Cats and SARS-CoV-2: A Scoping Review
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Data Sources and Search Strategy
2.2. Study Selection
2.3. Data Characterization, Summary, and Synthesis
3. Results
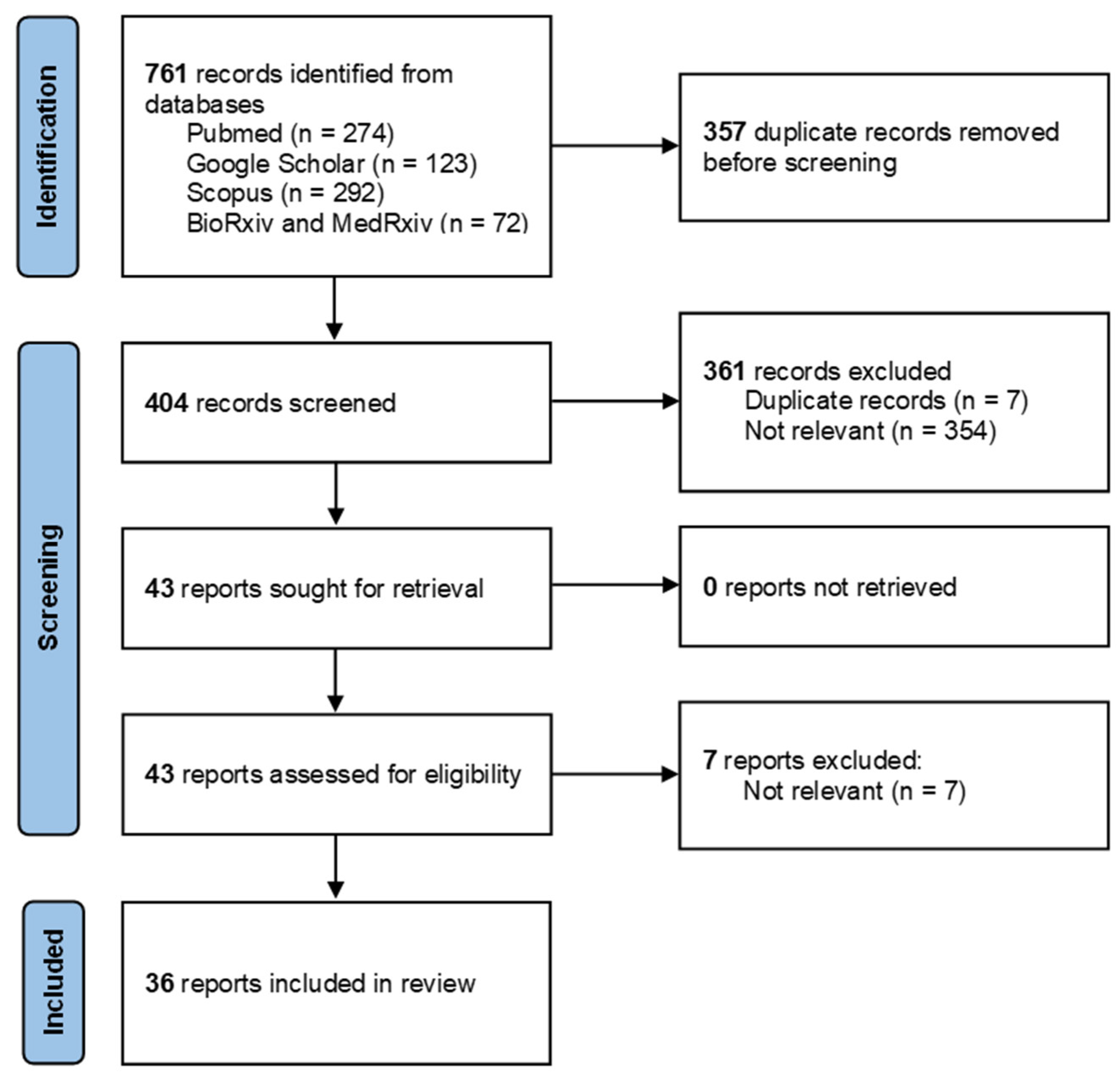
3.1. Article Selection
3.2. General Information about the Included Reports
3.3. Cats and SARS-CoV-2
3.3.1. Susceptibility of Cats to SARS-CoV-2
3.3.2. SARS-CoV-2-Induced Immunity and Seroprevalence among Cats
3.3.3. Manifestation of Infection
- cardiac abnormalities [22],
3.4. Humans to Cat Transmission
3.5. Cat to Cat Transmission
4. Discussion
4.1. Key Findings
4.1.1. Main Themes
4.1.2. Types of Evidence
4.1.3. Identified Gaps in Research
4.2. Positioning This Review within the Current Academic Debate
4.2.1. Mink-to-Cat Transmission
4.2.2. Seroprevalence of SARS-CoV-2 Antibodies among Cats
4.2.3. The Role of Subclinical Infections
4.2.4. Cat-to-Human Transmission
4.2.5. Cat-to-Cat Transmission
4.2.6. Evolution of SARS-CoV-2 in Cats
4.3. Zooming Out: The Wider Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- WHO. WHO-Convened Global Study of Origins of SARS-CoV-2: China Part; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Hassani, A.; Khan, G. Human-Animal Interaction and the Emergence of SARS-CoV-2. JMIR Public Health Surveill. 2020, 6, e22117. [Google Scholar] [CrossRef] [PubMed]
- Sharun, K.; Tiwari, R.; Natesan, S.; Dhama, K. SARS-CoV-2 infection in farmed minks, associated zoonotic concerns, and importance of the One Health approach during the ongoing COVID-19 pandemic. Vet. Q. 2021, 41, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Mahdy, M.A.A.; Younis, W.; Ewaida, Z. An Overview of SARS-CoV-2 and Animal Infection. Front. Vet. Sci. 2020, 7, 1084. [Google Scholar] [CrossRef]
- Dróżdż, M.; Krzyżek, P.; Dudek, B.; Makuch, S.; Janczura, A.; Paluch, E. Current State of Knowledge about Role of Pets in Zoonotic Transmission of SARS-CoV-2. Viruses 2021, 13, 1149. [Google Scholar] [CrossRef]
- Wu, L.; Chen, Q.; Liu, K.; Wang, J.; Han, P.; Zhang, Y.; Hu, Y.; Meng, Y.; Pan, X.; Qiao, C.; et al. Broad host range of SARS-CoV-2 and the molecular basis for SARS-CoV-2 binding to cat ACE2. Cell Discov. 2020, 6, 68. [Google Scholar] [CrossRef]
- He, S.; Han, J.; Lichtfouse, E. Backward transmission of COVID-19 from humans to animals may propagate reinfections and induce vaccine failure. Environ. Chem. Lett. 2021, 19, 763–768. [Google Scholar] [CrossRef]
- Dyer, O. COVID-19: Denmark to kill 17 million minks over mutation that could undermine vaccine effort. BMJ 2020, 371, m4338. [Google Scholar] [CrossRef]
- Stout, A.E.; André, N.M.; Jaimes, J.A.; Millet, J.K.; Whittaker, G.R. Coronaviruses in cats and other companion animals: Where does SARS-CoV-2/COVID-19 fit? Vet. Microbiol. 2020, 247, 108777. [Google Scholar] [CrossRef]
- Hosie, M.J.; Epifano, I.; Herder, V.; Orton, R.J.; Stevenson, A.; Johnson, N.; MacDonald, E.; Dunbar, D.; McDonald, M.; Howie, F.; et al. Detection of SARS-CoV-2 in respiratory samples from cats in the UK associated with human-to-cat transmission. Vet. Rec. 2021, 188, e247. [Google Scholar] [CrossRef]
- Singla, R.; Mishra, A.; Joshi, R.; Jha, S.; Sharma, A.R.; Upadhyay, S.; Sarma, P.; Prakash, A.; Medhi, B. Human animal interface of SARS-CoV-2 (COVID-19) transmission: A critical appraisal of scientific evidence. Vet. Res. Commun. 2020, 44, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Jin, Y.; Liu, Y.; Sun, J.; Hao, L.; Bai, J.; Huang, T.; Lin, D.; Jin, Y.; Tian, K. Serological survey of SARS-CoV-2 for experimental, domestic, companion and wild animals excludes intermediate hosts of 35 different species of animals. Transbound. Emerg. Dis. 2020, 67, 1745–1749. [Google Scholar] [CrossRef] [PubMed]
- Halfmann, P.J.; Hatta, M.; Chiba, S.; Maemura, T.; Fan, S.; Takeda, M.; Kinoshita, N.; Hattori, S.I.; Sakai-Tagawa, Y.; Iwatsuki-Horimoto, K.; et al. Transmission of SARS-CoV-2 in Domestic Cats. N. Engl. J. Med. 2020, 383, 592–594. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Wen, Z.; Zhong, G.; Yang, H.; Wang, C.; Huang, B.; Liu, R.; He, X.; Shuai, L.; Sun, Z.; et al. Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS–coronavirus 2. Science 2020, 368, 1016–1020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Page, M.J.; Mc Kenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Song, Z.; Xue, J.; Gao, H.; Liu, J.; Wang, J.; Guo, Q.; Zhao, B.; Qu, Y.; Qi, F.; et al. Susceptibility and Attenuated Transmissibility of SARS-CoV-2 in Domestic Cats. J. Infect. Dis. 2021, 223, 1313–1321. [Google Scholar] [CrossRef]
- Bessière, P.; Fusade-Boyer, M.; Walch, M.; Lèbre, L.; Brun, J.; Croville, G.; Boullier, S.; Cadiergues, M.C.; Guérin, J.L. Household cases suggest that cats belonging to owners with COVID-19 have a limited role in virus transmission. Viruses 2021, 13, 673. [Google Scholar] [CrossRef]
- Brandão, L.N.S.; Homochiski, D.P.; de Oliveira, L.B.; de Oliveira, R.M.S.; Maciel, S.A. Detection of SARS-CoV-2 by the RT-qPCR in domestic cats. Res. Soc. Dev. 2021, 10, e40110918184. [Google Scholar] [CrossRef]
- Braun, K.M.; Moreno, G.K.; Halfmann, P.J.; Hodcroft, E.B.; Baker, D.A.; Boehm, E.C.; Weiler, A.M.; Haj, A.K.; Hatta, M.; Chiba, S.; et al. Transmission of SARS-CoV-2 in domestic cats imposes a narrow bottleneck. PLoS Pathog. 2021, 17, e1009373. [Google Scholar] [CrossRef]
- Chaintoutis, S.C.; Siarkou, V.I.; Mylonakis, M.E.; Kazakos, G.M.; Skeva, P.N.; Bampali, M.; Dimitriou, M.; Dovrolis, N.; Polizopoulou, Z.S.; Karakasiliotis, I.; et al. Limited cross-species transmission and absence of mutations associated with SARS-CoV-2 adaptation in cats: A case study of infection in a small household setting. Transbound. Emerg. Dis. 2021, 69, 1606–1616. [Google Scholar] [CrossRef]
- Curukoglu, A.; Ergoren, M.C.; Ozgencil, F.E.; Sayiner, S.; Ince, M.E.; Sanlidag, T. First direct human-to-cat transmission of the SARS-CoV-2 B.1.1.7 variant. Aust. Vet. J. 2021, 99, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Gaudreault, N.N.; Trujillo, J.D.; Carossino, M.; Meekins, D.A.; Morozov, I.; Madden, D.W.; Indran, S.V.; Bold, D.; Balaraman, V.; Kwon, T.; et al. SARS-CoV-2 infection, disease and transmission in domestic cats. Emerg. Microbes Infect. 2020, 9, 2322–2332. [Google Scholar] [CrossRef] [PubMed]
- Gaudreault, N.N.; Carossino, M.; Morozov, I.; Trujillo, J.D.; Meekins, D.A.; Madden, D.W.; Cool, K.; Artiaga, B.L.; McDowell, C.; Bold, D.; et al. Experimental re-infected cats do not transmit SARS-CoV-2. Emerg. Microbes Infect. 2021, 10, 638–650. [Google Scholar] [CrossRef] [PubMed]
- Jara, L.M.; Ferradas, C.; Schiaffino, F.; Sánchez-Carrión, C.; Martínez-Vela, A.; Ulloa, A.; Isasi-Rivas, G.; Montalván, A.; Sarmiento, L.G.; Fernández, M.; et al. Evidence of neutralizing antibodies against SARS-CoV-2 in domestic cats living with owners with a history of COVID-19 in Lima—Peru. One Health 2021, 13, 100318. [Google Scholar] [CrossRef]
- Keller, M.; Hagag, I.T.; Balzer, J.; Beyer, K.; Kersebohm, J.C.; Sadeghi, B.; Wernike, K.; Höper, D.; Wylezich, C.; Beer, M. Detection of SARS-CoV-2 variant B. 1.1. 7 in a cat in Germany. Res. Vet. Sci. 2021, 140, 229–232. [Google Scholar] [CrossRef]
- Klaus, J.; Palizzotto, C.; Zini, E.; Meli, M.L.; Leo, C.; Egberink, H.; Zhao, S.; Hofmann-Lehmann, R. SARS-CoV-2 infection and antibody response in a symptomatic cat from italy with intestinal b-cell lymphoma. Viruses 2021, 13, 527. [Google Scholar] [CrossRef]
- Klaus, J.; Meli, M.L.; Willi, B.; Nadeau, S.; Beisel, C.; Stadler, T.; Eth Sars-Co, V.S.T.; Egberink, H.; Zhao, S.; Lutz, H.; et al. Detection and Genome Sequencing of SARS-CoV-2 in a Domestic Cat with Respiratory Signs in Switzerland. Viruses 2021, 13, 496. [Google Scholar] [CrossRef]
- Michelitsch, A.; Hoffmann, D.; Wernike, K.; Beer, M. Occurrence of antibodies against SARS-CoV-2 in the domestic cat population of Germany. Vaccines 2020, 8, 772. [Google Scholar] [CrossRef]
- Michelitsch, A.; Schön, J.; Hoffmann, D.; Beer, M.; Wernike, K. The Second Wave of SARS-CoV-2 Circulation-Antibody Detection in the Domestic Cat Population in Germany. Viruses 2021, 13, 1009. [Google Scholar] [CrossRef]
- Musso, N.; Costantino, A.; La Spina, S.; Finocchiaro, A.; Andronico, F.; Stracquadanio, S.; Liotta, L.; Visalli, R.; Emmanuele, G. New SARS-CoV-2 Infection Detected in an Italian Pet Cat by RT-qPCR from Deep Pharyngeal Swab. Pathogens 2020, 9, 746. [Google Scholar] [CrossRef]
- Natale, A.; Mazzotta, E.; Mason, N.; Ceglie, L.; Mion, M.; Stefani, A.; Fincato, A.; Bonfante, F.; Bortolami, A.; Monne, I.; et al. SARS-CoV-2 natural infection in a symptomatic cat: Diagnostic, clinical and medical management in a one health vision. Animals 2021, 11, 1640. [Google Scholar] [CrossRef] [PubMed]
- Neira, V.; Brito, B.; Agüero, B.; Berrios, F.; Valdés, V.; Gutierrez, A.; Ariyama, N.; Espinoza, P.; Retamal, P.; Holmes, E.C.; et al. A household case evidences shorter shedding of SARS-CoV-2 in naturally infected cats compared to their human owners. Emerg. Microbes Infect. 2021, 10, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Pagani, G.; Lai, A.; Bergna, A.; Rizzo, A.; Stranieri, A.; Giordano, A.; Paltrinieri, S.; Lelli, D.; Decaro, N.; Rusconi, S.; et al. Human-to-Cat SARS-CoV-2 Transmission: Case Report and Full-Genome Sequencing from an Infected Pet and Its Owner in Northern Italy. Pathogens 2021, 10, 252. [Google Scholar] [CrossRef] [PubMed]
- Segalés, J.; Puig, M.; Rodon, J.; Avila-Nieto, C.; Carrillo, J.; Cantero, G.; Terrón, M.T.; Cruz, S.; Parera, M.; Noguera-Julián, M.; et al. Detection of SARS-CoV-2 in a cat owned by a COVID-19-affected patient in Spain. Proc. Natl. Acad. Sci. USA 2020, 117, 24790–24793. [Google Scholar] [CrossRef] [PubMed]
- Spada, E.; Vitale, F.; Bruno, F.; Castelli, G.; Reale, S.; Perego, R.; Baggiani, L.; Proverbio, D. A pre-and during pandemic survey of SARS-CoV-2 infection in stray colony and shelter cats from a high endemic area of Northern Italy. Viruses 2021, 13, 618. [Google Scholar] [CrossRef]
- Stranieri, A.; Lauzi, S.; Giordano, A.; Galimberti, L.; Ratti, G.; Decaro, N.; Brioschi, F.; Lelli, D.; Gabba, S.; Amarachi, N.L.; et al. Absence of SARS-CoV-2 RNA and anti-SARS-CoV-2 antibodies in stray cats. Transbound. Emerg. Dis. 2021. [Google Scholar] [CrossRef]
- Subotsina, I.; Gromov, I.; Kupryianav, I. Features of clinical and pathomorphological picture in spontaneous infection of a domestic cat (lat. Félis cátus) with SARS-CoV-2 coronavirus. Nauk. Vìsn. Vet. Med. 2021, 165, 79–91. [Google Scholar] [CrossRef]
- Van der Leij, W.J.R.; Broens, E.M.; Hesselink, J.W.; Schuurman, N.; Vernooij, J.C.M.; Egberink, H.F. Serological Screening for Antibodies against SARS-CoV-2 in Dutch Shelter Cats. Viruses 2021, 13, 1634. [Google Scholar] [CrossRef]
- Villanueva-Saz, S.; Giner, J.; Tobajas, A.P.; Pérez, M.D.; González-Ramírez, A.M.; Macías-León, J.; González, A.; Verde, M.; Yzuel, A.; Hurtado-Guerrero, R.; et al. Serological evidence of SARS-CoV-2 and co-infections in stray cats in Spain. Transbound. Emerg. Dis. 2021, 9, 1056–1064. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, H.; Gao, J.; Huang, K.; Yang, Y.; Hui, X.; He, X.; Li, C.; Gong, W.; Zhang, Y.; et al. A serological survey of SARS-CoV-2 in cat in Wuhan. Emerg. Microbes Infect. 2020, 9, 2013–2019. [Google Scholar] [CrossRef]
- Zoccola, R.; Beltramo, C.; Magris, G.; Peletto, S.; Acutis, P.; Bozzetta, E.; Radovic, S.; Zappulla, F.; Porzio, A.M.; Gennero, M.S.; et al. First detection of an Italian human-to-cat outbreak of SARS-CoV-2 Alpha variant—lineage B.1.1.7. One Health 2021, 13, 100295. [Google Scholar] [CrossRef] [PubMed]
- Barrs, V.R.; Peiris, M.; Tam, K.W.S.; Law, P.Y.T.; Brackman, C.J.; To, E.M.W.; Yu, V.Y.T.; Chu, D.K.W.; Perera, R.; Sit, T.H.C. SARS-CoV-2 in Quarantined Domestic Cats from COVID-19 Households or Close Contacts, Hong Kong, China. Emerg. Infect. Dis. 2020, 26, 3071–3074. [Google Scholar] [CrossRef] [PubMed]
- Carlos, R.S.A.; Mariano, A.P.M.; Maciel, B.M.; Gadelha, S.R.; de Melo Silva, M.; Belitardo, E.; Rocha, D.; de Almeida, J.P.P.; Pacheco, L.G.C.; Aguiar, E.; et al. First genome sequencing of SARS-CoV-2 recovered from an infected cat and its owner in Latin America. Transbound. Emerg. Dis. 2021, 68, 3070–3074. [Google Scholar] [CrossRef] [PubMed]
- Chiba, S.; Halfmann, P.J.; Hatta, M.; Maemura, T.; Fan, S.; Armbrust, T.; Swartley, O.M.; Crawford, L.K.; Kawaoka, Y. Protective Immunity and Persistent Lung Sequelae in Domestic Cats after SARS-CoV-2 Infection. Emerg. Infect. Dis. 2021, 27, 660–663. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Liu, Y.; Sun, C.; Bai, J.; Sun, J.; Hao, L.; Li, X.; Tian, K. SARS-CoV-2 Serological Survey of Cats in China before and after the Pandemic. Virol. Sin. 2020, 35, 846–848. [Google Scholar] [CrossRef]
- Garigliany, M.; Van Laere, A.S.; Clercx, C.; Giet, D.; Escriou, N.; Huon, C.; Van Der Werf, S.; Eloit, M.; Desmecht, D. SARS-CoV-2 natural transmission from human to Cat, Belgium, March 2020. Emerg. Infect. Dis. 2020, 26, 3069–3071. [Google Scholar] [CrossRef]
- Schulz, C.; Wylezich, C.; Wernike, K.; Gründl, M.; Dangel, A.; Baechlein, C.; Hoffmann, D.; Röhrs, S.; Hepner, S.; Ackermann, N.; et al. Prolonged SARS-CoV-2 RNA shedding from therapy cat after cluster outbreak in retirement home. Emerg. Infect. Dis. 2021, 27, 1974–1976. [Google Scholar] [CrossRef]
- Akhmetzhanov, A.R.; Linton, N.M.; Nishiura, H. Rising evidence of COVID-19 transmission potential to and between animals: Do we need to be concerned? medRxiv 2020. [Google Scholar] [CrossRef]
- Fritz, M.; Nesi, N.; Denolly, S.; Boson, B.; Legros, V.; Rosolen, S.G.; Briend-Marchal, A.; Gouilh, M.A.; Leroy, E.M. New detection of SARS-CoV-2 in two cats height months after COVID-19 outbreak appearance in France. bioRxiv 2021. [Google Scholar] [CrossRef]
- Hale, V.L.; Dennis, P.M.; McBride, D.S.; Nolting, J.M.; Madden, C.; Huey, D.; Ehrlich, M.; Grieser, J.; Winston, J.; Lombardi, D.; et al. SARS-CoV-2 infection in free-ranging white-tailed deer. Nature 2021, 602, 481–486. [Google Scholar] [CrossRef]
- Giraldo-Ramirez, S.; Rendon-Marin, S.; Jaimes, J.A.; Martinez-Gutierrez, M.; Ruiz-Saenz, J. SARS-CoV-2 Clinical Outcome in Domestic and Wild Cats: A Systematic Review. Animals 2021, 11, 2056. [Google Scholar] [CrossRef] [PubMed]
- Hosie, M.J.; Hofmann-Lehmann, R.; Hartmann, K.; Egberink, H.; Truyen, U.; Addie, D.D.; Belák, S.; Boucraut-Baralon, C.; Frymus, T.; Lloret, A.; et al. Anthropogenic Infection of Cats during the 2020 COVID-19 Pandemic. Viruses 2021, 13, 185. [Google Scholar] [CrossRef] [PubMed]
- Sharun, K.; Dhama, K.; Pawde, A.M.; Gortázar, C.; Tiwari, R.; Bonilla-Aldana, D.K.; Rodriguez-Morales, A.J.; de la Fuente, J.; Michalak, I.; Attia, Y.A. SARS-CoV-2 in animals: Potential for unknown reservoir hosts and public health implications. Vet. Q. 2021, 41, 181–201. [Google Scholar] [CrossRef] [PubMed]
- Van Aart, A.E.; Velkers, F.C.; Fischer, E.A.J.; Broens, E.M.; Egberink, H.; Zhao, S.; Engelsma, M.; Hakze-van der Honing, R.W.; Harders, F.; de Rooij, M.M.T.; et al. SARS-CoV-2 infection in cats and dogs in infected mink farms. Transbound. Emerg. Dis. 2021, 1865. [Google Scholar] [CrossRef]
- Oreshkova, N.; Molenaar, R.J.; Vreman, S.; Harders, F.; Oude Munnink, B.B.; Hakze-van der Honing, R.W.; Gerhards, N.; Tolsma, P.; Bouwstra, R.; Sikkema, R.S.; et al. SARS-CoV-2 infection in farmed minks, the Netherlands, April and May 2020. Eurosurveillance 2020, 25, 2001005. [Google Scholar] [CrossRef]
- Boklund, A.; Hammer, A.S.; Quaade, M.L.; Rasmussen, T.B.; Lohse, L.; Strandbygaard, B.; Jørgensen, C.S.; Olesen, A.S.; Hjerpe, F.B.; Petersen, H.H.; et al. SARS-CoV-2 in Danish Mink Farms: Course of the Epidemic and a Descriptive Analysis of the Outbreaks in 2020. Animals 2021, 11, 164. [Google Scholar] [CrossRef]
- Tan, C.W.; Chia, W.N.; Qin, X.; Liu, P.; Chen, M.I.C.; Tiu, C.; Hu, Z.; Chen, V.C.-W.; Young, B.E.; Sia, W.R.; et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2–spike protein–protein interaction. Nat. Biotechnol. 2020, 38, 1073–1078. [Google Scholar] [CrossRef]
- Totton, S.C.; Sargeant, J.M.; O’Connor, A.M. How could we conclude cat-to-human transmission of SARS-CoV-2? Zoonoses Public Health 2021, 68, 67–68. [Google Scholar] [CrossRef]
- Li, P.; Wang, Y.; Lavrijsen, M.; Lamers, M.M.; de Vries, A.C.; Rottier, R.J.; Bruno, M.J.; Peppelenbosch, M.P.; Haagmans, B.L.; Pan, Q. SARS-CoV-2 Omicron variant is highly sensitive to molnupiravir, nirmatrelvir, and the combination. Cell Res. 2022, 32, 322–324. [Google Scholar] [CrossRef]
- Chan, J.F.-W.; To, K.K.-W.; Tse, H.; Jin, D.-Y.; Yuen, K.-Y. Interspecies transmission and emergence of novel viruses: Lessons from bats and birds. Trends Microbiol. 2013, 21, 544–555. [Google Scholar] [CrossRef]
- Baum, A.; Fulton, B.O.; Wloga, E.; Copin, R.; Pascal, K.E.; Russo, V.; Giordano, S.; Lanza, K.; Negron, N.; Ni, M.; et al. Antibody cocktail to SARS-CoV-2 spike protein prevents rapid mutational escape seen with individual antibodies. Science 2020, 369, 1014–1018. [Google Scholar] [CrossRef] [PubMed]
- Dieterle, M.E.; Haslwanter, D.; Bortz, R.H., 3rd; Wirchnianski, A.S.; Lasso, G.; Vergnolle, O.; Abbasi, S.A.; Fels, J.M.; Laudermilch, E.; Florez, C.; et al. A replication-competent vesicular stomatitis virus for studies of SARS-CoV-2 spike-mediated cell entry and its inhibition. bioRxiv 2020. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yang, C.; Xu, X.-f.; Xu, W.; Liu, S.-w. Structural and functional properties of SARS-CoV-2 spike protein: Potential antivirus drug development for COVID-19. Acta Pharmacol. Sin. 2020, 41, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- NCIRD. Science Brief: Omicron (B.1.1.529) Variant. In CDC COVID-19 Science Briefs; Centers for Disease Control and Prevention (US): Atlanta, GA, USA, 2021. [Google Scholar]
- Bashor, L.; Gagne, R.B.; Bosco-Lauth, A.M.; Bowen, R.A.; Stenglein, M.; VandeWoude, S. SARS-CoV-2 evolution in animals suggests mechanisms for rapid variant selection. Proc. Natl. Acad. Sci. USA 2021, 118, e2105253118. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Lin, W.; Dong, W.; Xu, J. Origin and evolutionary analysis of the SARS-CoV-2 Omicron variant. J. Biosaf. Biosecurity 2022, 4, 33–37. [Google Scholar] [CrossRef]
- Burki, T. The origin of SARS-CoV-2 variants of concern. Lancet Infect. Dis. 2022, 22, 174–175. [Google Scholar] [CrossRef]
- Kupferschmidt, K. Where did ‘weird’ Omicron come from? Science 2021, 374, 1179. [Google Scholar] [CrossRef]
- Devaux, C.A.; Pinault, L.; Delerce, J.; Raoult, D.; Levasseur, A.; Frutos, R. Spread of Mink SARS-CoV-2 Variants in Humans: A Model of Sarbecovirus Interspecies Evolution. Front. Microbiol. 2021, 12, 2842. [Google Scholar] [CrossRef]
- Larsen, H.D.; Fonager, J.; Lomholt, F.K.; Dalby, T.; Benedetti, G.; Kristensen, B.; Urth, T.R.; Rasmussen, M.; Lassaunière, R.; Rasmussen, T.B.; et al. Preliminary report of an outbreak of SARS-CoV-2 in mink and mink farmers associated with community spread, Denmark, June to November 2020. Eurosurveillance 2021, 26, 2100009. [Google Scholar] [CrossRef]
- Griffin, B.; Baker, H.J. Domestic Cats as Laboratory Animals. In Laboratory Animal Medicine, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2002; pp. 459–482. [Google Scholar] [CrossRef]
- Osenkowski, P.; Green, C.; Tjaden, A.; Cunniff, P. Evaluation of Educator &Student Use of & Attitudes toward Dissection & Dissection Alternatives. Am. Biol. Teach. 2015, 77, 340–346. [Google Scholar] [CrossRef]
- Sikkema, R.; Schrijver, R.; Dewar, D.; Vries, H.; Bergevoet, R.H.M.; Messori, S.; Barnard, S.; D’Albenzio, S. Study on the Welfare of Dogs and Cats Involved in Commercial Practices; IBF International Consulting, Wageningen University & Research Centre and Istituto Zooprofilattico Sperimentale dell’Abruzzo e del Molise “G. Caporale”: Wageningen, The Netherlands, 2015. [Google Scholar]
- Krzysztof, R.; Włodarczyk, M.; Chorbiński, P.; Płoneczka, K. Viral infections in cats in Wrocław city. Med. Weter. 2004, 60, 841–844. [Google Scholar]
- Chandler, J.C.; Bevins, S.N.; Ellis, J.W.; Linder, T.J.; Tell, R.M.; Jenkins-Moore, M.; Root, J.J.; Lenoch, J.B.; Robbe-Austerman, S.; DeLiberto, T.J.; et al. SARS-CoV-2 exposure in wild white-tailed deer. Proc. Natl. Acad. Sci. USA 2021, 118, e2114828118. [Google Scholar] [CrossRef] [PubMed]
- Palermo, P.M.; Orbegozo, J.; Watts, D.M.; Morrill, J.C. SARS-CoV-2 Neutralizing Antibodies in White-Tailed Deer from Texas. Vector Borne Zoonotic Dis 2022, 22, 62–64. [Google Scholar] [CrossRef] [PubMed]
- Vandegrift, K.J.; Yon, M.; Surendran-Nair, M.; Gontu, A.; Amirthalingam, S.; Nissly, R.H.; Levine, N.; Stuber, T.; DeNicola, A.J.; Boulanger, J.R.; et al. Detection of SARS-CoV-2 Omicron variant (B.1.1.529) infection of white-tailed deer. bioRxiv 2022. [Google Scholar] [CrossRef]
- Fenollar, F.; Mediannikov, O.; Maurin, M.; Devaux, C.; Colson, P.; Levasseur, A.; Fournier, P.-E.; Raoult, D. Mink, SARS-CoV-2, and the Human-Animal Interface. Front. Microbiol. 2021, 12, 663815. [Google Scholar] [CrossRef]
- Oude Munnink, B.B.; Sikkema, R.S.; Nieuwenhuijse, D.F.; Molenaar, R.J.; Munger, E.; Molenkamp, R.; van der Spek, A.; Tolsma, P.; Rietveld, A.; Brouwer, M.; et al. Transmission of SARS-CoV-2 on mink farms between humans and mink and back to humans. Science 2021, 371, 172–177. [Google Scholar] [CrossRef]
- Xia, C.; Lam, S.S.; Sonne, C. Ban unsustainable mink production. Science 2020, 370, 539. [Google Scholar] [CrossRef]
- Spotte, S. Free-Ranging Cats: Behavior, Ecology, Management; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Frutos, R.; Gavotte, L.; Devaux, C.A. Understanding the origin of COVID-19 requires to change the paradigm on zoonotic emergence from the spillover to the circulation model. Infect. Genet. Evol. 2021, 95, 104812. [Google Scholar] [CrossRef]
- Frutos, R.; Serra-Cobo, J.; Chen, T.; Devaux, C.A. COVID-19: Time to exonerate the pangolin from the transmission of SARS-CoV-2 to humans. Infect. Genet. Evol. 2020, 84, 104493. [Google Scholar] [CrossRef]
- Costagliola, A.; Liguori, G.; d’Angelo, D.; Costa, C.; Ciani, F.; Giordano, A. Do Animals Play a Role in the Transmission of Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2)? A Commentary. Animals 2020, 11, 16. [Google Scholar] [CrossRef]
- Davis, M.F.; Innes, G.K. The Cat’s in the Bag: Despite Limited Cat-to-Cat Severe Acute Respiratory Syndrome Coronavirus 2 Transmission, One Health Surveillance Efforts Are Needed. J. Infect. Dis. 2021, 223, 1309–1312. [Google Scholar] [CrossRef] [PubMed]
- Murphy, H.L.; Ly, H. Understanding the prevalence of SARS-CoV-2 (COVID-19) exposure in companion, captive, wild, and farmed animals. Virulence 2021, 12, 2777–2786. [Google Scholar] [CrossRef] [PubMed]
- Hosie, M.J.; Hartmann, K.; Hofmann-Lehmann, R.; Addie, D.D.; Truyen, U.; Egberink, H.; Tasker, S.; Frymus, T.; Pennisi, M.G.; Möstl, K. SARS-Coronavirus (CoV)-2 and Cats. Available online: http://www.abcdcatsvets.org/sars-coronavirus-2-and-cats/ (accessed on 14 February 2022).
- Sharun, K.; Tiwari, R.; Saied, A.A.; Dhama, K. SARS-CoV-2 vaccine for domestic and captive animals: An effort to counter COVID-19 pandemic at the human-animal interface. Vaccine 2021, 39, 7119–7122. [Google Scholar] [CrossRef] [PubMed]

| Topic | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Language | English | Language other than English |
| Publication date | 2019–2021 | Before 2019 |
| Study design | All study designs | |
| Research type | Primary research | Secondary research |
| Topic area |
|
|
| Study participants |
|
|
| Publication type |
|
|
| First Author | Year | Location | Study Aim | Study Type | N * | Reference |
|---|---|---|---|---|---|---|
| Peer-reviewed articles | ||||||
| Bao | 2021 | China (Beijing) | Evaluation of the cat-to-cat transmissibility of SARS-CoV-2 upon serial passaging | Case Study | 18 | [17] |
| Bessière | 2021 | France (Toulouse) | Enhancing understanding of cats’ role in COVID-19 epidemiology through monitoring of viral shedding in five different cats and their quarantined, COVID-19 positive owners | Case Study | 5 | [18] |
| Brandão | 2021 | Brazil (Alta Floresta city) | Reporting SARS-CoV-2 detection using PCR testing and clinical manifestations in two domestic cats | Case Study | 2 | [19] |
| Braun | 2021 | USA (Wisconsin) | Characterization of viral genetic diversity arising, persisting, and being transmitted in domestic cats to model the evolutionary capacity of SARS-CoV-2 within and between hosts | Experimental | 6 | [20] |
| Chaintoutis | 2021 | Greece (Thessaloniki) | Reporting the first case of SARS-CoV-2 infection in a cat in Latin America | Case Study | 3 | [21] |
| Curukoglu | 2021 | Northern Cyprus | Investigation of the course of SARS-CoV-2 infection in naturally exposed cats in small household setting | Case Study | 1 | [22] |
| Gaudreault | 2020 | USA (Manhattan, Kansas) | In-depth study of SARS-CoV-2 infection, associated disease and transmission in domestic cats | Experimental | 10 | [23] |
| Gaudreault | 2021 | USA (Manhattan, Kansas) | Investigation of possibility of SARS-CoV-2 re-infection in previously infected cats | Experimental | 11 | [24] |
| Hosie | 2021 | United Kingdom | Finding evidence of SARS-CoV-2 infection in cats from the UK | Case Study | 2 | [11] |
| Jara | 2021 | Peru (Lima) | Demonstrating presence of neutralizing antibodies against SARS-CoV-2 in cats whose owners have been infected with SARS-CoV-2 | Cross-sectional | 41 | [25] |
| Keller | 2021 | Germany | Reporting of first case of a cat infected with SARS-CoV-2 alpha variant of concern | Case Study | 1 | [26] |
| Klaus | 2021 | Italy | Investigation of the case of a cat with SARS-CoV-2 with underlying B-cell lymphoma | Case Study | 1 | [27] |
| Klaus | 2021 | Switzerland (Zurich) | Reporting of first SARS-CoV-2 infections in cats in a COVID-19 affected household in Switzerland | Case Study | 2 | [28] |
| Michelitsch | 2020 | Germany | Assessing the incidence of naturally occurring human-to-cat transmission of SARS-CoV-2 through serological testing | Cross-sectional | 920 | [29] |
| Michelitsch | 2021 | Germany | Monitoring the occurrence of SARS-CoV-2 transmission between humans and cats during second pandemic wave | Cross-sectional | 1173 | [30] |
| Musso | 2020 | Italy | Reporting the first natural infection of a cat with SARS-CoV-2 in Italy | Case Study | 1 | [31] |
| Natale | 2021 | Italy | Reporting and describing of a case of symptomatic, natural SARS-CoV-2 infection in a cat | Case Study | 1 | [32] |
| Neira | 2021 | Chile (Santiago City) | Reporting of case of three cats in a household with SARS-CoV-2 infection | Case Study | 3 | [33] |
| Pagani | 2021 | Italy | Description of case of human-to-cat SARS-CoV-2 transmission and full-genome analysis of virus from human and cat | Case Study | 1 | [34] |
| Segalés | 2020 | Spain | Description of case of a cat with severe respiratory symptoms and thrombocytopenia in a SARS-CoV-2-infected household | Case Study | 2 | [35] |
| Spada | 2021 | Italy (Lombardy) | Investigation of SARS-CoV-2 infection in a stray cat population through serological survey and PCR testing | Cross-sectional | 99 | [36] |
| Stranieri | 2021 | Ital (Lodi province) | Evaluation of the presence of SARS-CoV-2 RNA and antibodies against SARS-CoV-2 in free roaming cats belonging to colonies in an area highly affected by the COVID-19 pandemic | Cross-sectional | 99 | [37] |
| Subotsina | 2021 | Belarus | Determination of clinical, pathoanatomical, and histological features in domestic cats with SARS-CoV-2 infection | Case Study | 15 | [38] |
| Van der Leij | 2021 | Netherlands | Determination of seroprevalence of SARS-CoV-2 in Dutch shelter cats | Cross-sectional | 240 | [39] |
| Villanueva-Saz | 2021 | Spain (Zaragoza) | Furthering the knowledge about the role played by stray cats in the context of SARS-CoV-2 and possible predisposing of cats to SARS-CoV-2 infection through concomitant infections | Cross-sectional | 114 | [40] |
| Zhang | 2020 | China (Wuhan) | Investigation of SARS-CoV-2 infection in cats during COVID-19 outbreak in Wuhan using serological detection methods | Cross-sectional | 141 | [41] |
| Zoccola | 2021 | Italy | Description of Italian human-to-cat transmission of SARS-CoV-2 alpha variant of concern | Case Study | 1 | [42] |
| Scientific communication items | ||||||
| Barrs | 2020 | China (Hong-Kong) | Reporting PCR testing results for cats from COVID-19 households | Cross-sectional | 50 | [43] |
| Carlos | 2021 | Brazil | Reporting the first case of SARS-CoV-2 infection in a cat in Latin America | Case Study | 1 | [44] |
| Chiba | 2021 | USA (Wisconsin) | Further characterization of the biology of SARS-CoV-2 in cats through experimental inoculation of cats with the virus and subsequent histopathological examination and re-infection experiment | Experimental | 17 | [45] |
| Deng | 2020 | China | Elucidating the role of domestic cats in SARS-CoV-2 transmission in China through serological survey | Cross-sectional | 423 | [46] |
| Gargliany | 2020 | Belgium | Investigation of SARS-CoV-2 infection and illness in a domestic cat in Belgium | Case Study | 1 | [47] |
| Halfmann | 2020 | Germany (Hamburg) | Evaluation of nasal shedding of SARS-CoV-2 from inoculated cats and subsequent transmission by direct contact to naïve cats | Experimental | 6 | [14] |
| Schulz | 2021 | Germany | Reporting the case of three domestic cats in a retirement home and likely natural human-to-cat SARS-CoV-2 transmission | Case Study | 3 | [48] |
| Unpublished pre-prints | ||||||
| Akhmetzhanov | 2020 | China (Wuhan) | Resolving some uncertainty around SARS-CoV-2 transmission potential to and between cats and quantification of transmission strength. | Case Study | 143 | [49] |
| Fritz | 2021 | France | Investigation of clinical and biological features of SARS-CoV-2 infection in two mildly symptomatic cats from SARS-CoV-2 household | Case Study | 2 | [50] |
| First Author | Year | Seropre-Valence | N Positive Samples (N Total Samples) * | Method ** | Type of Cat | Timeframe | Study Place | Reference |
|---|---|---|---|---|---|---|---|---|
| Deng | 2020 | 0% | 0 (423) | ELISA | Domestic | February 2020–April 2020 | China | [46] |
| Jara | 2021 | 31.7% | 13 (41) | ELISA | Domestic with owners with COVID-19 history | August 2020–April 2021 | Lima, Peru | [25] |
| Michelitsch | 2020 | 0.7% | 6 (920) | ELISA + VNT | Domestic | April 2020–September 2020 | Germany | [29] |
| Michelitsch | 2021 | 1.4% | 16 (1173) | ELISA + VNT | Domestic | September 2020–February 2021 | Germany | [30] |
| Spada | 2021 | 1.0% | 1 (99) | ELISA | Stray, shelter | December 2019–February 2021 | Italy (Lombardy) | [36] |
| Stranieri | 2021 | 0% | 0 (99) | ELISA + VNT | Stray, free-roaming domestic | December 2019–December 2020 | Italy (Lodi province) | [37] |
| Van der Leij | 2021 | 0.8% | 2 (240) | ELISA + VNT | Shelter | August 2020–February 2021 | Netherlands | [39] |
| Villanueva-Saz | 2021 | 3.5% | 4 (114) | ELISA | Stray | January 2020–October 2020 | Zaragoza, Spain | [40] |
| Zhang | 2020 | 10.8% | 11 (102) | ELISA + VNT | Shelter, domestic, domestic with owners with COVID-19 history | January 2020–March 2020 | Wuhan, China | [41] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doliff, R.; Martens, P. Cats and SARS-CoV-2: A Scoping Review. Animals 2022, 12, 1413. https://doi.org/10.3390/ani12111413
Doliff R, Martens P. Cats and SARS-CoV-2: A Scoping Review. Animals. 2022; 12(11):1413. https://doi.org/10.3390/ani12111413
Chicago/Turabian StyleDoliff, Ramona, and Pim Martens. 2022. "Cats and SARS-CoV-2: A Scoping Review" Animals 12, no. 11: 1413. https://doi.org/10.3390/ani12111413
APA StyleDoliff, R., & Martens, P. (2022). Cats and SARS-CoV-2: A Scoping Review. Animals, 12(11), 1413. https://doi.org/10.3390/ani12111413






