Hair Cortisol and DHEA-S in Foals and Mares as a Retrospective Picture of Feto-Maternal Relationship under Physiological and Pathological Conditions
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Population
2.2. Clinical Data and Sample Collection
2.3. Hormone Analysis
2.4. Statistical Analysis
3. Results
3.1. Clinical Data
3.2. CORT and DHEA-S Concentrations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Toribio, R.E. Endocrine dysregulation in critically ill foals and horses. Vet. Clin. N. Am. Equine Pract. 2011, 27, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Kamin, H.S.; Kertes, D.A. Cortisol and DHEA in development and psychopathology. Horm. Behav. 2017, 89, 69–85. [Google Scholar] [CrossRef] [PubMed]
- Santschi, E.M.; LeBlanc, M.M.; Weston, P.G. Progestagen, oestrone sulphate and cortisol concentrations in pregnant mares during medical and surgical disease. J. Reprod. Fertil. Suppl. 1991, 44, 627–634. [Google Scholar] [PubMed]
- Canisso, I.F.; Ball, B.A.; Esteller-Vico, A.; Williams, N.M.; Squires, E.L.; Troedsson, M.H. Changes in maternal androgens and oestrogens in mares with experimentally-induced ascending placentitis. Equine Vet. J. 2017, 49, 244–249. [Google Scholar] [CrossRef]
- Gold, J.R.; Divers, T.J.; Barton, M.H.; Lamb, S.V.; Place, N.J.; Mohammed, H.O.; Bain, F.T. Plasma adrenocorticotropin, cortisol, and adrenocorticotropin/cortisol ratios in septic and normal-term foals. J. Vet. Intern. Med. 2007, 21, 791–796. [Google Scholar]
- Hurcombe, S.D.A.; Toribio, R.E.; Slovis, N.; Kohn, C.W.; Refsal, K.; Saville, W.; Mudge, M.C. Blood arginine vasopressin, adrenocorticotropin hormone, and cortisol concentrations at admission in septic and critically ill foals and their association with survival. J. Vet. Intern. Med. 2008, 22, 639–647. [Google Scholar] [CrossRef]
- Castagnetti, C.; Rametta, M.; Popeia, R.T.; Govoni, N.; Mariella, J. Plasma levels of ACTH and cortisol in normal and critically-ill neonatal foals. Vet. Res. Commun. 2008, 32, 127–129. [Google Scholar] [CrossRef]
- Hart, K.A.; Slovis, N.M.; Barton, M.H. Hypothalamic-pituitary-adrenal axis dysfunction in hospitalized neonatal foals. J. Vet. Intern. Med. 2009, 23, 901–912. [Google Scholar] [CrossRef]
- Aleman, M.R.; Pickles, K.J.; Conley, A.J.; Standley, S.; Haggett, E.; Toth, B.; Madigan, J.E. Abnormal plasma neuroactive progestagen derivatives in ill, neonatal foals presented to the neonatal intensive care unit. Equine Vet. J. 2013, 45, 3. [Google Scholar] [CrossRef]
- Heimbürge, S.; Kanitz, E.; Otten, W. The use of hair cortisol for the assessment of stress in animals. Gen. Comp. Endocrinol. 2019, 270, 10–17. [Google Scholar] [CrossRef]
- Comin, A.; Veronesi, M.C.; Montillo, M.; Faustini, M.; Valentini, S.; Cairoli, F.; Prandi, A. Hair cortisol level as a retrospective marker of hypothalamic–pituitary–adrenal axis activity in horse foals. Vet. J. 2012, 194, 131–132. [Google Scholar] [CrossRef] [PubMed]
- Montillo, M.; Comin, A.; Corazzin, M.; Peric, T.; Faustini, M.; Veronesi, M.C.; Valentini, S.; Bustaffa, M.; Prandi, A. The effect of temperature, rainfall, and light conditions on hair cortisol concentrations in newborn foals. J. Equine Vet. Sci. 2014, 34, 774–778. [Google Scholar] [CrossRef]
- Duran, M.C.; Janz, D.M.; Waldner, C.L.; Campbell, J.R.; Marques, F.J. Hair cortisol concentration as a stress biomarker in horses: Associations with body location and surgical castration. J. Equine Vet. Sci. 2017, 55, 27–33. [Google Scholar] [CrossRef]
- Banse, H.E.; Getachew, F.; Levy, M.; Smits, J. Influence of season and pituitary pars intermedia dysfunction on hair cortisol concentration in horses. Domest. Anim. Endocrinol. 2020, 72, 106375. [Google Scholar] [CrossRef]
- Prinsloo, M.; Hynd, P.; Franklin, S.; Weaver, S.; van den Boom, R. Hair cortisol concentration is inversely related to the severity of equine squamous gastric disease. Vet. J. 2019, 249, 58–59. [Google Scholar] [CrossRef]
- Placci, M.; Marliani, G.; Sabioni, S.; Gabai, G.; Mondo, E.; Borghetti, P.; De Angelis, E.; Accorsi, P.A. Natural horse boarding vs traditional stable: A comparison of hormonal, hematological and immunological parameters. J. Appl. Anim. Welf. Sci. 2020, 23, 366–377. [Google Scholar] [CrossRef]
- Olvera-Maneu, S.; Carbajal, A.; Gardela, J.; Lopez-Bejar, M. Hair Cortisol, testosterone, dehydroepiandrosterone sulfate and their ratios in stallions as a retrospective measure of hypothalamic–pituitary–adrenal and hypothalamic–pituitary–gonadal axes activity: Exploring the influence of seasonality. Animals 2021, 11, 2202. [Google Scholar] [CrossRef]
- Roberts, S.J. Examination for pregnancy. In Veterinary Obstetrics and Genital Disease, 3rd ed.; Woodstock, V.T., Ed.; Theriogenology; Literary Licensing, LLC: Whitefish, MT, USA, 1986; pp. 14–32. [Google Scholar]
- Ginther, O.J. Embriology and placentation. In Reproductive Biology of the Mare, 2nd ed.; Ginther, O.J., Ed.; Equiservices: Cross Plains, WI, USA, 1993; pp. 345–418. [Google Scholar]
- Russell, E.; Koren, G.; Rieder, M.; Van Uum, S. Hair cortisol as a biological marker of chronic stress: Current status, future directions and unanswered questions. Psychoneuroendocrinology 2012, 37, 589–601. [Google Scholar] [CrossRef]
- Ousey, J.C. Peripartal endocrinology in the mare and foetus. Reprod. Domest. Anim. 2004, 39, 222–231. [Google Scholar] [CrossRef]
- Santschi, E.M.; Vaala, W.E. Identification of the high-risk pregnancy. In Equine Reproduction, 2nd ed.; McKinnon, A.O., Squires, E.L., Vaala, W.E., Varner, D.D., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2011; pp. 5–15. [Google Scholar]
- Ellero, N.; Lanci, A.; Ferlizza, E.; Andreani, G.; Mariella, J.; Isani, G.; Castagnetti, C. Activities of matrix metalloproteinase-2 and-9 in amniotic fluid at parturition in mares with normal and high-risk pregnancy. Theriogenology 2021, 172, 116–122. [Google Scholar] [CrossRef]
- Vaala, W.E.; House, J.K.; Madigan, J.E. Initial management and physical examination of the neonate. In Large Animal Internal Medicine; Smith, B.P., Ed.; Mosby: St. Louis, MO, USA, 2002; pp. 277–293. [Google Scholar]
- Knottenbelt, D.C.; Holdstock, N.; Madigan, J.E. Equine Neonatology Medicine and Surgery; Saunders: Edimbourgh, UK, 2004; pp. 155–363. [Google Scholar]
- Pirrone, A.; Panzani, S.; Govoni, N.; Castagnetti, C.; Veronesi, M.C. Thyroid hormone concentrations in foals affected by perinatal asphyxia syndrome. Theriogenology 2013, 80, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Toribio, R.E. Equine neonatal encephalopathy: Facts, evidence, and opinions. Vet. Clinic. N. Am. Equine Pract. 2019, 35, 363–378. [Google Scholar] [CrossRef] [PubMed]
- Ellero, N.; Freccero, F.; Lanci, A.; Morini, M.; Castagnetti, C.; Mariella, J. Rhabdomyolysis and acute renal failure associated with oxytetracycline administration in two neonatal foals affected by flexural limb deformity. Vet. Sci. 2020, 7, 160. [Google Scholar] [CrossRef] [PubMed]
- Probo, M.; Peric, T.; Fusi, J.; Prandi, A.; Faustini, M.; Veronesi, M.C. Hair cortisol and dehydroepiandrosterone sulfate concentrations in healthy beef calves from birth to 6 months of age. Theriogenology 2021, 175, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Peric, T.; Comin, A.; Corazzin, M.; Montillo, M.; Cappa, A.; Campanile, G.; Prandi, A. Hair cortisol levels in Holstein Friesian and Crossbreed F1 heifers. J. Diary Sci. 2013, 96, 3023–3027. [Google Scholar] [CrossRef]
- Arena, I.; Marliani, G.; Sabioni, S.; Gabai, G.; Bucci, D.; Accorsi, P.A. Assessment of horses’ welfare: Behavioral, hormonal, and husbandry aspects. J. Vet. Behav. 2021, 41, 82–90. [Google Scholar] [CrossRef]
- Romero-Gonzalez, B.; Caparros-Gonzalez, R.A.; Gonzalez-Perez, R.; Delgado-Puertas, P.; Peralta-Ramirez, M.I. Newborn infants’ hair cortisol levels reflect chronic maternal stress during pregnancy. PLoS ONE 2018, 13, e0200279. [Google Scholar] [CrossRef]
- Hoffman, M.C.; D’Anna-Hernandez, K.; Benitez, P.; Ross, R.G.; Laudenslager, M.L. Cortisol during human fetal life: Characterization of a method for processing small quantities of newborn hair from 26 to 42 weeks gestation. Dev. Psychobiol. 2017, 59, 123–127. [Google Scholar] [CrossRef]
- Rossdale, P.D.; Ousey, J.C.; Cottrill, C.M.; Chavatte, P.; Allen, W.R.; McGladdery, A.J. Effects of placental pathology on maternal plasma progestagen and mammary secretion calcium concentrations and on neonatal adrenocortical function in the horse. J. Reprod. Fert. Suppl. 1991, 44, 579–590. [Google Scholar]
- Nathanielsz, P.W.; Rossdale, P.D.; Silver, M.; Comline, R.S. Studies on fetal, neonatal and maternal cortisol metabolism in the mare. J. Reprod. Fert. Suppl. 1975, 23, 625–630. [Google Scholar]
- Fleeger, J.L.; Harms, P.G.; Dunn, E.L.; Atkins, D.T. Levels of deoxycorticosterone and 21-hydroxy-5α-pregnane-3, 20-dione in the peripheral circulation of the prepartum and postpartum mare. Biol. Reprod. 1979, 21, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Cudd, T.A.; LeBlanc, M.; Silver, M.; Norman, W.; Madison, J.; Keller-Wood, M.; Wood, C.E. Ontogeny and ultradian rhythms of adrenocorticotropin and cortisol in the late-gestation fetal horse. J. Endocrinol. 1995, 144, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Ousey, J.C.; Rossdale, P.D.; Palmer, L.; Grainger, L.; Houghton, E. Effects of maternally administered Depot ACTH1–24 on fetal maturation and the timing of parturition in the mare. Equine Vet. J. 2000, 32, 489–496. [Google Scholar] [CrossRef]
- Chavatte, P.; Rossdale, P.D.; Tait, A.D. 11 beta-hydroxysteroid dehydrogenase (11 beta HSD) in equine placenta. Am. Assoc. Equine Pract. 1995, 41, 264–265. [Google Scholar]
- Clarke, K.A.; Ward, J.W.; Forhead, A.J.; Giussani, D.A.; Fowden, A.L. Regulation of 11beta-hydroxysteroid dehydrogenase type 2 activity in ovine placenta by fetal cortisol. J. Endocrinol. 2002, 172, 527–534. [Google Scholar] [CrossRef][Green Version]
- Nagel, C.; Erber, R.; Bergmaier, C.; Wulf, M.; Aurich, J.; Möstl, E.; Aurich, C. Cortisol and progestin release, heart rate and heart rate variability in the pregnant and postpartum mare, fetus and newborn foal. Theriogenology 2012, 78, 759–767. [Google Scholar] [CrossRef]
- Gabai, G.; Mongillo, P.; Giaretta, E.; Marinelli, L. Do dehydroepiandrosterone (DHEA) and its sulfate (DHEAS) play a role in the stress response in domestic animals? Front. Vet. Sci. 2020, 7, 847. [Google Scholar] [CrossRef]
- Legacki, E.L.; Ball, B.A.; Corbin, C.J.; Loux, S.C.; Scoggin, K.E.; Stanley, S.D.; Conley, A.J. Equine fetal adrenal, gonadal and placental steroidogenesis. Reproduction 2017, 154, 445–454. [Google Scholar] [CrossRef]
- Legacki, E.L.; Scholtz, E.L.; Ball, B.A.; Stanley, S.D.; Berger, T.; Conley, A.J. The dynamic steroid landscape of equine pregnancy mapped by mass spectrometry. Reproduction 2016, 151, 421–430. [Google Scholar] [CrossRef]
- Satué, K.; Marcilla, M.; Medica, P.; Ferlazzo, A.; Fazio, E. Testosterone, androstenedione and dehydroepiandrosterone concentrations in pregnant Spanish Purebred mare. Theriogenology 2019, 123, 62–67. [Google Scholar] [CrossRef]
- Pluchino, N.; Drakopoulos, P.; Bianchi-Demicheli, F.; Wenger, J.M.; Petignat, P.; Genazzani, A.R. Neurobiology of DHEA and effects on sexuality, mood and cognition. J. Steroid Biochem. Mol. Biol. 2015, 145, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Whitham, J.C.; Bryant, J.L.; Miller, L.J. Beyond glucocorticoids: Integrating dehydroepiandrosterone (DHEA) into animal welfare research. Animals 2020, 10, 1381. [Google Scholar] [CrossRef] [PubMed]
- Almeida, P.E.; Weber, P.S.D.; Burton, J.L.; Zanella, A.J. Depressed DHEA and increased sickness response behaviors in lame dairy cows with inflammatory foot lesions. Domest. Anim. Endocrinol. 2008, 34, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Gundlach, N.H.; Feldmann, M.; Gundelach, Y.; Gil, M.A.; Siebert, U.; Hoedemaker, M.; Schmicke, M. Dehydroepiandrosterone and cortisol/dehydroepiandrosterone ratios in dairy cattle with postpartum metritis. Res. Vet. Sci. 2017, 115, 530–533. [Google Scholar] [CrossRef]
- Gardela, J.; Carbajal, A.; Tallo-Parra, O.; Olvera-Maneu, S.; Álvarez-Rodríguez, M.; Jose-Cunilleras, E.; López-Béjar, M. Temporary relocation during rest periods: Relocation stress and other factors influence hair cortisol concentrations in horses. Animals 2020, 10, 642. [Google Scholar] [CrossRef]
- Sauveroche, M.; Henriksson, J.; Theodorsson, E.; Holm, A.C.S.; Roth, L.S. Hair cortisol in horses (Equus caballus) in relation to management regimes, personality, and breed. J. Vet. Behav. 2020, 37, 1–7. [Google Scholar] [CrossRef]
- Mazzola, S.M.; Colombani, C.; Pizzamiglio, G.; Cannas, S.; Palestrini, C.; Della Costa, E.; Gazzonis, A.L.; Bionda, A.; Crepaldi, P. Do you think I am living well? A four-season hair cortisol analysis on leisure horses in different housing and management conditions. Animals 2021, 11, 2141. [Google Scholar] [CrossRef]
- Bennett, A.; Hayssen, V. Measuring cortisol in hair and saliva from dogs: Coat color and pigment differences. Domest. Anim. Endocrinol. 2010, 39, 171–180. [Google Scholar] [CrossRef]
- Grigg, E.K.; Nibblett, B.M.; Robinson, J.Q.; Smits, J.E. Evaluating pair versus solitary housing in kennelled domestic dogs (Canis familiaris) using behaviour and hair cortisol: A pilot study. Vet. Rec. Open 2017, 4, e000193. [Google Scholar] [CrossRef]
- Casal, N.; Manteca, X.; Peña, R.; Bassols, A.; Fàbrega, E. Analysis of cortisol in hair samples as an indicator of stress in pigs. J. Vet. Behav. 2017, 19, 1–6. [Google Scholar] [CrossRef]
- Schubach, K.M.; Cooke, R.F.; Brandão, A.P.; Lippolis, K.D.; Silva, L.G.T.; Marques, R.S.; Bohnert, D.W. Impacts of stocking density on development and puberty attainment of replacement beef heifers. Animal 2017, 11, 2260–2267. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.R.B.; Lobeck-Luchterhand, K.M.; Cerri, R.L.A.; Haines, D.M.; Ballou, M.A.; Endres, M.I.; Chebel, R.C. Effects of prepartum stocking density on innate and adaptive leukocyte responses and serum and hair cortisol concentrations. Vet. Immunol. Immunopathol. 2016, 169, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Peric, T.; Comin, A.; Corazzin, M.; Montillo, M.; Canavese, F.; Stebel, M.; Prandi, A. Relocation and hair cortisol concentrations in New Zealand white rabbits. J. Appl. Anim. Welf. Sci. 2017, 20, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Comin, A.; Prandi, A.; Peric, T.; Corazzin, M.; Dovier, S.; Bovolenta, S. Hair cortisol levels in dairy cows from winter housing to summer highland grazing. Livest. Sci. 2011, 138, 69–73. [Google Scholar] [CrossRef]

| Breed | Age (Years) | Parity (n) | Type of Pregnancy (N/HR/AN) | Gestation Length (Days) | |
|---|---|---|---|---|---|
| Group H (n = 56) | Stb n = 34 Other n = 22 | 11 ± 5 (4–24) | 4 ± 3 (1–14) | N | 340 ± 11 (321–371) |
| Group S (n = 51) | Stb n = 31 Other n = 20 | 11 ± 4 (4–21) | 3 ± 3 (1–10) | HR n = 13 AN n = 38 | 332 ± 16 (281–360) |
| Sex | Weight (kg) | Apgar score | Age at admission (days) | Outcome | |
| Group H (n = 56) | Male n = 23 Female n = 33 | 50 ± 8 (38–73) | 10 ± 1 (8–10) | 3 ± 4 (0–13) | Sv |
| Group S (n = 51) | Male n = 27 Female n = 24 | 45 ± 8 (25–62) | 8 ± 2 (0–10) | 2 ± 3 (0–13) | Sv n = 44 NSv n = 7 |
| Group H (n = 56 Pairs; 112 Animals) | Group S (n = 51 Pairs; 102 Animals) | |
|---|---|---|
| Foal CORT (pg/mg) | 71.5 ± 38.2 (27.7–258.9) | 62.2 ± 36.1 (6.3–200.7) |
| Foal DHEA-S (pg/mg) | 19.2 ± 44.0 (2.2–333.9) | 43.1 ± 69.0 (4.5–285.3) * |
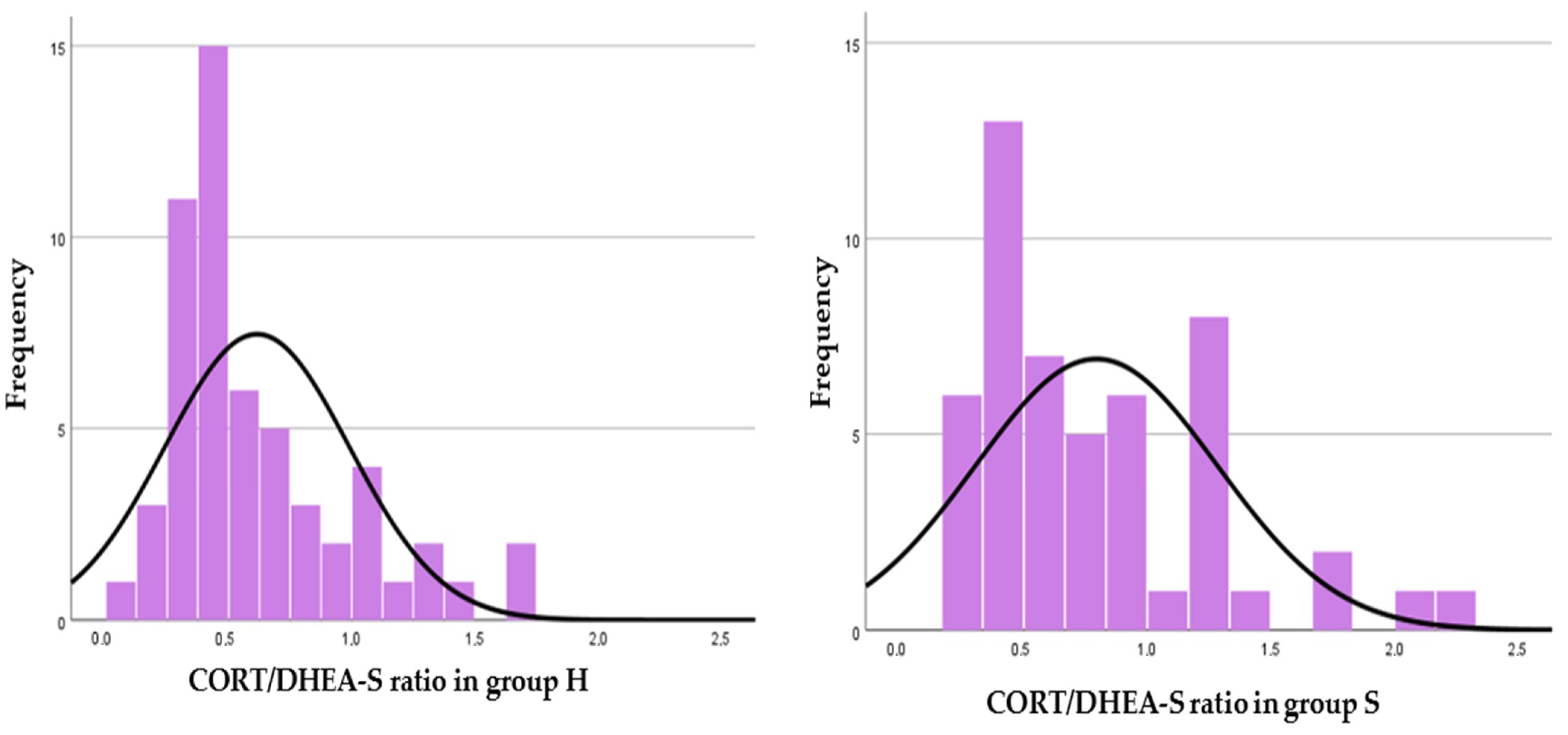
| Foal CORT/DHEA-S ratio | 7.8 ± 6.7 (0.3–38.9) | 4.1 ± 3.8 (0.2–17.7) * |
| Mare CORT (pg/mg) | 5.4 ± 2.4 (2.6–18.5) | 6.6 ± 4.9 (2.1–28.2) |
| Mare DHEA-S (pg/mg) | 12.1 ± 14.1 (2.6–105.7) | 9.0 ± 3.9 (3.1–20.9) |
| Mare CORT/DHEA-S ratio | 0.6 ± 0.4 (0.1–1.7) | 0.8 ± 0.5 (0.2–2.3) * |
| Foal/Mare CORT ratio | 14.9 ± 10.4 (3.2–76.2) | 11.6 ± 7.3 (2.0–28.2) |
| Foal/Mare DHEA-S ratio | 1.8 ± 2.9 (0.1–22.3) | 5.1 ± 7.7 (0.4–32.7) * |
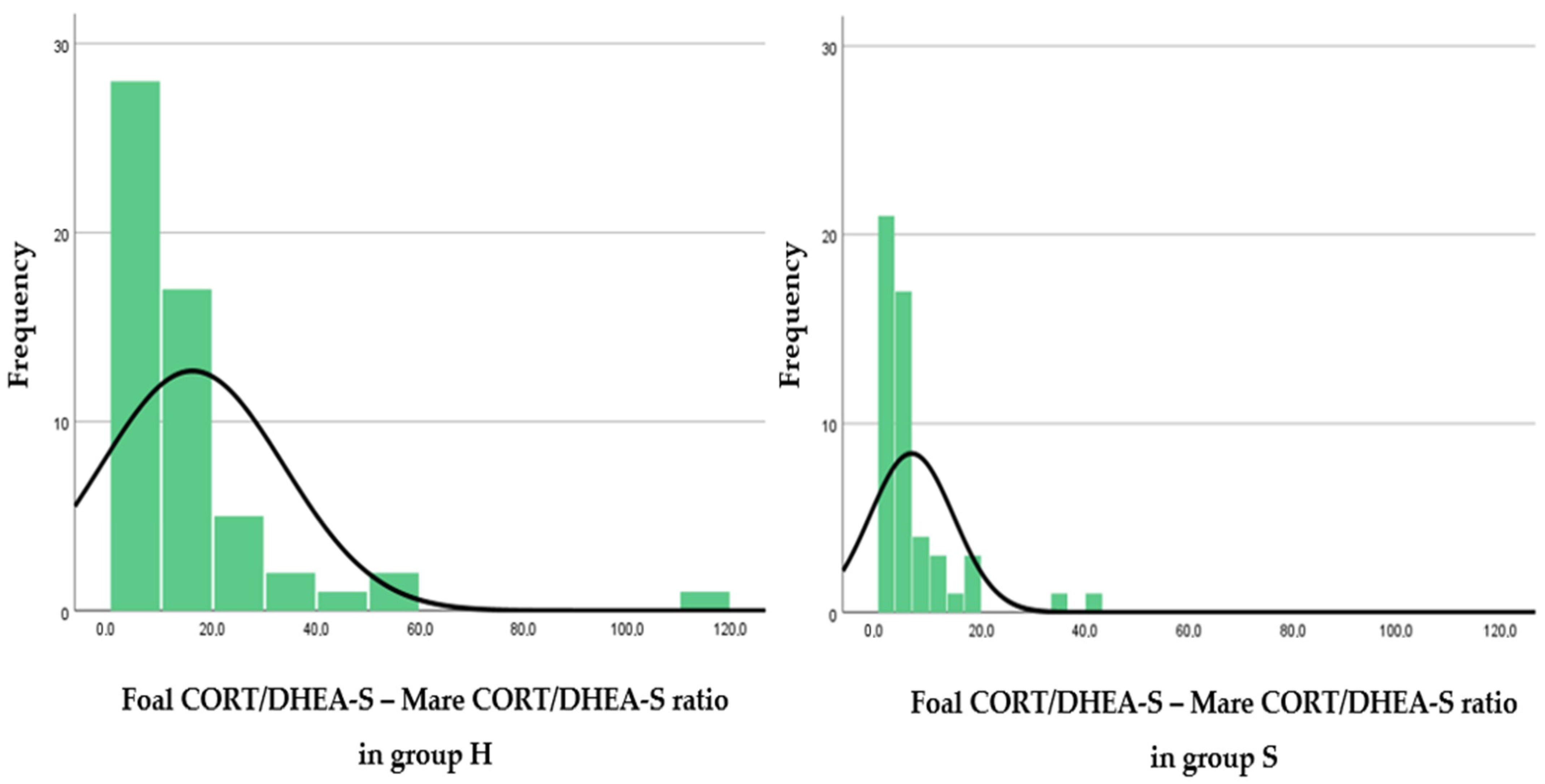
| Foal CORT/DHEA-S - Mare CORT/DHEA-S ratio | 16.0 ± 17.6 (1.0–113.1) | 6.5 ± 8.1 (0.4–42.0) |
| Group HH: Hospital (n = 30 Pairs; 60 Animals) | Group HB: Breeding Farm (n = 26 Pairs; 52 Animals) | |
|---|---|---|
| Foal CORT (pg/mg) | 71.9 ± 39.4 (43.1–258.9) | 71.0 ± 37.5 (27.7–201.2) |
| Foal DHEA-S (pg/mg) | 12.0 ± 7.4 (2.2–33.3) | 27.5 ± 63.7 (2.7–333.9) |
| Foal CORT/DHEA-S ratio | 9.0 ± 8.3 (2.0–39.5) | 6.3 ± 4.2 (0.3–15.5) |
| Mare CORT (pg/mg) | 5.4 ± 2.8 (2.8–18.5) | 5.4 ± 2.0 (2.6–11.3) |
| Mare DHEA-S (pg/mg) | 9.7 ± 3.7 (2.6–17.5) | 15.0 ± 20.1 (2.8–105.7) |
| Mare CORT/DHEA-S ratio | 0.7 ± 0.4 (0.2–1.7) | 0.6 ± 0.3 (0.1–1.5) |
| Foal/Mare CORT ratio | 15.4 ± 12.3 (3.2–76.2) | 14.2 ± 7.9 (5.1–42.8) |
| Foal/Mare DHEA-S ratio | 1.3 ± 0.8 (0.3–4.1) | 2.3 ± 4.2 (0.1–22.3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lanci, A.; Mariella, J.; Ellero, N.; Faoro, A.; Peric, T.; Prandi, A.; Freccero, F.; Castagnetti, C. Hair Cortisol and DHEA-S in Foals and Mares as a Retrospective Picture of Feto-Maternal Relationship under Physiological and Pathological Conditions. Animals 2022, 12, 1266. https://doi.org/10.3390/ani12101266
Lanci A, Mariella J, Ellero N, Faoro A, Peric T, Prandi A, Freccero F, Castagnetti C. Hair Cortisol and DHEA-S in Foals and Mares as a Retrospective Picture of Feto-Maternal Relationship under Physiological and Pathological Conditions. Animals. 2022; 12(10):1266. https://doi.org/10.3390/ani12101266
Chicago/Turabian StyleLanci, Aliai, Jole Mariella, Nicola Ellero, Alice Faoro, Tanja Peric, Alberto Prandi, Francesca Freccero, and Carolina Castagnetti. 2022. "Hair Cortisol and DHEA-S in Foals and Mares as a Retrospective Picture of Feto-Maternal Relationship under Physiological and Pathological Conditions" Animals 12, no. 10: 1266. https://doi.org/10.3390/ani12101266
APA StyleLanci, A., Mariella, J., Ellero, N., Faoro, A., Peric, T., Prandi, A., Freccero, F., & Castagnetti, C. (2022). Hair Cortisol and DHEA-S in Foals and Mares as a Retrospective Picture of Feto-Maternal Relationship under Physiological and Pathological Conditions. Animals, 12(10), 1266. https://doi.org/10.3390/ani12101266






