Turnover Rates and Diet–Tissue Discrimination Factors of Nitrogen and Carbon Stable Isotopes in Seahorse Hippocampus reidi Juveniles Following a Laboratory Diet Shift
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Broodstock
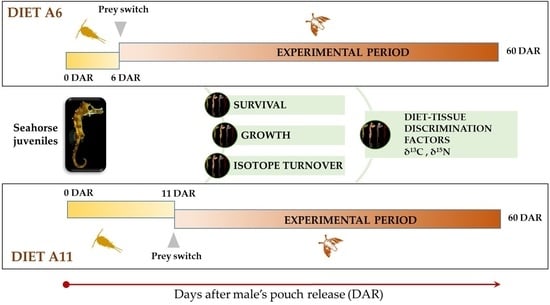
2.2. Experimental Design
- -
- First feeding (0 to 5 DAR): Two daily doses of cultivated copepods Acartia tonsa (1 copepod mL−1);
- -
- Artemia feeding (6 to 60 DAR): Two daily doses of Artemia nauplii (1–2 Artemia mL−1).
- -
- Diet A11
- -
- First feeding (0 to 10 DAR): Two daily doses of cultivated copepods Acartia tonsa (1 copepod mL−1);
- -
- Artemia feeding (11 to 60 DAR): Two daily doses of Artemia nauplii (1–2 Artemia mL−1).
2.3. Sampling and Analyses
2.4. Data Treatment
- -
- Growth-based model G [21]
- -
- Development-based model D (adapted from [23])
2.5. Bioethics
3. Results
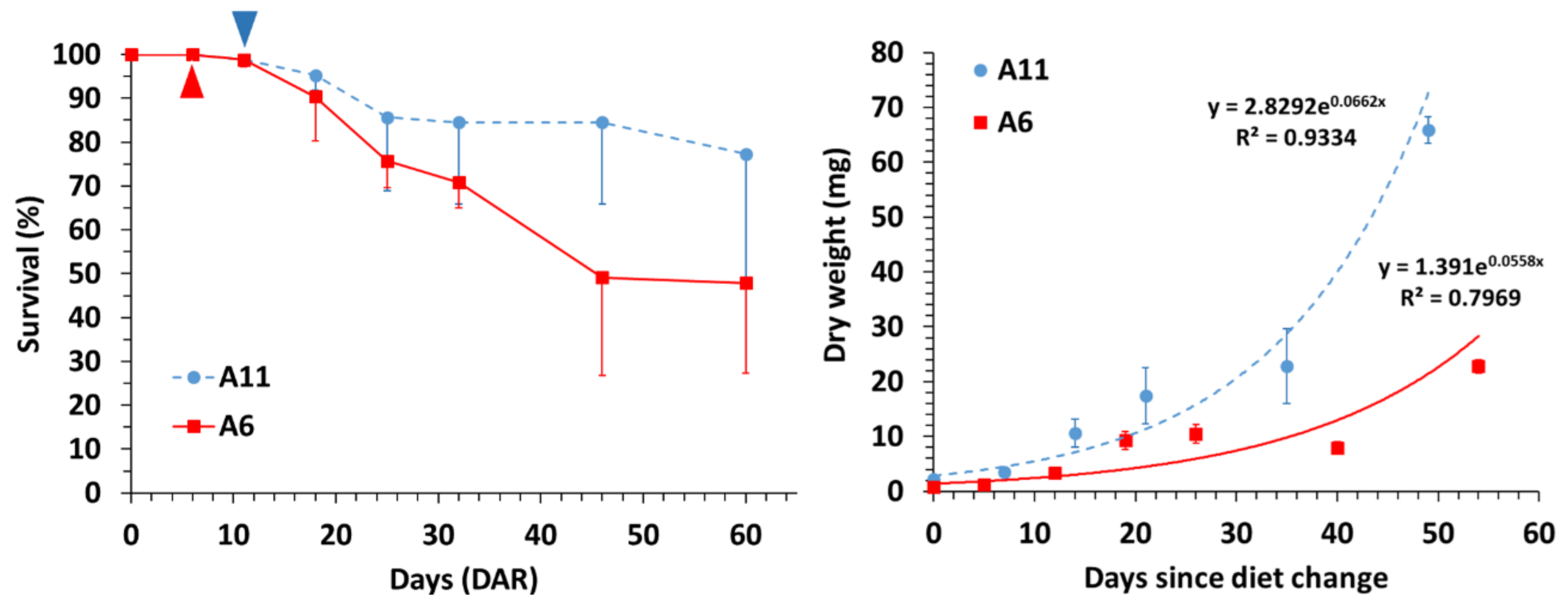
3.1. Survival and Growth
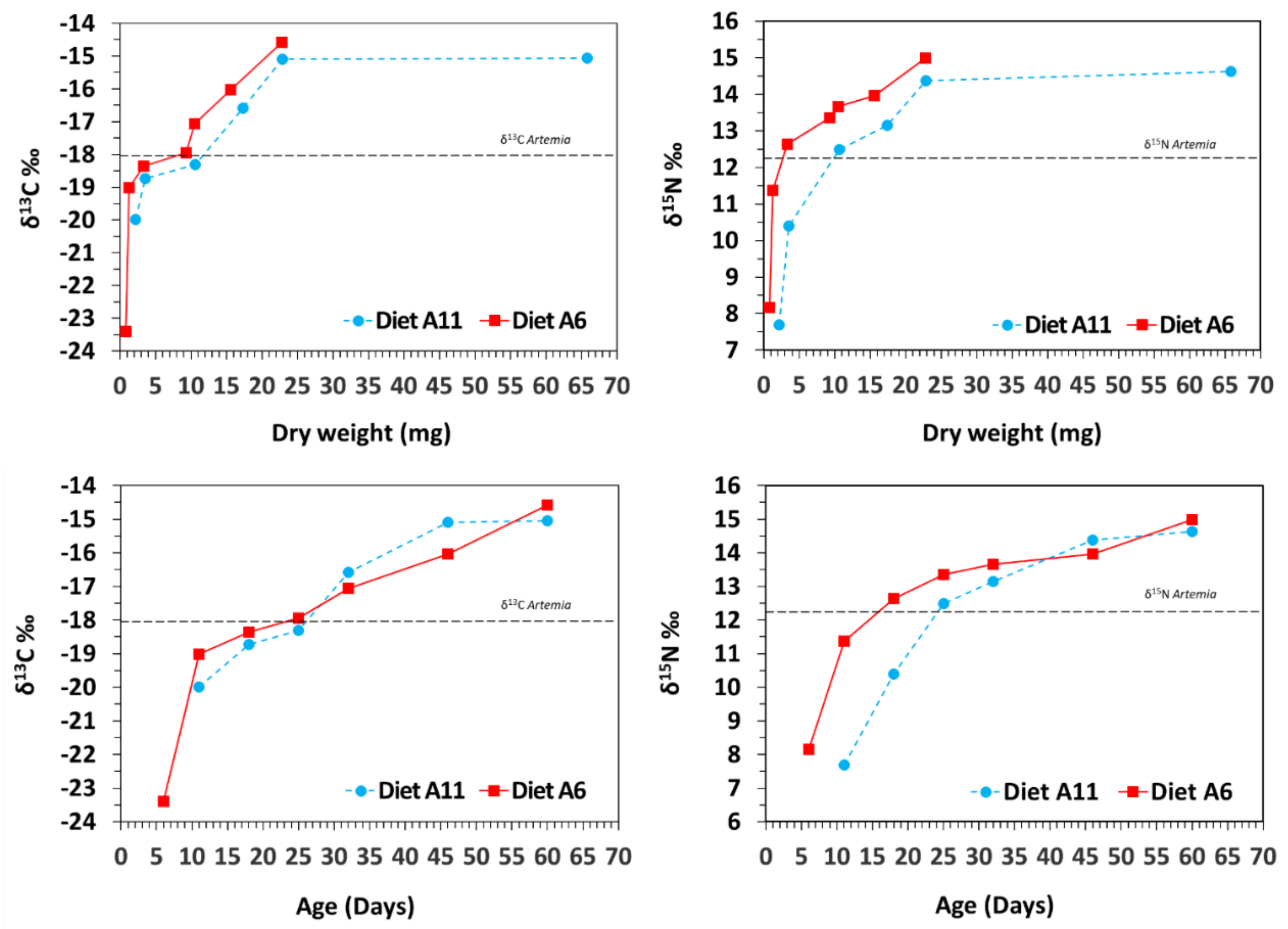
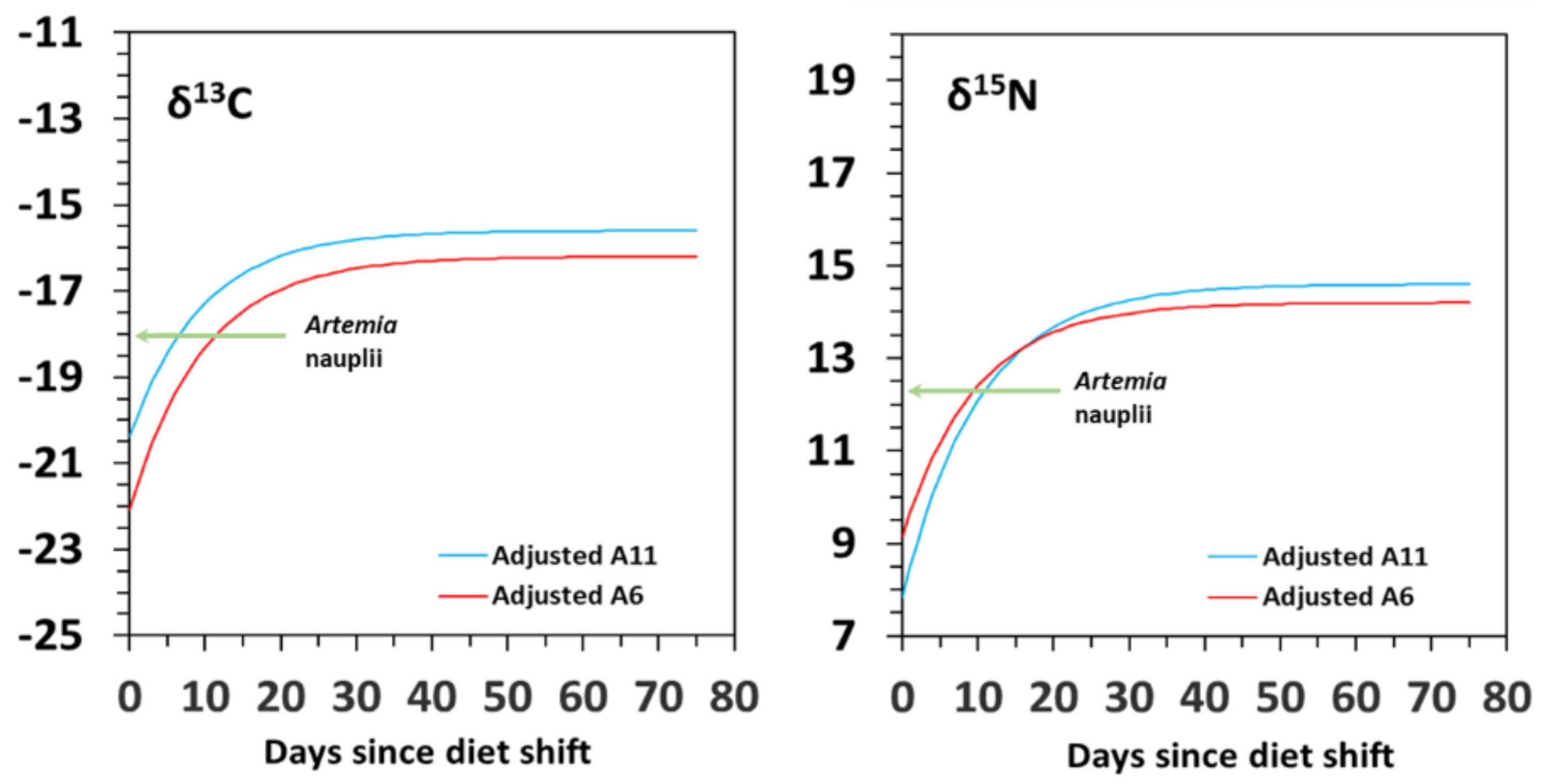
3.2. Isotopic Variation with Ontogeny
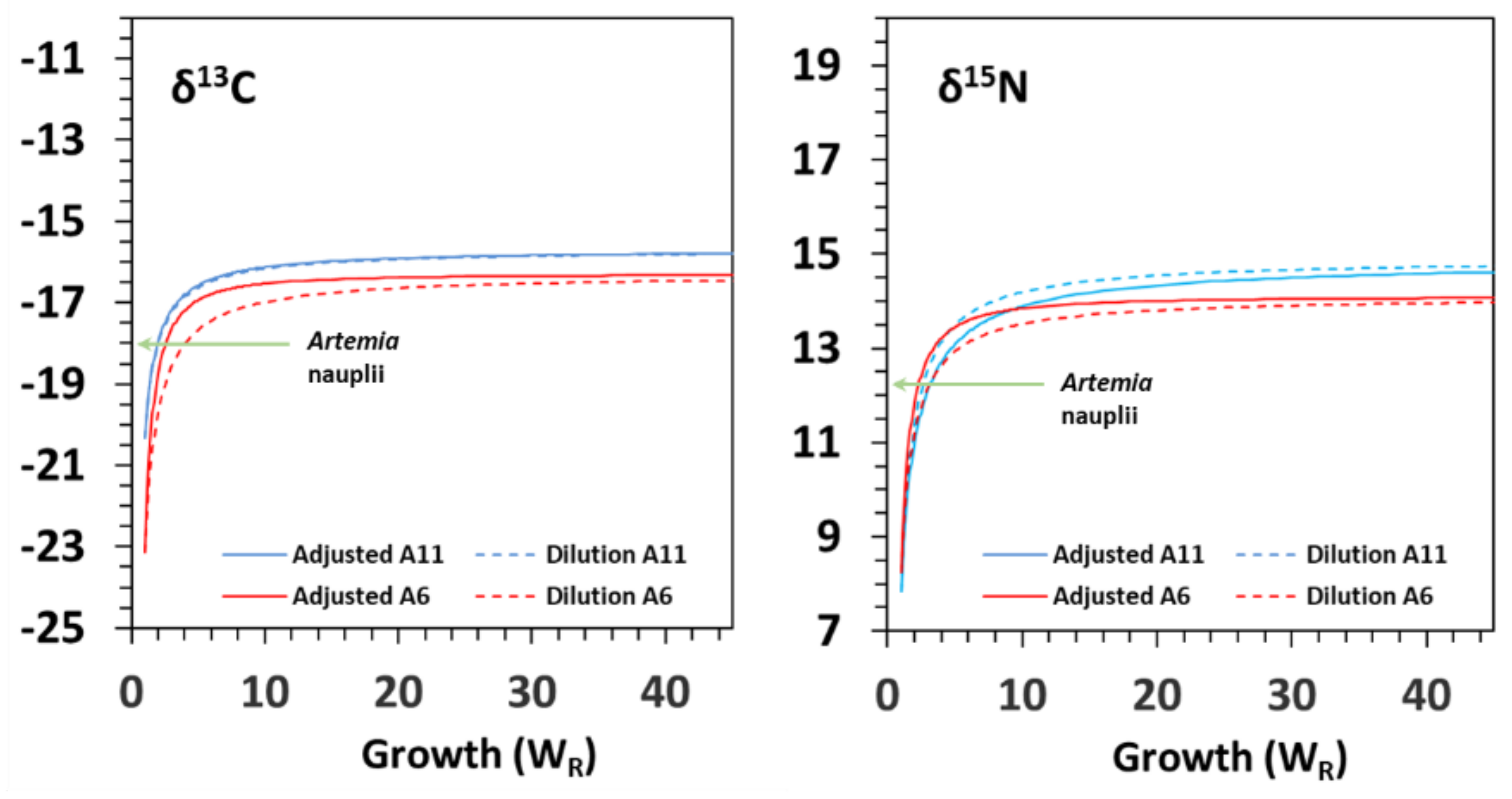
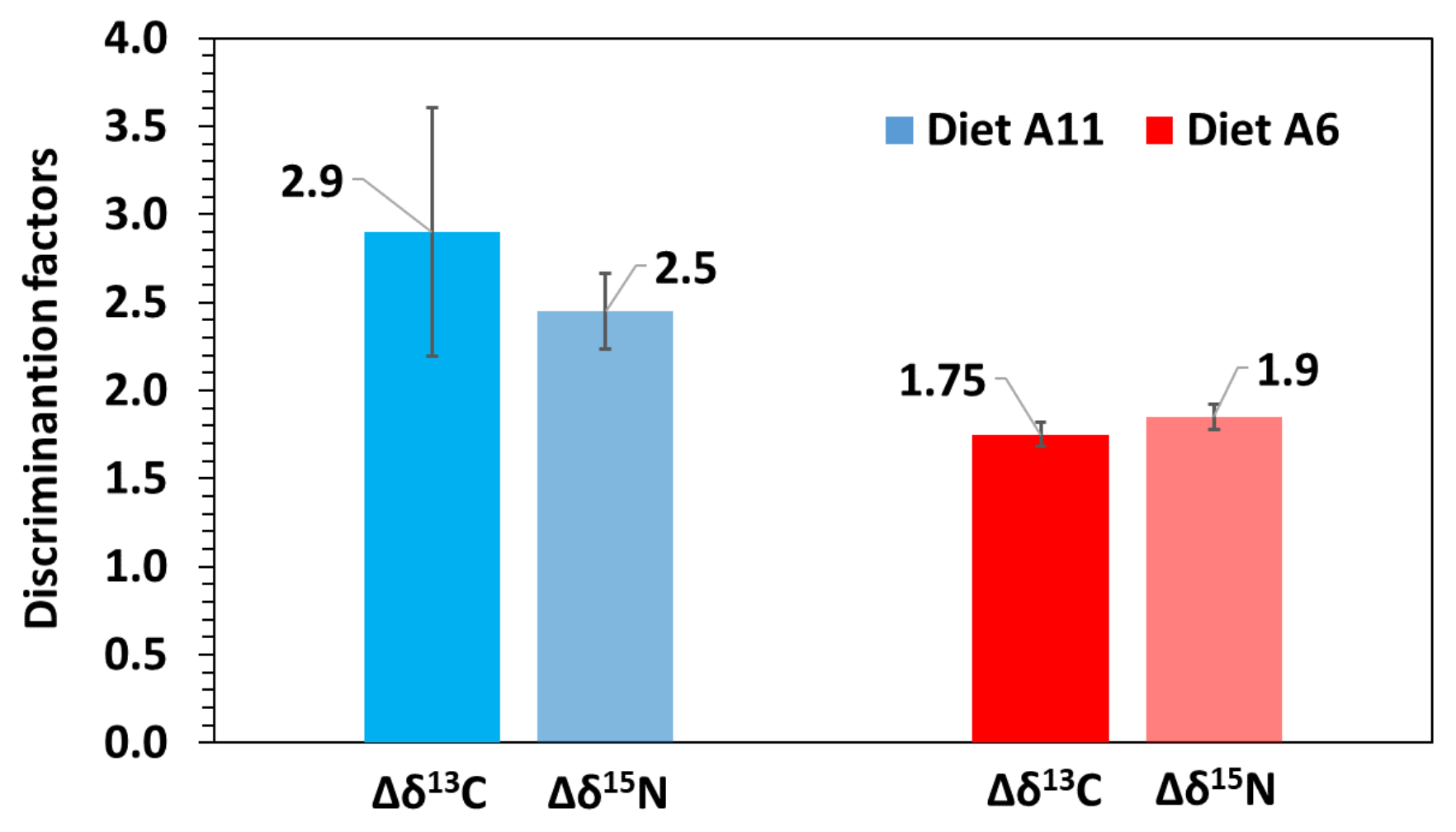
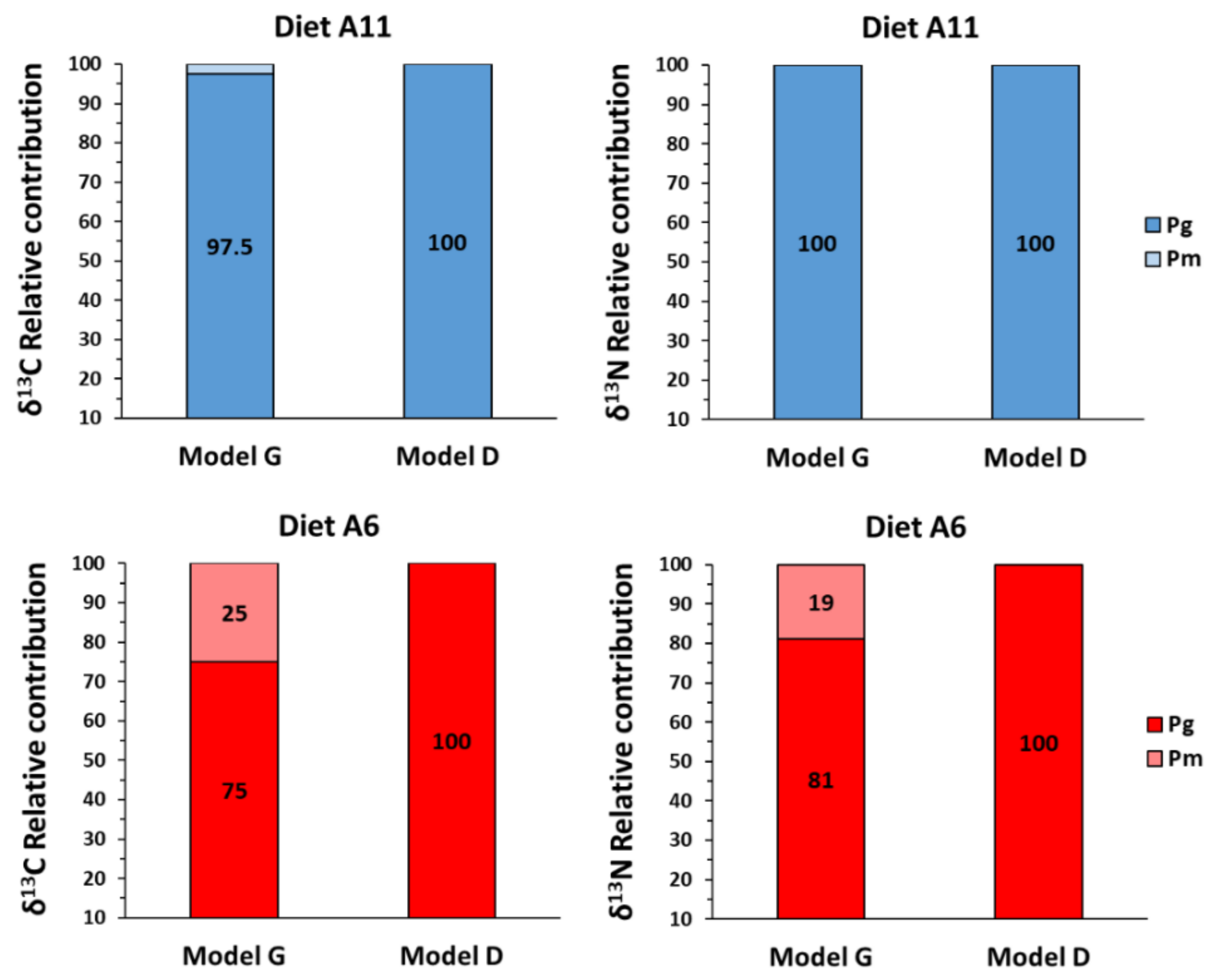
3.3. Turnover and Discrimination Factors
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peterson, B.J.; Howarth, R.W.; Garritt, R.H. Multiple stable isotopes used to trace the flow of organic matter in estuarine food webs. Science 1985, 227, 1361–1363. [Google Scholar] [CrossRef] [PubMed]
- Kurata, K.; Minami, H.; Kikuchi, E. Stable isotope anlysis of food sources for salt marsh snails. Mar. Ecol. Prog. Ser. 2001, 223, 167–177. [Google Scholar] [CrossRef][Green Version]
- Jones, J.I.; Waldron, S. Combined stable isotope and gut contents analysis of food webs in plant-dominated, shallow lakes. Freshw. Biol. 2003, 48, 1396–1407. [Google Scholar] [CrossRef]
- Gamboa-Delgado, J.; Cañavate, J.P.; Zerolo, R.; Le Vay, L. Natural carbon stable isotope ratios as indicators of the relative contribution of live and inert diets to growth in larval Senegalese sole (Solea senegalensis). Aquaculture 2008, 280, 190–197. [Google Scholar] [CrossRef]
- Kharlamenko, V.I.; Kiyashko, S.I.; Rodkina, S.A.; Imbs, A.B. Determination of food sources of marine invertebrates from a subtidal sand community using analyses of fatty acids and stable isotopes. Russ. J. Mar. Biol. 2008, 34, 101–109. [Google Scholar] [CrossRef]
- DeNiro, M.J.; Epstein, S. You are what you eat (plus a few‰): The carbon isotope cycle in food chains. Geol. Soc. Am. Abs. Prog. 1976, 8, 834–835. [Google Scholar]
- Pinnegar, J.K.; Polunin, V.C. Differential fractionation of δ13C and δ15N among fish tissues: Implications for the study of trophic interactions. Funct. Ecol. 1999, 13, 225–231. [Google Scholar] [CrossRef]
- DeNiro, M.J.; Epstein, S. Influence of diet on the distribution of carbon isotopes in animals. Geochim. Cosmochim. Acta 1978, 42, 495–506. [Google Scholar] [CrossRef]
- DeNiro, M.J.; Epstein, S. Influence of diet on the distribution of nitrogen isotopes in animals. Geochim. Cosmochim. Acta 1981, 45, 341–351. [Google Scholar] [CrossRef]
- Olive, P.J.W.; Pinnegar, J.K.; Polunin, N.V.C.; Richards, G.; Welch, R. Isotope trophic-step fractionation: A dynamic equilibrium model. J. Anim. Ecol. 2003, 72, 608–617. [Google Scholar] [CrossRef]
- Martínez del Rio, C.; Wolf, B.O. Mass balance models for animal isotopic ecology. In Physiological and Ecological Adaptations to Feeding in Vertebrates; Starck, M.A., Wang, T., Eds.; Science Publishers: Enfield, UK, 2005; pp. 141–174. [Google Scholar]
- Fry, B. Stable Isotope Ecology; Springer: New York, NY, USA, 2006. [Google Scholar]
- Post, D.M. Using stable isotopes to estimate trophic position: Models, methods, and assumptions. Ecology 2002, 83, 703–718. [Google Scholar] [CrossRef]
- Hobson, K.A.; Welch, H.E. Cannibalism and trophic structure in a high Arctic lake: Insights from stable-isotope analysis. Can. J. Fish. Aquat. Sci. 1995, 52, 1195–1201. [Google Scholar] [CrossRef]
- Ponsard, S.; Averbuch, P. Should growing and adult animals fed on the same diet show different delta 15N values? Rapid Commun. Mass. Sp. 1999, 13, 1305–1310. [Google Scholar] [CrossRef]
- Overman, N.C.; Parrish, D.L. Stable isotope composition of walleye: N-15 accumulation with age and area-specific differences in delta C-13. Can. J. Fish. Aquat. Sci. 2001, 58, 1253–1260. [Google Scholar] [CrossRef]
- Pinnegar, J.K.; Campbell, N.; Polunin, N.V.C. Unusual stable isotope fractionation patterns observed for fish host-parasite trophic relationships. J. Fish. Biol. 2001, 59, 494–503. [Google Scholar]
- Vanderklift, M.A.; Ponsard, S. Sources of variation in consumer-diet δ15N enrichment: A meta-analysis. Oecologia 2003, 136, 169–182. [Google Scholar] [CrossRef]
- Barnes, C.; Sweeting, C.J.; Jennings, S.; Barry, J.T.; Polunin, N.V.C. Effect of temperature and ration size on carbon and nitrogen stable isotope trophic fractionation. Funct. Ecol. 2007, 21, 356–362. [Google Scholar] [CrossRef]
- Planas, M.; Chamorro, A.; Paltrinieri, A.; Campos, S.; Nedelec, K.; Hernández-Urcera, J. Effect of diet on breeders and inheritance in Syngnathids: Application of isotopic experimentally derived data to field studies. Mar. Ecol. Prog. Ser. 2020, 650, 107–123. [Google Scholar] [CrossRef]
- Fry, B.; Arnold, C. Rapid 13C/12C turnover during growth of brown shrimp (Penaeus aztecus). Oecologia 1982, 172, 21–34. [Google Scholar] [CrossRef]
- Hobson, K.A.; Clark, R.G. Assessing avian diets using stable isotopes I: Turnover of 13C in tissues. Condor 1992, 94, 181–188. [Google Scholar] [CrossRef]
- Hesslein, R.H.; Hallard, K.A.; Ramlal, P. Replacement of sulphur, carbon and nitrogen in tissue of growing broad whitefish (Coregonus nasus) in response to a change in diet traced by δ34S, δ13C and δ15N. Can. J. Fish. Aquat. Sci. 1993, 50, 2071–2076. [Google Scholar] [CrossRef]
- Herzka, S.Z.; Holt, G.J. Changes in isotopic composition of red drum (Sciaenops ocellatus) larvae in response to dietary shifts: Potential applications to settlement studies. Can. J. Fish. Aquat. Sci. 2000, 57, 137–147. [Google Scholar] [CrossRef]
- MacAvoy, S.E.; Macko, S.A.; Garman, G.C. Isotopic turnover in aquatic predators: Quantifying the exploitation of migratory prey. Can. J. Fish. Aquat. Sci. 2001, 58, 923–932. [Google Scholar] [CrossRef]
- Bosley, K.L.; Witting, D.A.; Chambers, R.C.; Wainright, S.C. Estimating turnover rates of carbon and nitrogen in recently metamorphosed winter flounder Pseudopleuronectes americanus with stable isotopes. Mar. Ecol. Prog. Ser. 2002, 236, 233–240. [Google Scholar] [CrossRef]
- Tominaga, O.; Uno, N.; Seikai, T. Influence of diet shift from formulated feed to live mysids on the carbon and nitrogen stable isotope ratio (δ13C and δ15N) in dorsal muscles of juvenile Japanese flounders, Paralichthys olivaceus. Aquaculture 2003, 218, 265–276. [Google Scholar] [CrossRef]
- Gannes, L.Z.; O’Brien, D.M.; Martínez del Rio, C. Stable isotopes in animal ecology: Assumptions, caveats, and a call for more laboratory experiments. Ecology 1997, 78, 1271–1276. [Google Scholar] [CrossRef]
- Rosa, I.L.; Dias, T.L.; Baum, J.K. Threatened fishes of the world: Hippocampus reidi Ginsburg, 1933 (Syngnathidae). Environ. Biol. Fish. 2002, 64, 378. [Google Scholar] [CrossRef]
- Dias, T.L.P.; Rosa, I.L. Habitat preferences of a seahorse species, Hippocampus reidi (Teleostei: Syngnathidae) in Brazil. Aquaculture 2003, 6, 165–176. [Google Scholar]
- Hora, M.d.S.C.d.; Joyeux, J.C.; Rodrigues, R.V.; Sousa-Santos, L.P.d.; Gomes, L.C.; Tsuzuki, M.Y. Tolerance and growth of the longsnout seahorse Hippocampus reidi at different salinities. Aquaculture 2016, 463, 1–6. [Google Scholar] [CrossRef]
- Cohen, F.P.A.; Valenti, W.C.; Planas, M.; Calado, R. Seahorse aquaculture, biology and conservation: Knowledge gaps and research opportunities. Rev. Fish. Sci. Aquac. 2017, 25, 100–111. [Google Scholar] [CrossRef]
- Koning, S.; Hoeksema, B.W. Diversity of seahorse species (Hippocampus spp.) in the international aquarium trade. Diversity 2021, 13, 187. [Google Scholar] [CrossRef]
- Olivotto, I.; Planas, M.; Simões, N.; Holt, G.J.; Avella, A.M.; Calado, R. Advances in breeding and rearing marine ornamentals. J. World Aquacul. Soc. 2011, 42, 135–166. [Google Scholar] [CrossRef]
- Blanco, A.; Planas, M.; Moyano, F.J. Ontogeny of digestive enzymatic capacities in juvenile seahorses Hippocampus guttulatus fed on different live diets. Aquacult. Res. 2015, 47, 3558–3569. [Google Scholar] [CrossRef]
- Randazzo, B.; Rolla, L.; Ofelio, C.; Planas, M.; Gioacchini, G.; Vargas, A.; Giorgini, E.; Olivotto, I. The influence of diet on the early development of two seahorse species (H. guttulatus and H. reidi): Traditional and innovative approaches. Aquaculture 2018, 490, 75–90. [Google Scholar] [CrossRef]
- Planas, M.; Chamorro, A.; Quintas, P.; Vilar, A. Establishment and maintenance of threatened long-snouted seahorse, Hippocampus guttulatus, broodstock in captivity. Aquaculture 2008, 283, 19–28. [Google Scholar] [CrossRef]
- Planas, M.; Silva, C.; Quintas, P.; Chamorro, A.; Piñero, S. Ongrowing and enhancement of n-3 HUFA profile in adult Artemia: Short- vs. long-time enrichment. J. Appl. Phycol. 2017, 29, 1409–1420. [Google Scholar] [CrossRef]
- Blanco, A.; Chamorro, A.; Planas, M. Implications of physical key factors in the early rearing of the long-snouted seahorse Hippocampus guttulatus. Aquaculture 2014, 433, 214–222. [Google Scholar] [CrossRef]
- Post, D.M.; Layman, C.A.; Arrington, D.A.; Takimoto, G.; Quattrochi, J.; Montaña, C.G. Getting to the fat of the matter: Models, methods and assumptions for dealing with lipids in stable isotope analyses. Oecologia 2007, 152, 179–189. [Google Scholar] [CrossRef]
- Logan, J.M.; Jardine, T.D.; Miller, T.J.; Bunn, S.E.; Cunjak, R.A.; Lutcavage, M.E. Lipid corrections in carbon and nitrogen stable isotope analyses: Comparison of chemical extraction and modelling methods. J. Anim. Ecol. 2008, 77, 838–846. [Google Scholar] [CrossRef]
- Martínez del Rio, C.; Anderson-Sprecher, R. Beyond the reaction progress variable: The meaning and significance of isotopic incorporation data. Oecologia 2008, 156, 765–772. [Google Scholar] [CrossRef]
- Xia, B.; Gao, Q.F.; Li, H.M.; Dong, S.L.; Wang, F. Turnover and fractionation of nitrogen stable isotope in tissues of grass carp Ctenopharyngodon idellus. Aquacult. Environ. Interact. 2013, 3, 177–186. [Google Scholar] [CrossRef]
- Winter, E.R.; Nolan, E.T.; Busst, G.M.A.; Britton, J.R. Estimating stable isotope turnover rates of epidermal mucle and dorsal muscle for an omnivorous fish using a diet-switch experiment. Hydrobiologia 2019, 828, 245–258. [Google Scholar] [CrossRef]
- Kleibert, M. Body size and metabolism. Hilgardia 1932, 6, 315–351. [Google Scholar] [CrossRef]
- Boecklen, W.J.; Yarnes, C.T.; Cook, B.A.; James, A.C. On the use of stable isotopes in trophic ecology. Annu. Rev. Ecol. Evol. Syst. 2011, 42, 411–440. [Google Scholar] [CrossRef]
- Buchheister, A.; Latour, R.J. Turnover and fractionation of carbon and nitrogen stable isotopes in tissues of a migratory coastal predator, summer flounder (Paralichthys dentatus). Can. J. Fish. Aquat. Sci. 2010, 67, 445–461. [Google Scholar] [CrossRef]
- Tieszen, L.L.; Boutton, T.W.; Tesdahl, K.G.C.; Slade, N.A. Fractionation and turnover of stable carbon isotopes in animal tissues: Implications for δ13C analysis of diet. Oecologia 1983, 57, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Payne, M.; Rippingale, R. Rearing West Australian seahorse, Hippocampus subelongatus, juveniles on copepod nauplii and enriched Artemia. Aquaculture 2000, 188, 353–361. [Google Scholar] [CrossRef]
- Olivotto, I.; Avella, M.A.; Sampaolesi, G.; Piccinetti, C.; Navarro Ruiz, P.; Carnevali, O.; Ruiz, P.N. Breeding and rearing the longsnout seahorse Hippocampus reidi: Rearing and feeding studies. Aquaculture 2008, 283, 92–96. [Google Scholar] [CrossRef]
- Blanco, A.; Planas, M. Mouth growth and prey selection in juveniles of the European long-snouted seahorse, Hippocampus guttulatus. J. World Aquacult. Soc. 2015, 46, 596–607. [Google Scholar] [CrossRef]
- Novelli, B.; Socorro, J.A.; Caballero, M.J.; Otero-Ferrer, F.; Segade-Botella, A.; Molina Domínguez, L. Development of seahorse (Hippocampus reidi, Ginsburg 1933): Histological and histochemical study. Fish. Physiol. Biochem. 2015, 41, 1233–1251. [Google Scholar] [CrossRef]
- Valladares, S.; Planas, M. Application of effective day degrees in the assessment of stable isotope patterns in developing seahorses under different temperatures. Animals 2020, 10, 1571. [Google Scholar] [CrossRef] [PubMed]
- Harvey, C.J.; Hanson, P.C.; Essington, T.E.; Brown, P.B.; Kitchell, J.F. Using bioenergetics models to predict stable isotope ratios in fishes. Can. J. Fish. Aquat. Sci. 2002, 59, 115–124. [Google Scholar] [CrossRef]
- Suarez-Bregua, P.; Rosendo, S.; Comesaña, P.; Sánchez-Ruiloba, L.; Morán, P.; Planas, M.; Rotllant, J. Dynamic changes in DNA methylation during seahorse (Hippocampus reidi) postnatal development and settlement. Front. Zool. 2021, 18, 52. [Google Scholar] [CrossRef] [PubMed]
- Ofelio, C.; Díaz, A.O.; Radaelli, G.; Planas, M. Histological characterization of early developmental stages in the seahorse Hippocampus guttulatus. J. Fish. Biol. 2018, 93, 72–87. [Google Scholar] [CrossRef]
- Ofelio, C.; Cohen, S.; Adriaens, D.; Radaelli, G.; Díaz, A.O. Histochemistry of goblet cells and micro-computed tomography to study the digestive system in the long-snouted seahorse Hippocampus guttulatus. Aquaculture 2019, 502, 400–409. [Google Scholar] [CrossRef]
- Beardsley, H.; Britton, J.R. Contribution of temperature and nutrient loading to growth rate variation of three cyprinid fishes in a lowland river. Aquat. Ecol. 2012, 46, 143–152. [Google Scholar] [CrossRef]
- Busst, G.M.A.; Britton, J.R. Tissue-specific turnover rates of the nitrogen stable isotope as functions of time and growth in a cyprinid fish. Hydrobiologia 2018, 805, 49–60. [Google Scholar] [CrossRef]
- Xia, B.; Gao, Q.F.; Dong, S.L.; Wang, F. Carbon stable isotope turnover and fractionation in grass carp Ctenopharyngodon idella tissues. Aquat. Biol. 2013, 19, 207–2016. [Google Scholar] [CrossRef]
- Caut, S.; Ângulo, E.; Courchamp, F. Variation in discrimination factors (Δ 15N and Δ 13C): The effect of diet isotopic values and applications for diet reconstruction. J. Appl. Ecol. 2009, 46, 443–453. [Google Scholar] [CrossRef]
- Le Vay, L.; Gamboa-Delgado, J. Naturally-occurring stable isotopes as direct measures of larval feeding efficiency, nutrient incorporation and turnover. Aquaculture 2011, 315, 95–103. [Google Scholar] [CrossRef]
- Uriarte, A.; García, A.; Ortega, A.; de la Gándara, F.; Quintanilla, J.; Laiz-Carrión, R. Isotopic discrimination factors and nitrogen turnover rates in reared Atlantic bluefin tuna larvae (Thunnus thynnus): Effects of maternal transmission. Sci. Mar. 2016, 80, 447–456. [Google Scholar] [CrossRef]
- Varela, J.L.; Larrañaga, A.; Medina, A. Prey-muscle carbon and nitrogen stable-isotope discrimination factors in Atlantic bluefin tuna (Thunnus thynnus). J. Exp. Mar. Biol. Ecol. 2011, 406, 21–28. [Google Scholar] [CrossRef]
- Logan, J.; Haas, H.; Deegan, L.; Gaines, E. Turnover rates of nitrogen stable isotopes in the salt marsh mummichog, Fundulus heteroclitus, following a laboratory diet switch. Oecologia 2006, 147, 91–395. [Google Scholar] [CrossRef] [PubMed]
- Cunha, I.; Saborido-Rey, F.; Planas, M. Fish larvae nutritional condition assessment using multivariate analysis on nucleic acids and protein levels. Biol. Bull. 2007, 204, 339–349. [Google Scholar] [CrossRef]
- Tarboush, R.A.; MacAvoy, S.E.; Macko, S.A.; Connaughton, V. Contribution of catabolic tissue replacement to the turnover of stable isotopes in Danio rerio. Can. J. Zool. 2006, 84, 1453–1460. [Google Scholar] [CrossRef]
- Heady, W.N.; Moore, J.W. Tissue turnover and stable isotope clocks to quantify resource shifts in anadromous rainbow trout. Oecologia 2013, 172, 21–34. [Google Scholar] [CrossRef]
- Schubert, P.; Vog, L.; Ede, K.; Hauff, T.; Wilk, T. Effects of feed species and HUFA composition on survival and growth of the longsnout seahorse (Hippocampus reidi). Front. Mar. Sci. 2016, 3, 53. [Google Scholar] [CrossRef]
| Diet | Days | Survival | DW (mg) | SL (mm) | C:N | |||
|---|---|---|---|---|---|---|---|---|
| (DAR) | (%) | Mean | sd | Mean | sd | Mean | sd | |
| A6 | 6 | 100 | 0.80 | 0.02 | 12.33 | 0.32 | 5.75 | 0.00 |
| 11 | 98.8 | 1.30 | 0.39 | 15.95 | 2.52 | 3.03 | 0.06 | |
| 18 | 90.4 | 3.33 | 0.07 | 20.19 | 0.10 | 3.20 | 0.03 | |
| 25 | 75.7 | 9.28 | 2.30 | 26.41 | 4.12 | 3.07 | 0.37 | |
| 32 | 70.9 | 10.50 | 1.66 | 29.32 | 2.34 | 2.88 | 0.04 | |
| 46 | 49.2 | 15.61 | 4.17 | 31.81 | 5.07 | 2.80 | 0.01 | |
| 60 | 47.9 | 22.78 | 3.11 | 35.01 | 1.80 | 2.85 | 0.04 | |
| A11 | 11 | 98.8 | 2.20 | 0.14 | 18.15 | 0.35 | 3.14 | 0.02 |
| 18 | 95.2 | 3.53 | 0.85 | 22.05 | 2.39 | 3.07 | 0.06 | |
| 25 | 85.7 | 10.63 | 0.04 | 26.74 | 0.40 | 3.29 | 0.10 | |
| 32 | 85.4 | 17.38 | 6.29 | 33.82 | 4.66 | 3.14 | 0.20 | |
| 46 | 84.5 | 22.85 | 2.58 | 36.94 | 3.08 | 3.05 | 0.17 | |
| 60 | 77.4 | 65.85 | 7.41 | 51.21 | 5.87 | 2.91 | 0.08 | |
| δ13C | Model G | δYeq | SE | c | SE | R2 | G50 | G95 | D50 | D95 | Δδ |
| A6 | −16.3 | 0.5 | −1.474 | 0.489 | 0.831 | 1.6 | 7.6 | 8.4 | 36.3 | 1.7 | |
| A11 | −15.7 | 0.4 | −1.037 | 0.103 | 0.776 | 2.0 | 43.7 | 10.1 | 74.4 | 3.4 | |
| Model D | δYeq | SE | m | SE | R2 | G50 | G95 | D50 | D95 | Δδ | |
| A6 | −16.2 | 0.9 | 0.040 | 0.070 | 0.816 | 2.5 | 8.8 | 6.8 | 29.4 | 1.8 | |
| A11 | −15.6 | 0.8 | 0.035 | 0.047 | 0.828 | 2.0 | 8.5 | 6.6 | 28.6 | 2.4 | |
| δ15N | Model G | δYeq | SE | c | SE | R2 | G50 | G95 | D50 | D95 | Δδ |
| A6 | 14.1 | 0.3 | −1.326 | 0.269 | 0.934 | 1.7 | 9.6 | 9.3 | 40.3 | 1.8 | |
| A11 | 14.9 | 0.5 | −0.844 | 0.176 | 0.959 | 2.3 | 34.8 | 12.4 | 57.3 | 2.6 | |
| Model D | δYeq | SE | m | SE | R2 | G50 | G95 | D50 | D95 | Δδ | |
| A6 | 14.2 | 0.5 | 0.041 | 0.018 | 0.916 | 2.5 | 8.6 | 6.7 | 28.9 | 1.9 | |
| A11 | 14.6 | 0.1 | 0.030 | 0.008 | 0.944 | 2.0 | 9.4 | 6.9 | 30.1 | 2.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Urcera, J.; Dias Carneiro, M.D.; Planas, M. Turnover Rates and Diet–Tissue Discrimination Factors of Nitrogen and Carbon Stable Isotopes in Seahorse Hippocampus reidi Juveniles Following a Laboratory Diet Shift. Animals 2022, 12, 1232. https://doi.org/10.3390/ani12101232
Hernández-Urcera J, Dias Carneiro MD, Planas M. Turnover Rates and Diet–Tissue Discrimination Factors of Nitrogen and Carbon Stable Isotopes in Seahorse Hippocampus reidi Juveniles Following a Laboratory Diet Shift. Animals. 2022; 12(10):1232. https://doi.org/10.3390/ani12101232
Chicago/Turabian StyleHernández-Urcera, Jorge, Mario Davi Dias Carneiro, and Miquel Planas. 2022. "Turnover Rates and Diet–Tissue Discrimination Factors of Nitrogen and Carbon Stable Isotopes in Seahorse Hippocampus reidi Juveniles Following a Laboratory Diet Shift" Animals 12, no. 10: 1232. https://doi.org/10.3390/ani12101232
APA StyleHernández-Urcera, J., Dias Carneiro, M. D., & Planas, M. (2022). Turnover Rates and Diet–Tissue Discrimination Factors of Nitrogen and Carbon Stable Isotopes in Seahorse Hippocampus reidi Juveniles Following a Laboratory Diet Shift. Animals, 12(10), 1232. https://doi.org/10.3390/ani12101232