kg/d | 56.1 | 55.8 | 1.24 | 0.62 |
| Milk composition, g/100 g | ||||
| Protein | 3.10 | 2.97 | 0.059 | 0.13 |
| Fat | 3.80 | 3.83 | 0.245 | 0.92 |
| Lactose | 4.85 | 4.93 | 0.134 | 0.67 |
| Urea nitrogen, mg/dL | 13.8 | 14.1 | 0.51 | 0.61 |
| Item 1 | L-SCC | H-SCC | SEM | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Coccygeal Artery | Coccygeal Vein | Mammary Vein | Coccygeal Artery | Coccygeal Vein | Mammary Vein | Vessel | SCC | Interaction | ||
| Na+, mmol/L | 136.9 | 136.5 | 137.2 | 136.3 | 135.9 | 136.9 | 0.30 | 0.26 | 0.24 | 0.94 |
| K+, mmol/L | 3.76 b | 4.03 a | 3.65 b | 3.87 b | 4.23 a | 3.79 b | 0.0390 | <0.01 | <0.01 | 0.80 |
| TCO2, mmol/L | 30.9 b | 31.7 b | 32.5 a | 30.8 b | 31.2 b | 33.2 a | 0.33 | <0.01 | 0.94 | 0.56 |
| iCa, mmol/L | 1.19 b | 1.20 a | 1.14 b | 1.19 b | 1.23 a | 1.15 b | 0.101 | <0.01 | 0.40 | 0.74 |
| HCT | 0.245 | 0.236 | 0.236 | 0.244 | 0.234 | 0.232 | 0.006 | 0.62 | 0.07 | 0.51 |
| HGB, g/dL | 8.30 | 8.33 | 8.34 | 8.10 | 7.97 | 7.88 | 0.115 | 0.91 | 0.04 | 0.79 |
| pH | 7.49 a | 7.45 b | 7.51 a | 7.50 a | 7.43 b | 7.50 a | 0.009 | <0.01 | 0.57 | 0.60 |
| pCO2, mmHg | 37.6 | 45.3 a | 40.7 b | 35.9 | 44.8 a | 41.2 b | 0.45 | <0.01 | 0.35 | 0.35 |
| pO2, mmHg | 107 a | 31.2 | 40.3 | 101 b | 31.1 | 33.6 | 1.40 | <0.01 | 0.03 | 0.29 |
| HCO3−, mmol/L | 29.8 b | 30.4 b | 31.3 a | 29.6 b | 29.8 b | 31.9 a | 0.31 | <0.01 | 0.85 | 0.53 |
| BEecf, mmol/L | 6.70 b | 5.60 | 7.90 a | 7.00 b | 5.70 | 8.70 a | 0.446 | <0.01 | 0.28 | 0.72 |
| Saturation of O2 % | 98.2 a | 60.0 | 77.8 b | 98.7 a | 60.3 | 69.3 | 2.13 | <0.01 | 0.15 | 0.05 |
| Item 1 | L-SCC | H-SCC | SEM | p-Value |
|---|---|---|---|---|
| Coccygeal artery | 11.4 | 11.2 | 0.27 | 0.54 |
| Coccygeal vein | 10.6 | 11.1 | 0.54 | 0.56 |
| Mammary vein | 9.15 a | 7.72 b | 0.224 | <0.01 |
| Item 1 | L-SCC | H-SCC | SEM | p-Value |
|---|---|---|---|---|
| Oxidative-stress-related variables | ||||
| MDA, nmol/L | 9.52 b | 10.45 a | 0.26 | 0.02 |
| GSH-Px, U/mL | 138.1 | 128.3 | 5.39 | 0.21 |
| SOD, U/mL | 119.0 a | 104.8 b | 2.76 | <0.01 |
| T-AOC, U/mL | 14.0 | 11.9 | 0.73 | 0.06 |
| Mammary-gland permeability | ||||
| Na+, mmol/L | 19.2 | 20.6 | 0.71 | 0.21 |
| K+, mmol/L | 37.8 | 35.7 | 0.81 | 0.08 |
| Na+/K+ | 0.51 | 0.57 | 0.024 | 0.07 |
| BSA, g/L | 0.72 | 0.78 | 0.034 | 0.25 |
| Plasmin, U/L | 2.58 b | 3.17 a | 0.21 | 0.05 |
| LDH, U/L | 223 b | 254 a | 8.5 | 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, J.; Peng, W.; Hu, Z.; Cai, J.; Liu, J.; Wang, D. Multiple-Vessel-Based Blood Gas Profiles Analysis Revealed the Potential of Blood Oxygen in Mammary Vein as Indicator of Mammary Gland Health Risk of High-Yielding Dairy Cows. Animals 2022, 12, 1484. https://doi.org/10.3390/ani12121484
Feng J, Peng W, Hu Z, Cai J, Liu J, Wang D. Multiple-Vessel-Based Blood Gas Profiles Analysis Revealed the Potential of Blood Oxygen in Mammary Vein as Indicator of Mammary Gland Health Risk of High-Yielding Dairy Cows. Animals. 2022; 12(12):1484. https://doi.org/10.3390/ani12121484
Chicago/Turabian StyleFeng, Juan, Wenchao Peng, Zhenzhen Hu, Jie Cai, Jianxin Liu, and Diming Wang. 2022. "Multiple-Vessel-Based Blood Gas Profiles Analysis Revealed the Potential of Blood Oxygen in Mammary Vein as Indicator of Mammary Gland Health Risk of High-Yielding Dairy Cows" Animals 12, no. 12: 1484. https://doi.org/10.3390/ani12121484
APA StyleFeng, J., Peng, W., Hu, Z., Cai, J., Liu, J., & Wang, D. (2022). Multiple-Vessel-Based Blood Gas Profiles Analysis Revealed the Potential of Blood Oxygen in Mammary Vein as Indicator of Mammary Gland Health Risk of High-Yielding Dairy Cows. Animals, 12(12), 1484. https://doi.org/10.3390/ani12121484






