Effects of Tannic Acid Supplementation on Growth Performance, Oocyst Shedding, and Gut Health of in Broilers Infected with Eimeria Maxima
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of E. maxima Inoculum
2.2. Experimental Design and Growth Performance
2.3. Oocyst Shedding and Fecal Consistency
2.4. In Vivo Gut Permeability
2.5. Tissue and Digesta Sample Collection
2.6. Total Glutathione (GSH) and Oxidized Glutathione (GSSH) Assays, and Total Antioxidant Capacity (TAC) Assay
2.7. Apparent Ileal Digestibility of Nutrients
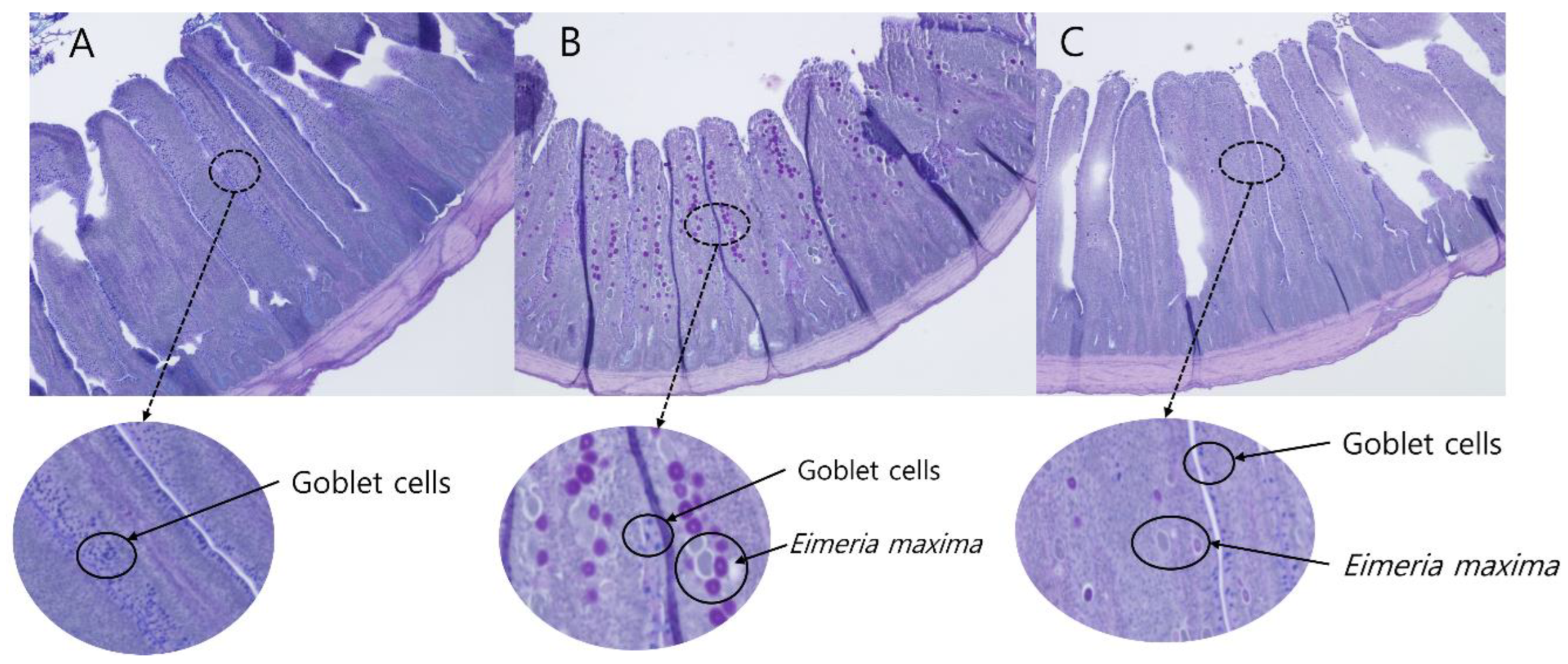
2.8. Intestinal Morphology and Goblet Cell Counting Analyses
2.9. RNA Extraction and Real-Time Polymerase Chain Reaction (PCR) Analysis
2.10. Statistical Analyses
3. Results
3.1. Growth Performance
3.2. Fecal Moisture Content and Oocyst Shedding
3.3. Gut Permeability and Jejunal Lesion
3.4. Total Antioxidant Capacity (TAC), Total Glutathione (GSH), Reduced GSH, and Oxidized Glutathione (GSSG)
3.5. Apparent Ileal Digestibility of Nutrients
3.6. Jejunal Morphology and Goblet Cell Density
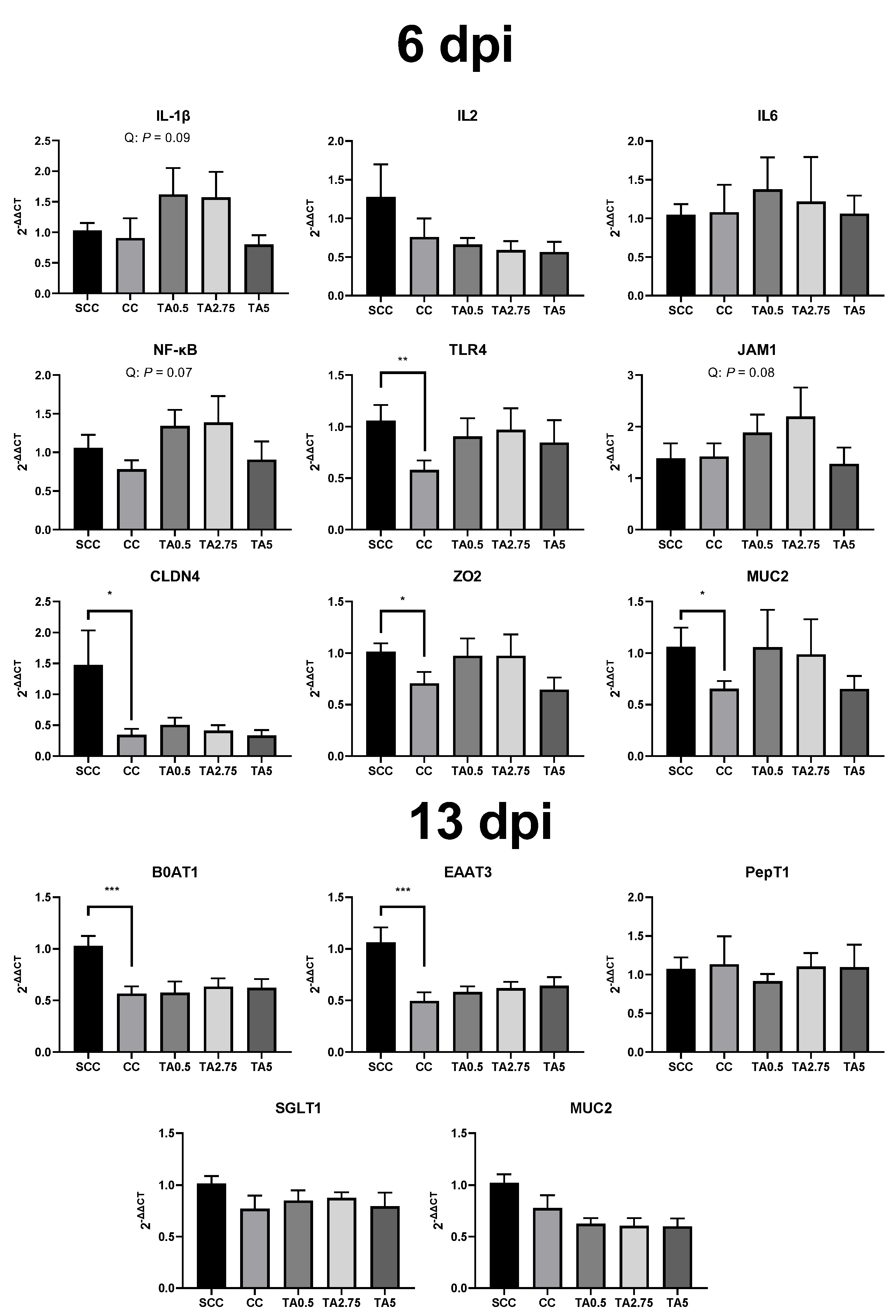
3.7. Relative mRNA Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdullahi, A.; Yu, X.; Fu, Y.; Wang, M.; Qi, N.; Xia, M.; Kallon, S.; Pan, W.; Shi, X.; Fang, Y. Effects of dietary supplement of organic acids induced protective immunity against coccidiosis. Iran. J. Appl. Anim. Sci. 2020, 10, 119–129. [Google Scholar]
- Chapman, H. Milestones in avian coccidiosis research: A review. Poult. Sci. 2014, 93, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Clark, E.L.; Macdonald, S.E.; Thenmozhi, V.; Kundu, K.; Garg, R.; Kumar, S.; Ayoade, S.; Fornace, K.M.; Jatau, I.D.; Moftah, A. Cryptic Eimeria genotypes are common across the southern but not northern hemisphere. Int. J. Parasitol. 2016, 46, 537–544. [Google Scholar] [CrossRef]
- Li, C.; Yan, X.; Lillehoj, H.S.; Oh, S.; Liu, L.; Sun, Z.; Gu, C.; Lee, Y.; Xianyu, Z.; Zhao, H. Eimeria maxima-induced transcriptional changes in the cecal mucosa of broiler chickens. Parasites Vectors 2019, 12, 285. [Google Scholar] [CrossRef] [PubMed]
- Waldenstedt, L.; Elwinger, K.; Lunden, A.; Thebo, P.; Uggla, A. Sporulation of Eimeria maxima oocysts in litter with different moisture contents. Poult. Sci. 2001, 80, 1412–1415. [Google Scholar] [CrossRef]
- Yin, G.; Lin, Q.; Wei, W.; Qin, M.; Liu, X.; Suo, X.; Huang, Z. Protective immunity against Eimeria tenella infection in chickens induced by immunization with a recombinant C-terminal derivative of EtIMP1. Vet. Immunol. Immunopathol. 2014, 162, 117–121. [Google Scholar] [CrossRef]
- Teng, P.-Y.; Yadav, S.; de Souza Castro, F.L.; Tompkins, Y.H.; Fuller, A.L.; Kim, W.K. Graded Eimeria challenge linearly regulated growth performance, dynamic change of gastrointestinal permeability, apparent ileal digestibility, intestinal morphology, and tight junctions of broiler chickens. Poult. Sci. 2020, 99, 4203–4216. [Google Scholar] [CrossRef]
- Peek, H.; Landman, W. Coccidiosis in poultry: Anticoccidial products, vaccines and other prevention strategies. Vet. Q. 2011, 31, 143–161. [Google Scholar] [CrossRef]
- Teng, P.-Y.; Choi, J.; Yadav, S.; Tompkins, Y.; Kim, W.K. Effects of low-crude protein diets supplemented with arginine, glutamine, threonine, and methionine on regulating nutrient absorption, intestinal health, and growth performance of Eimeria-infected chickens. Poult. Sci. 2021, 100, 101427. [Google Scholar] [CrossRef]
- Levine, R.; Horst, G.; Tonda, R.; Lumpkins, B.; Mathis, G. Evaluation of the effects of feeding dried algae containing beta-1, 3-glucan on broilers challenged with Eimeria. Poult. Sci. 2018, 97, 3494–3500. [Google Scholar] [CrossRef]
- Yadav, S.; Teng, P.-Y.; Singh, A.; Choi, J.; Kim, W. Influence of Brassica spp. rapeseed and canola meal, and supplementation of bioactive compound (AITC) on growth performance, intestinal-permeability, oocyst shedding, lesion score, histomorphology, and gene expression of broilers challenged with E. maxima. Poult. Sci. 2022, 101, 101583. [Google Scholar] [CrossRef] [PubMed]
- Abbas, R.Z.; Munawar, S.H.; Manzoor, Z.; Iqbal, Z.; Khan, M.N.; Saleemi, M.K.; Zia, M.A.; Yousaf, A. Anticoccidial effects of acetic acid on performance and pathogenic parameters in broiler chickens challenged with Eimeria tenella. Pesqui. Vet. Bras. 2011, 31, 99–103. [Google Scholar] [CrossRef]
- Teng, P.-Y.; Fuller, A.L.; Kim, W.K. Evaluation of nitro compounds as feed additives in diets of Eimeria-challenged broilers in vitro and in vivo. Poult. Sci. 2020, 99, 1320–1325. [Google Scholar] [CrossRef] [PubMed]
- McCann, M.; Newell, E.; Preston, C.; Forbes, K. The use of mannan-oligosaccharides and/or tannin in broiler diets. Int. J. Poult. Sci. 2006, 5, 873–879. [Google Scholar] [CrossRef]
- Cejas, E.; Pinto, S.; Prosdocimo, F.; Batallé, M.; Barrios, H.; Tellez, G.; De Franceschi, M. Evaluation of quebracho red wood (Schinopsis lorentzii) polyphenolic vegetable extracts for the reduction of coccidiosis in broiler chicks. Int. J. Poultry Sci. 2011, 10, 344–349. [Google Scholar] [CrossRef]
- Hooge, D.M.; Mathis, G.F.; Lumpkins, B.; Ponebsek, J.; Moran, D. Dose-Responses of Broiler Chicks, Given Live Coccidia Vaccine on Day of Hatch, to Diets Supplemented with Various Levels of Farmatano (Sweet Chestnut Wood Tannins) or BMD33lStafac ‘3 in a 42-Day Pen Trial on Built-Up Litter. Int. J. Poult. Sci. 2012, 11, 474–481. [Google Scholar] [CrossRef][Green Version]
- Lu, P.; Choi, J.; Yang, C.; Mogire, M.; Liu, S.; Lahaye, L.; Adewole, D.; Rodas-Gonzalez, A.; Yang, C. Effects of antibiotic growth promotor and dietary protease on growth performance, apparent ileal digestibility, intestinal morphology, meat quality and intestinal gene expression in broiler chickens: A comparison. J. Anim. Sci. 2020, 98, skaa254. [Google Scholar] [CrossRef]
- Scalbert, A. Antimicrobial properties of tannins. Phytochemistry 1991, 30, 3875–3883. [Google Scholar] [CrossRef]
- Chung, K.-T.; Lu, Z.; Chou, M. Mechanism of inhibition of tannic acid and related compounds on the growth of intestinal bacteria. Food Chem. Toxicol. 1998, 36, 1053–1060. [Google Scholar] [CrossRef]
- Kaur, G.; Athar, M.; Alam, M.S. Quercus infectoria galls possess antioxidant activity and abrogates oxidative stress-induced functional alterations in murine macrophages. Chem.-Biol. Interact. 2008, 171, 272–282. [Google Scholar] [CrossRef]
- Hamiza, O.O.; Rehman, M.U.; Tahir, M.; Khan, R.; Khan, A.Q.; Lateef, A.; Ali, F.; Sultana, S. Amelioration of 1, 2 Dimethylhydrazine (DMH) induced colon oxidative stress, inflammation and tumor promotion response by tannic acid in Wistar rats. Asian Pac. J. Cancer Prev. 2012, 13, 4393–4402. [Google Scholar] [CrossRef] [PubMed]
- Tonda, R.; Rubach, J.; Lumpkins, B.; Mathis, G.; Poss, M. Effects of tannic acid extract on performance and intestinal health of broiler chickens following coccidiosis vaccination and/or a mixed-species Eimeria challenge. Poult. Sci. 2018, 97, 3031–3042. [Google Scholar] [CrossRef] [PubMed]
- Mansoori, B.; Modirsanei, M. Effects of dietary tannic acid and vaccination on the course of coccidiosis in experimentally challenged broiler chicken. Vet. Parasitol. 2012, 187, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Teng, P.-Y.; Choi, J.; Tompkins, Y.; Lillehoj, H.; Kim, W. Impacts of increasing challenge with Eimeria maxima on the growth performance and gene expression of biomarkers associated with intestinal integrity and nutrient transporters. Vet. Res. 2021, 52, 81. [Google Scholar] [CrossRef] [PubMed]
- Teng, P.-Y.; Yadav, S.; Dos Santos, T.S.; Fuller, A.L.; Kim, W.K. 2-Nitro-1-propanol improved nutrient digestibility and oocyst shedding but not growth performance of Eimeria-challenged broilers. Poult. Sci. 2020, 99, 4314–4322. [Google Scholar] [CrossRef]
- Koo, B.; Choi, J.; Yang, C.; Nyachoti, C.M. Diet complexity and l-threonine supplementation: Effects on growth performance, immune response, intestinal barrier function, and microbial metabolites in nursery pigs. J. Anim. Sci. 2020, 98, skaa125. [Google Scholar] [CrossRef]
- Johnson, J.; Reid, W.M. Anticoccidial drugs: Lesion scoring techniques in battery and floor-pen experiments with chickens. Exp. Parasitol. 1970, 28, 30–36. [Google Scholar] [CrossRef]
- Choi, J.; Wang, L.; Liu, S.; Lu, P.; Zhao, X.; Liu, H.; Lahaye, L.; Santin, E.; Liu, S.; Nyachoti, M. Effects of a microencapsulated formula of organic acids and essential oils on nutrient absorption, immunity, gut barrier function, and abundance of enterotoxigenic Escherichia coli F4 in weaned piglets challenged with E. coli F4. J. Anim. Sci. 2020, 98, skaa259. [Google Scholar] [CrossRef]
- Short, F.; Gorton, P.; Wiseman, J.; Boorman, K. Determination of titanium dioxide added as an inert marker in chicken digestibility studies. Anim. Feed Sci. Technol. 1996, 59, 215–221. [Google Scholar] [CrossRef]
- Lin, Y.; Olukosi, O.A. Qualitative and quantitative profiles of jejunal oligosaccharides and cecal short-chain fatty acids in broiler chickens receiving different dietary levels of fiber, protein and exogenous enzymes. J. Sci. Food Agric. 2021, 101, 5190–5201. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, research0034.1. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Ko, H.; Tompkins, Y.H.; Teng, P.-Y.; Lourenco, J.M.; Callaway, T.R.; Kim, W.K. Effects of Eimeria tenella Infection on Key Parameters for Feed Efficiency in Broiler Chickens. Animals 2021, 11, 3428. [Google Scholar] [CrossRef] [PubMed]
- Redondo, L.M.; Chacana, P.A.; Dominguez, J.E.; Fernandez Miyakawa, M.E.D. Perspectives in the use of tannins as alternative to antimicrobial growth promoter factors in poultry. Front. Microbiol. 2014, 5, 118. [Google Scholar] [CrossRef]
- Van der Aar, P.; Molist, F.v.; Van Der Klis, J. The central role of intestinal health on the effect of feed additives on feed intake in swine and poultry. Anim. Feed Sci. Technol. 2017, 233, 64–75. [Google Scholar] [CrossRef]
- Cha, J.O.; Zhao, J.; Yang, M.S.; Kim, W.I.; Cho, H.S.; Lim, C.W.; Kim, B. Oocyst-shedding patterns of three Eimeria species in chickens and shedding pattern variation depending on the storage period of Eimeria tenella oocysts. J. Parasitol. 2018, 104, 18–22. [Google Scholar] [CrossRef]
- Kaleem, Q.M.; Akhtar, M.; Awais, M.M.; Saleem, M.; Zafar, M.; Iqbal, Z.; Muhammad, F.; Anwar, M.I. Studies on Emblica officinalis derived tannins for their immunostimulatory and protective activities against coccidiosis in industrial broiler chickens. Sci. World J. 2014, 2014, 378473. [Google Scholar] [CrossRef]
- Walker, R.A.; Sharman, P.A.; Miller, C.M.; Lippuner, C.; Okoniewski, M.; Eichenberger, R.M.; Ramakrishnan, C.; Brossier, F.; Deplazes, P.; Hehl, A.B. RNA Seq analysis of the Eimeria tenella gametocyte transcriptome reveals clues about the molecular basis for sexual reproduction and oocyst biogenesis. BMC Genom. 2015, 16, 94. [Google Scholar] [CrossRef]
- Miska, K.B.; Fetterer, R.H. The effect of Eimeria maxima infection on the expression of amino acid and sugar transporters aminopeptidase, as well as the di-and tri-peptide transporter PepT1, is not solely due to decreased feed intake. Poult. Sci. 2018, 97, 1712–1721. [Google Scholar] [CrossRef]
- Karamać, M. Chelation of Cu (II), Zn (II), and Fe (II) by tannin constituents of selected edible nuts. Int. J. Mol. Sci. 2009, 10, 5485–5497. [Google Scholar] [CrossRef]
- Lee, S.H. Intestinal permeability regulation by tight junction: Implication on inflammatory bowel diseases. Intest. Res. 2015, 13, 11. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Teng, P.-Y.; Kim, W.K.; Applegate, T.J. Assay considerations for fluorescein isothiocyanate-dextran (FITC-d): An indicator of intestinal permeability in broiler chickens. Poult. Sci. 2021, 100, 101202. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Applegate, T.J.; Liu, S.; Guo, Y.; Eicher, S.D. Supplemental dietary L-arginine attenuates intestinal mucosal disruption during a coccidial vaccine challenge in broiler chickens. Br. J. Nutr. 2014, 112, 1098–1109. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Guo, P.; Zhou, Q. Role of TLR4/NF-κB in damage to intestinal mucosa barrier function and bacterial translocation in rats exposed to hypoxia. PLoS ONE 2012, 7, e46291. [Google Scholar] [CrossRef] [PubMed]
- Memon, F.; Yang, Y.; Lv, F.; Soliman, A.; Chen, Y.; Sun, J.; Wang, Y.; Zhang, G.; Li, Z.; Xu, B. Effects of probiotic and Bidens pilosa on the performance and gut health of chicken during induced Eimeria tenella infection. J. Appl. Microbiol. 2021, 131, 425–434. [Google Scholar] [CrossRef]
- Koo, B.; Nyachoti, C.M. Effects of thermally oxidized canola oil and tannic acid supplementation on nutrient digestibility and microbial metabolites in finishing pigs. J. Anim. Sci. 2019, 97, 2468–2478. [Google Scholar] [CrossRef]
- Choi, J.; Li, W.; Schindell, B.; Ni, L.; Liu, S.; Zhao, X.; Gong, J.; Nyachoti, M.; Yang, C. Molecular cloning, tissue distribution and the expression of cystine/glutamate exchanger (xCT, SLC7A11) in different tissues during development in broiler chickens. Anim. Nutr. 2020, 6, 107–114. [Google Scholar] [CrossRef]
- Jobe, M.C.; Ncobela, C.N.; Kunene, N.W.; Opoku, A.R. Effects of Cassia abbreviata extract and stocking density on growth performance, oxidative stress and liver function of indigenous chickens. Trop. Anim. Health Prod. 2019, 51, 2567–2574. [Google Scholar] [CrossRef]
- Peng, K.; Lv, X.; Zhao, H.; Chen, B.; Chen, X.; Huang, W. Antioxidant and intestinal recovery function of condensed tannins in Lateolabrax maculatus responded to in vivo and in vitro oxidative stress. Aquaculture 2022, 547, 737399. [Google Scholar] [CrossRef]
- Marzo, F.; Urdaneta, E.; Santidrian, S. Liver proteolytic activity in tannic acid-fed birds. Poult. Sci. 2002, 81, 92–94. [Google Scholar] [CrossRef]
- Wang, M.; Huang, H.; Liu, S.; Zhuang, Y.; Yang, H.; Li, Y.; Chen, S.; Wang, L.; Yin, L.; Yao, Y. Tannic acid modulates intestinal barrier functions associated with intestinal morphology, antioxidative activity, and intestinal tight junction in a diquat-induced mouse model. RSC Adv. 2019, 9, 31988–31998. [Google Scholar] [CrossRef] [PubMed]
- Wadolkowski, E.A.; Laux, D.C.; Cohen, P.S. Colonization of the streptomycin-treated mouse large intestine by a human fecal Escherichia coli strain: Role of adhesion to mucosal receptors. Infect. Immun. 1988, 56, 1036–1043. [Google Scholar] [CrossRef] [PubMed]
- Edelman, S.; Leskelä, S.; Ron, E.; Apajalahti, J.; Korhonen, T.K. In vitro adhesion of an avian pathogenic Escherichia coli O78 strain to surfaces of the chicken intestinal tract and to ileal mucus. Vet. Microbiol. 2003, 91, 41–56. [Google Scholar] [CrossRef]
- Collier, C.; Hofacre, C.; Payne, A.; Anderson, D.; Kaiser, P.; Mackie, R.; Gaskins, H. Coccidia-induced mucogenesis promotes the onset of necrotic enteritis by supporting Clostridium perfringens growth. Vet. Immunol. Immunopathol. 2008, 122, 104–115. [Google Scholar] [CrossRef] [PubMed]
| Items | D 0 to 15 | D 15 to 28 |
|---|---|---|
| Ingredients (kg/ton) | ||
| Corn | 651.95 | 700.80 |
| Soybean meal (480 g crude protein /kg) | 294.94 | 241.76 |
| Defluorinated phosphate | 15.78 | 15.84 |
| Filler 1 (sand and tannic acid) | 10.00 | 13.99 |
| Soybean oil | 7.93 | 10.00 |
| Limestone | 7.16 | 6.11 |
| DL-Methionine 99% | 3.17 | 2.85 |
| L-Lysine HCl 78% | 3.01 | 2.80 |
| Vitamin premix 2 | 2.50 | 2.50 |
| Common salt | 1.55 | 1.79 |
| L-Threonine | 1.20 | 0.80 |
| Mineral premix 3 | 0.80 | 0.77 |
| Total | 1000 | 1000 |
| Calculated energy and nutrient value, % | ||
| Metabolizable energy, Mcal/kg | 3000 | 3100 |
| Crude protein, % | 20.60 | 18.37 |
| SID 4 methionine, % | 0.61 | 0.55 |
| SID 4 total sulfur amino acids, % | 0.88 | 0.80 |
| SID 4 lysine, % | 1.17 | 1.02 |
| SID 4 threonine, % | 0.78 | 0.66 |
| Total calcium, % | 0.87 | 0.76 |
| Available phosphorus, % | 0.44 | 0.38 |
| Genes | Sequence, 5′ to 3′ | Amplicon |
|---|---|---|
| GAPDH | F: GCT AAG GCT GTG GGG AAA GT | 161 |
| R: TCA GCA GCA GCC TTC ACT AC | ||
| Beta actin | F: CAA CAC AGT GCT GTC TGG TGG TA | 205 |
| R: ATC GTA CTC CTG CTT GCT GAT CC | ||
| NFκB | F: GAA GGA ATC GTA CCG GGA ACA | 131 |
| R: CTC AGA GGG CCT TGT GAC AGT AA | ||
| IL1β | F: TGC CTG CAG AAG AAG CCT CG | 204 |
| R: GAC GGG CTC AAA AAC CTC CT | ||
| IL2 | F: TTG GCT GTA TTT CGG TAG CA | 169 |
| R: GTG CAC TCC TGG GTC TCA GT | ||
| IL6 | F: ATA AAT CCC GAT GAA GTG G | 146 |
| R: CTC ACG GTC TTC TCC ATA AA | ||
| JAM2 | F: AGC CTC AAA TGG GAT TGG ATT | 59 |
| R: CAT CAA CTT GCA TTC GCT TCA | ||
| ZO2 | F: ATC CAA GAA GGC ACC TCA GC | 100 |
| R: CAT CCT CCC GAA CAA TGC | ||
| CLDN4 | F: GAA GCG CTG AAA CGA TAC CA | 134 |
| R: TGC TTC TGT GCC TCA GTT TCC | ||
| SGLT1 | F: GCC ATG GCC AGG GCT TA | 71 |
| R: CAA TAA CCT GAT CTG TGC ACC AGT A | ||
| PEPT1 | F: CCC CTG AGG AGG ATC CTT | 66 |
| R: CAA AAG AGC AGC AAC GA | ||
| B0AT1 | F: GGG TTT TGT GTT GGC TTA GGA A | 60 |
| R: TCC ATG GCT CTG GCA GAG AT | ||
| EAAT3 | F: TGC TGC TTT GGA TTC CAG TGT | 79 |
| R: AGC AAT GAC TGT AGT GCA GAA GTA ATA TAT G | ||
| GLUT2 | F: TCA TTG TAG CTG AGC TGT T | 147 |
| R: CGA AGA CAA CGA ACA CAT AC | ||
| MUC2 | F: ATG CGA TGT TAA CAC AGG ACT C | 110 |
| R: GTG GAG CAC AGC AGA CTT TG |
| Eimeria maxima-Challenged 3 | Poly. Contrast | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Items | SCC 2 | CC | TA0.5 | TA2.75 | TA5 | SEM | p-Value | Lin. | Quad. |
| Pre-challenge | |||||||||
| BW | 462.1 | 440.8 a | 452.8 a | 432.8 ab | 404.7 b | 19.9 | <0.01 | <0.01 | 0.19 |
| ADG | 27.68 | 26.26 a | 27.06 a | 25.7 ab | 23.8 b | 1.4 | <0.01 | <0.01 | 0.22 |
| ADFI | 38.96 | 38.37 | 38.46 | 37.11 | 35.85 | 2.02 | 0.07 | 0.01 | 0.88 |
| FCR | 1.41 | 1.46 | 1.42 | 1.44 | 1.51 | 0.11 | 0.52 | 0.25 | 0.41 |
| Acute phase | |||||||||
| BW | 812.2 *** | 696.5 ab | 721.4 a | 691.9 ab | 638.5 b | 41.7 | <0.01 | <0.01 | 0.15 |
| ADG | 58.3 *** | 42.6 | 44.7 | 43.2 | 39 | 5.5 | 0.28 | 0.11 | 0.31 |
| ADFI | 115.4 *** | 86.8 | 87.5 | 92.3 | 87.9 | 7.51 | 0.52 | 0.59 | 0.18 |
| FCR | 1.99 | 2.08 | 1.96 | 2.18 | 2.33 | 0.48 | 0.80 | 0.36 | 0.90 |
| Recovery phase | |||||||||
| BW | 1273 | 1211 a | 1227 a | 1141 ab | 1062 b | 105.3 | <0.01 | <0.01 | 0.41 |
| ADG | 69.8 | 75.6 | 74.2 | 64.3 | 57.6 | 23.5 | 0.04 | <0.01 | 0.88 |
| ADFI | 118 ** | 130.2 | 128.1 | 124 | 121 | 11.7 | 0.47 | 0.12 | 0.82 |
| FCR | 1.75 | 1.75 | 1.76 | 1.94 | 2.2 | 0.32 | 0.04 | <0.01 | 0.72 |
| Eimeria maxima-Challenged 3 | Poly. Contrast | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Items | SCC 2 | CC | TA0.5 | TA2.75 | TA5 | SEM | p-Value | Lin. | Quad. |
| Ileal content | |||||||||
| 6 dpi | 81.64 | 82.46 | 84.24 | 82.02 | 82.69 | 2.03 | 0.22 | 0.44 | 0.63 |
| 13 dpi | 81.46 | 81.99 | 82.00 | 81.23 | 80.45 | 1.2 | 0.07 | 0.01 | 0.86 |
| Fecal content | |||||||||
| 3 to 5 dpi | 75.47 | 75.30 | 78.23 | 75.68 | 76.09 | 4.44 | 0.62 | 0.77 | 0.96 |
| 5 to 7 dpi | 78.84 | 76.77 a | 74.84 a | 68.69 b | 72.41 ab | 3.92 | <0.01 | 0.01 | <0.01 |
| 7 to 9 dpi | 78.85 | 78.00 | 79.65 | 76.00 | 77.28 | 2.54 | 0.09 | 0.13 | 0.22 |
| 9 to 11 dpi | 80.04 | 80.06 ab | 82.11 a | 79.74 ab | 77.26 b | 2.54 | 0.01 | <0.01 | 0.26 |
| 11 to 13 dpi | 77.77 | 79.32 a | 79.79 a | 77.24 ab | 74.80 b | 2.70 | <0.01 | <0.01 | 0.72 |
| Eimeria maxima-Challenged 2 | Poly. Contrast | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Items | SCC | CC | TA0.5 | TA2.75 | TA5 | SEM | p-Value | Lin. | Quad. |
| 5 to 7 dpi | N/D 3 | 2205.6 | 2198.6 | 371.3 | 426.1 | 2446.8 | 0.31 | 0.09 | 0.47 |
| 7 to 9 dpi | 6203.8 a | 686.3 b | 680.8 b | 452.1 b | 3719.2 | 0.02 | 0.04 | 0.14 | |
| 9 to 11 dpi | 827.2 | 1143.6 | 272.9 | 963.8 | 1140.7 | 0.52 | 0.78 | 0.26 | |
| Eimeria maxima-Challenged 3 | Poly. Contrasts | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Items | SCC 2 | CC | TA0.5 | TA2.75 | TA5 | SEM | p-Value | Lin. | Quad. |
| Serum FITC-D4 3 | 0.15 *** | 2.22 a | 1.76 ab | 1.06 b | 2.04 a | 0.10 | <0.01 | 0.46 | <0.01 |
| Jejunal lesion 4 | 0 *** | 0.75 | 0.96 | 0.78 | 0.82 | 0.02 | 0.62 | 0.84 | 0.96 |
| Eimeria maxima-Challenged 3 | Poly. Contrasts | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Items | SCC 2 | CC | TA0.5 | TA2.75 | TA5 | SEM | p-Value | Lin. | Quad. |
| 6 dpi jejunum | |||||||||
| TAC | 73.67 | 76.98 | 73.62 | 75.93 | 73.12 | 7.27 | 0.72 | 0.54 | 0.79 |
| Total GSH | 42.57 | 31.37 | 30.53 | 24.12 | 20.39 | 10.31 | 0.17 | 0.03 | 0.81 |
| GSSG | 2.81 | 3.38 | 1.49 | 1.31 | 1.37 | 1.42 | 0.03 | 0.05 | 0.13 |
| Reduced GSH 4 | 36.93 | 24.61 | 27.55 | 21.50 | 17.66 | 9.69 | 0.29 | 0.07 | 0.84 |
| Reduced GSH/GSSG | 14.67 | 12.77 | 20.03 | 18.32 | 14.61 | 7.98 | 0.32 | 0.86 | 0.20 |
| Liver | |||||||||
| Total GSH | 52.58 | 43.5 | 33.38 | 31.07 | 33.6 | 13.60 | 0.35 | 0.3 | 0.26 |
| GSSG | 2.35 | 3.13 | 2.36 | 2.42 | 2.06 | 1.47 | 0.59 | 0.28 | 0.82 |
| Reduced GSH | 47.87 * | 37.24 | 28.65 | 26.23 | 29.47 | 11.94 | 0.36 | 0.35 | 0.22 |
| Reduced GSH/GSSG | 29.43 ** | 13.16 | 13.23 | 14.16 | 17.54 | 9 | 0.78 | 0.33 | 0.74 |
| 13 dpi Jejunum | |||||||||
| TAC | 112.1 | 101.6 | 101.8 | 91.38 | 87.04 | 17.99 | 0.33 | 0.08 | 0.8 |
| Total GSH | 53.45 | 50.57 ab | 61.24 a | 35.07 b | 46.54 ab | 13.81 | 0.01 | 0.07 | 0.06 |
| GSSG | 5.45 | 4.70 | 5.20 | 3.57 | 4.10 | 1.30 | 0.13 | 0.10 | 0.22 |
| Reduced GSH | 42.54 | 41.18 ab | 50.83 a | 27.9 b | 38.33 ab | 12.75 | 0.02 | 0.10 | 0.07 |
| Reduced GSH/GSSG | 7.87 | 11.95 | 9.75 | 8.89 | 9.84 | 6.31 | 0.83 | 0.61 | 0.51 |
| Liver | |||||||||
| Total GSH | 40 | 44.15 | 41.37 | 38.95 | 39.3 | 5.36 | 0.27 | 0.11 | 0.30 |
| GSSG | 4 * | 5.95 | 5.01 | 5.5 | 5.49 | 2.18 | 0.88 | 0.95 | 0.81 |
| Reduced GSH | 32 | 32.24 | 31.35 | 27.96 | 28.31 | 5.14 | 0.32 | 0.1 | 0.39 |
| Reduced GSH/GSSG | 8.56 * | 5.93 | 6.31 | 5.99 | 5.84 | 1.78 | 0.96 | 0.77 | 0.88 |
| Eimeria maxima-Challenged 3 | Poly. Contrasts | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Items | SCC 2 | CC | TA0.5 | TA2.75 | TA5 | SEM | p Value | Lin. | Quad. |
| 6 dpi | |||||||||
| DM | 59.9 | 63.06 | 58.84 | 62.32 | 59.69 | 6.98 | 0.63 | 0.72 | 0.78 |
| OM | 65.45 | 65.45 | 61.95 | 65.04 | 63.93 | 6.67 | 0.76 | 0.94 | 0.94 |
| Ash | 12.5 ** | 29.18 a | 0.93 bc | 12.33 ab | −22.66 c | 16.64 | <0.01 | <0.01 | 0.2 |
| CP | 72 | 71.2 | 64.17 | 69.3 | 67.9 | 5.85 | 0.17 | 0.99 | 0.87 |
| 13 dpi | |||||||||
| DM | 69 ** | 73.85 b | 74.88 ab | 77.05 a | 69.67 c | 1.79 | <0.01 | <0.01 | <0.01 |
| OM | 72.05 ** | 75.73 b | 77 ab | 78.8 a | 71.6 c | 1.77 | <0.01 | <0.01 | <0.01 |
| Ash | 17.86 *** | 47.14 a | 37.54 b | 44.87 a | 32.2 b | 4.26 | 4.25 | <0.01 | 0.01 |
| CP | 68.59 | 73.3 | 68.32 | 69.14 | 68.03 | 5.73 | 0.3 | 0.23 | 0.58 |
| Eimeria maxima-Challenged 3 | Poly. Contrasts | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Items | SCC 2 | CC | TA0.5 | TA2.75 | TA5 | SEM | p-Value | Lin. | Quad. |
| 6 dpi | |||||||||
| VH | 1375 *** | 775.6 | 812.26 | 764.79 | 739.04 | 191.88 | 0.91 | 0.56 | 0.9 |
| Goblet cells per 100 μm VH | 7.48 | 7.82 | 7.63 | 7.37 | 9.15 | 1.7 | 0.23 | 0.14 | 0.14 |
| CD | 248.5 | 279.22 | 357.8 | 354.94 | 328.46 | 55.56 | 0.05 | 0.38 | 0.06 |
| Goblet cells per 100 μm CD | 4.05 | 3.94 | 3.74 | 3.62 | 3.97 | 0.49 | 0.5 | 0.83 | 0.14 |
| VH/CD | 5.8 *** | 2.87 | 2.49 | 2.22 | 2.39 | 0.82 | 0.51 | 0.31 | 0.3 |
| 13 dpi | |||||||||
| VH | 1344 ** | 973.9 | 1101.3 | 1210.8 | 1299.3 | 245.9 | 0.1 | 0.02 | 0.59 |
| Goblet cells per 100 μm VH | 5.12 *** | 8.53 | 10.12 | 8.35 | 8.67 | 1.75 | 0.24 | 0.42 | 0.83 |
| CD | 248.62 ** | 310.36 | 289.7 | 315.88 | 329.65 | 42.68 | 0.21 | 0.11 | 0.74 |
| Goblet cells per 100 μm CD | 2.72 *** | 4.46 | 4.34 | 4.14 | 3.87 | 0.96 | 0.69 | 0.24 | 0.99 |
| VH/CD | 5.81 *** | 3.22 | 4.09 | 3.95 | 4.07 | 0.85 | 0.20 | 0.21 | 0.45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.; Tompkins, Y.H.; Teng, P.-Y.; Gogal, R.M., Jr.; Kim, W.K. Effects of Tannic Acid Supplementation on Growth Performance, Oocyst Shedding, and Gut Health of in Broilers Infected with Eimeria Maxima. Animals 2022, 12, 1378. https://doi.org/10.3390/ani12111378
Choi J, Tompkins YH, Teng P-Y, Gogal RM Jr., Kim WK. Effects of Tannic Acid Supplementation on Growth Performance, Oocyst Shedding, and Gut Health of in Broilers Infected with Eimeria Maxima. Animals. 2022; 12(11):1378. https://doi.org/10.3390/ani12111378
Chicago/Turabian StyleChoi, Janghan, Yuguo Huo Tompkins, Po-Yun Teng, Robert M. Gogal, Jr., and Woo Kyun Kim. 2022. "Effects of Tannic Acid Supplementation on Growth Performance, Oocyst Shedding, and Gut Health of in Broilers Infected with Eimeria Maxima" Animals 12, no. 11: 1378. https://doi.org/10.3390/ani12111378
APA StyleChoi, J., Tompkins, Y. H., Teng, P.-Y., Gogal, R. M., Jr., & Kim, W. K. (2022). Effects of Tannic Acid Supplementation on Growth Performance, Oocyst Shedding, and Gut Health of in Broilers Infected with Eimeria Maxima. Animals, 12(11), 1378. https://doi.org/10.3390/ani12111378







