Simple Summary
The world’s population is projected to reach 10 billion by 2050. To meet the nutritional needs of this growing population, animal production must double by 2050. The production of fish and other aquatic animals is growing rapidly, but with an intensification of farming, the risk of infectious diseases is increasing, including bacterial diseases. In recent years, antibiotics and chemotherapeutic agents that can be used in aquaculture have been evidenced as no longer effective, resulting in a lack of effective treatment options and leading to higher animal mortality and economic losses for the farm. For this reason, new prevention and treatment options are being sought. One such method is the use of bacteriophages. These are viruses that attack bacteria, consequently destroying them. This is not a new idea, as the first scientific reports on the use of bacteriophages on animals in aquaculture were published 40 years ago but were abandoned after the invention of antibiotics. Now, they are rapidly gaining renewed interest. This paper summarizes the results of using bacteriophages in various aquaculture animals for the prevention and control of bacterial pathogens.
Abstract
To meet the nutritional requirements of our growing population, animal production must double by 2050, and due to the exhaustion of environmental capacity, any growth will have to come from aquaculture. Aquaculture is currently undergoing a dynamic development, but the intensification of production increases the risk of bacterial diseases. In recent years, there has been a drastic development in the resistance of pathogenic bacteria to antibiotics and chemotherapeutic agents approved for use, which has also taken place in aquaculture. Consequently, animal mortality and economic losses in livestock have increased. The use of drugs in closed systems is an additional challenge as it can damage biological filters. For this reason, there has been a growing interest in natural methods of combating pathogens. One of the methods is the use of bacteriophages both for prophylactic purposes and therapy. This work summarizes the diverse results of the in vivo application of bacteriophages for the prevention and control of bacterial pathogens in aquatic animals to provide a reference for further research on bacteriophages in aquaculture and to compare major achievements in the field.
1. Introduction
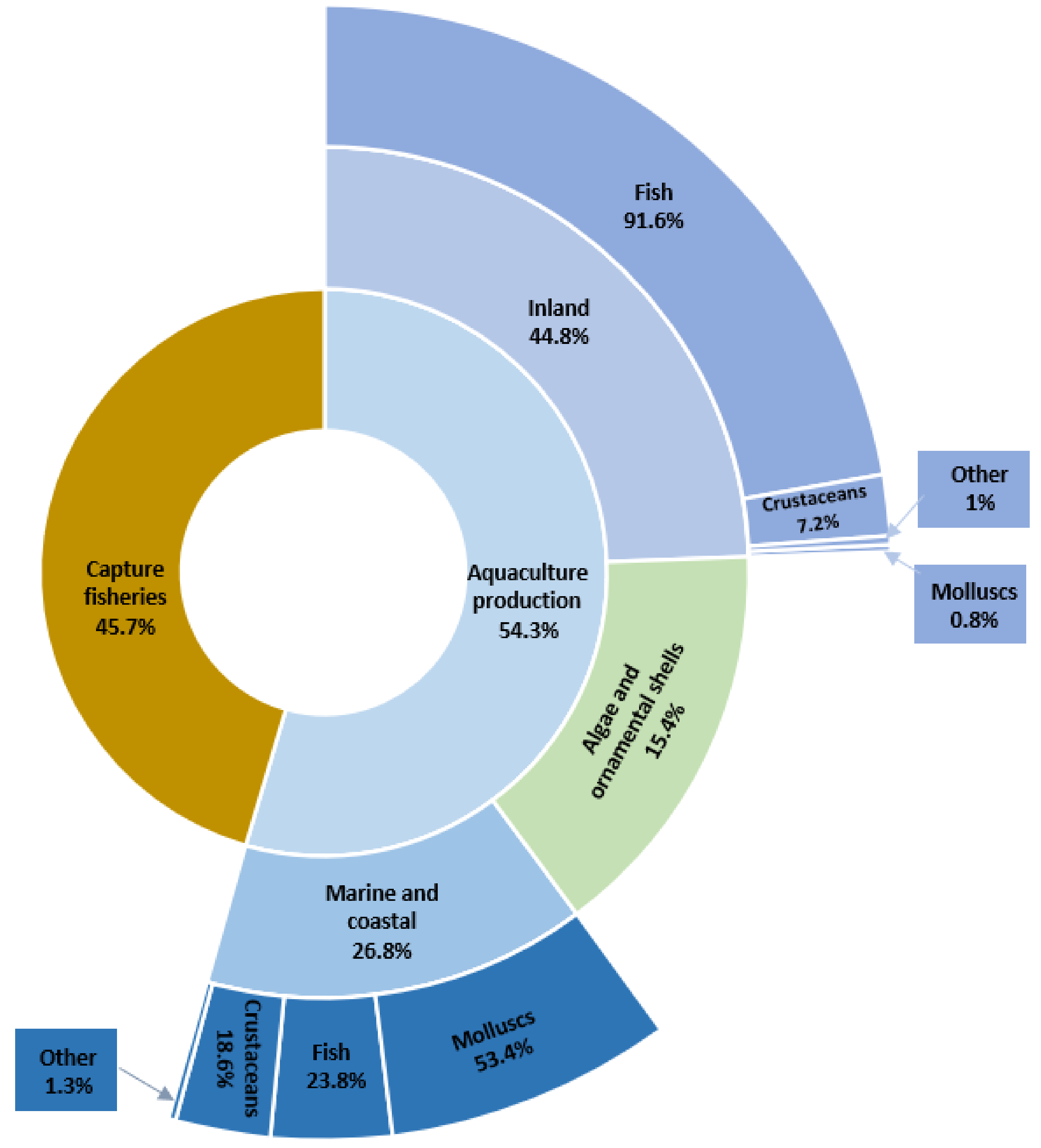
Agriculture, along with aquaculture, provides most of the food that the world population needs. Fish and aquaculture products are recognized not only as some of the healthiest foods on the planet but also as some of the least harmful to the environment [1]. According to estimates by the Food and Agriculture Organization of the United Nations (FAO), aquaculture is one of the fastest-growing food production sectors in the world. The latest statistics show that the total world production of aquatic organisms reached a record of 210.9 million tonnes in live weight in 2018. Aquaculture accounted for 54.3% of this production and consisted of 82.1 million tonnes of aquatic animals and 32.6 million tonnes of algae, ornamental shells, and pearls. The dominant segment was fish production (Figure 1). The total number of FAO-registered farm aquatic animal species in 2018 was 622. Sales of global aquaculture production excluding algae and ornamental shell production are estimated at $250 billion [1].
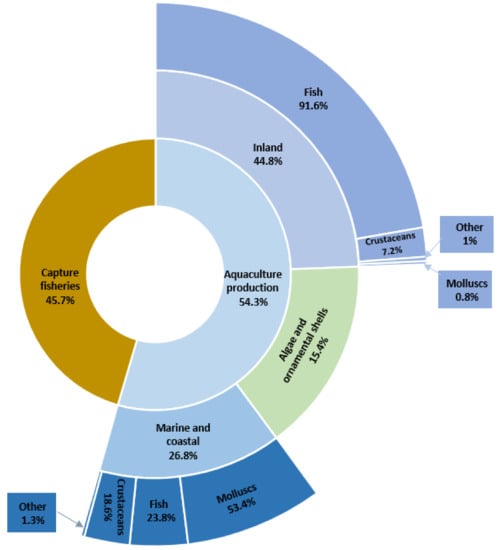
Figure 1.
Percentage of global catches and aquaculture in 2018 (based on [1]).
The rapid increase in aquaculture production raises concerns related to the quality and safety of aquatic-animal health. As in other livestock-production sectors, aquatic farming also uses intensive and semi-intensive practices, leading to a greater density of animals in small water spaces and significantly increasing the risk of developing infectious diseases [2]. Bacterial diseases affecting crops, fish, and crustaceans not only cause large economic losses to producers but can even cause food shortages, resulting in malnutrition in vulnerable populations [3].
Bacterial diseases which are routinely encountered in aquaculture and contribute to production failure or decline are mainly caused by Gram-negative bacteria such as Aeromonas (A.) hydrophila, A. salmonicida, Edwardsiella tarda, Flavobacterium psychrophilum, Pseudomonas fluorescens, and various Vibrio (V.) species. Much less often, diseases are caused by Gram-positive bacteria, such as Streptococcus iniae, Renibacterium salmoninarum, or Mycobacterium sp. [4,5,6]. Most of the bacterial pathogens that cause problems in aquaculture occur naturally in the aquatic environment, both freshwater and marine. External stressors, including transport, high stocking densities, poor water quality, and inadequate nutrition can predispose animals to disease.
The actual amount of antimicrobials used in food-producing animals is difficult to estimate due to incomplete information, but 2018 sales data for 31 EU member states indicate that 6400 tons of antimicrobials were used for veterinary purposes, primarily for food-producing animals [7]. In the United States, domestic sales and the distribution of medically important antimicrobial drugs approved for use in food-producing animals totaled 11,500 tons in 2019 [8]. This is a particularly important problem because almost all antimicrobial agents used in animal husbandry are similar in structure or are identical to those used in human medicine, which promotes the formation of multi-drug resistant strains and cross-resistance.
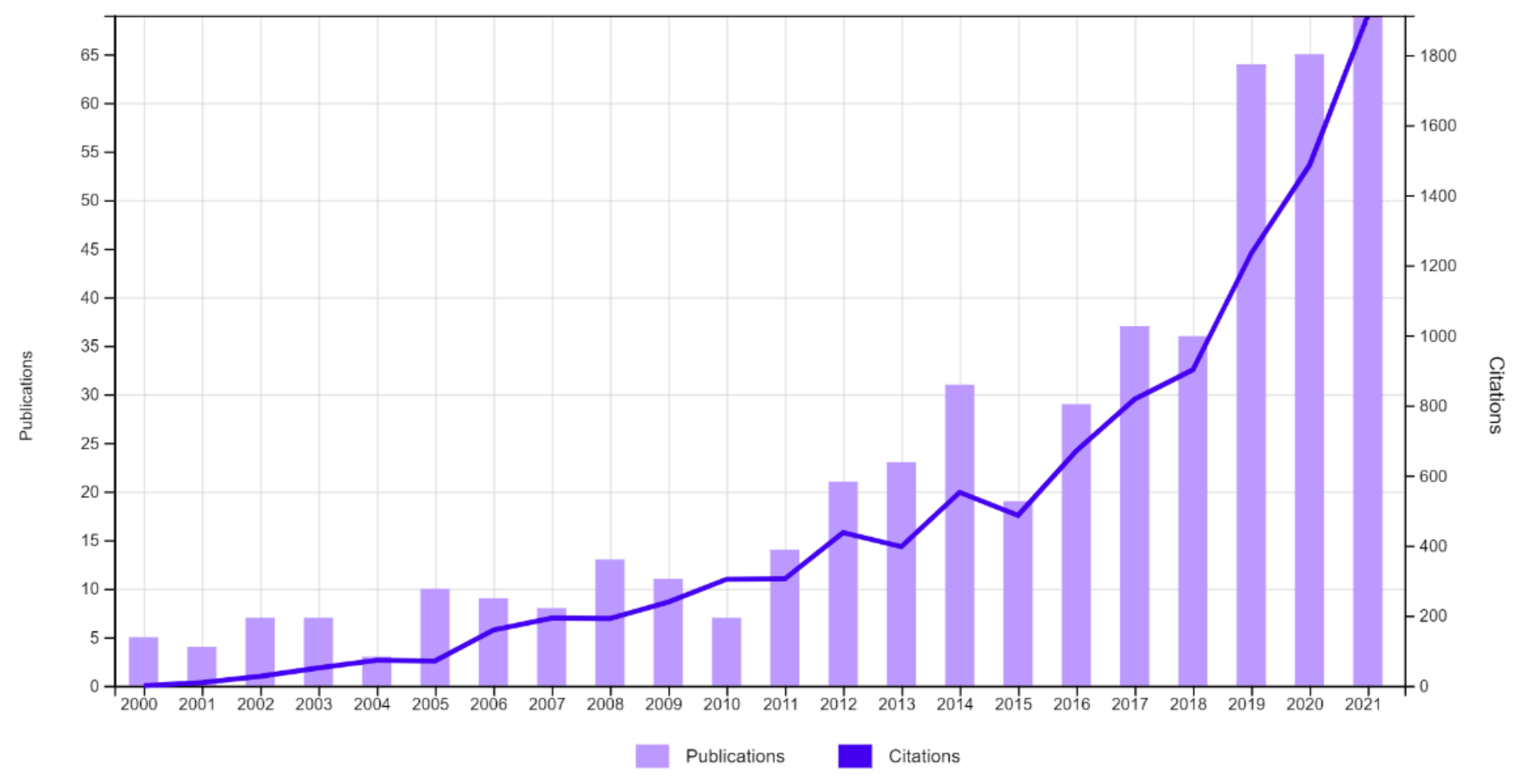
As we approach what could be a “post-antibiotic era” as announced by the WHO, there is growing interest in alternative tools that will reduce the use of antibiotics. One of the eco-friendly alternatives considered and a possible solution to the antimicrobial resistance crisis is the use of bacteriophages. The use of bacteriophages is not a new concept, and the results of its use have been described in human medicine, veterinary medicine, agriculture, and the food industry, but its use in aquatic animal husbandry has recently gained more interest. An analysis of the number of records containing the words “phage” and “aquaculture” in the Web of Science All Database from 2000 to 2021 reveals a rapid increase in the number of scientific reports related to the subject of bacteriophages in combination with the aquatic environment, which suggests a significant increase in interest in the need to research the subject (Figure 2).
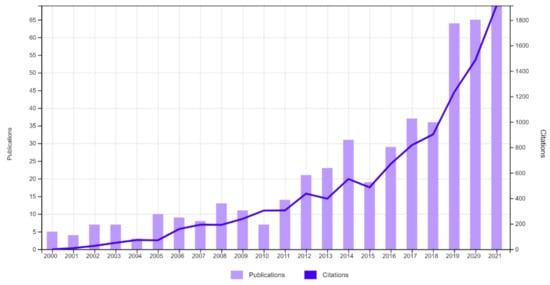
Figure 2.
The number of records for the words “phage” and “aquaculture” in the years 2000–2021 in the Web of Science All Database.
2. Antibiotic Resistance in Aquaculture
Antimicrobials in aquaculture are used for both prophylactic and therapeutic purposes and are usually administered to the entire population of sick, healthy, and vector animals through a process known as metaphylaxis. For this reason, the amount of antibiotics used in aquaculture is proportionally higher than that used in land-animal farming. Antimicrobial agents are administered to aquaculture animals mainly in feed, rarely by injection or immersion. Of the ingested antimicrobials, about 80% pass into the environment as unabsorbed in feces or, after absorption, in urine and other secretions. Additionally, if the fish are sick and anorexic, uneaten food containing drugs (possibly as much as 30%) is gravitationally deposited into sediments from which they can be flushed away by currents and carried to distant locations. Residual antibiotics remain in the sediment, thus changing the composition of the sediment microbiota, allowing for the development of antibiotic-resistant bacteria [9,10]. In surface waters, it is difficult to find an area where no antibiotic residues are detected. The exceptions are places near the source, where rivers or streams have not yet passed through urban or agricultural areas [11]. Some antibiotics can even be found in groundwater below 10 m [12].
The classes and amounts of antibiotics used in agriculture and aquaculture depend on the region of the world studied. Between 2008 and 2018, 67 different antibiotics were used in 11 of the world’s leading aquaculture-producing countries, with the main users being Vietnam (39), China (33), and Bangladesh (21) [13]. Compared to a report published 13 years ago [14], when an average of seven antibiotics were used between 1990 and 2007, a drastic increase has occurred, possibly due to the widespread prophylactic use of drugs in Vietnam and China. The lists of banned antibiotics in these countries were recently updated [15,16], but these products are still detected in aquaculture products.
The extensive and frequent use of antibiotics in aquaculture in the past has resulted in the development of pathogen resistance, representing one of the main challenges in aquaculture. The first recorded pathogen of fish showing resistance to antimicrobial agents (against sulfathiazole and tetracycline) was A. salmonicida [17]. Currently, such pathogens are isolated from many farmed fish and crustaceans around the world [18,19] (Table 1).

Table 1.
Examples of antimicrobial-resistant aquaculture pathogens.
The WHO report on the global surveillance of resistance states indicates that existing antimicrobials are becoming less effective [40,41]. At the same time, there is limited work focused on developing new ones. Although preventive vaccines are available against many bacterial infections, their use is still limited. If this trend continues, tools to combat resistant microorganisms will soon be exhausted [40]. The choice of effective therapeutic methods is therefore problematic at present. There exists a need to develop ways to protect aquaculture animals from pathogenic bacteria without the use of antibiotics. A holistic approach that considers the relationship between pathogen, host, and environment seems necessary in the long term.
3. Bacteriophages
Bacteriophages (bacterial viruses, phages) are the most abundant microorganisms on Earth. They occur almost everywhere, including in extreme environments [42], as well as in almost all niches of human and animal organisms [43]. They were independently discovered by Frederick W. Twort in England in 1915 and by Felix d’Herelle at the Pasture Institute in Paris in 1917 [44,45]. These are viruses that infect and multiply in bacteria and archaea. They are extremely varied in size, morphology, and genome organization, however, almost all the currently classified bacteriophages are assigned to only three families of the caudate bacteriophage, which is assigned to the order Caudovirales [46]. The differences between the three families are as follows: a long or short shrink tail (Myoviridae), a long non-shrink tail (Siphoviridae), and a short non-shrink tail (Podoviridae) [47].
3.1. The Life Cycle of Bacteriophages
Bacteriophages, similarly to other viruses, must infect the host cell to reproduce. They are very specific to their hosts and usually infect only one species of bacteria or even certain strains within a species. Bacteriophages can recognize several components of the bacterial cell structure as their receptors. These include, inter alia, outer membrane proteins, peptidoglycan (PG), teichoic acids, oligosaccharide, lipopolysaccharide (LPS), flagella, and fimbriae [48]. This means that they can only infect bacteria that have a target molecule to bind to [42,49].
The first step in a tightly programmed bacteriophage infection process is the production of polysaccharide-degrading enzymes, also known as polysaccharide depolymerases. These can be released into the environment, associated with the tail or capsid of the bacteriophage, and are used for the enzymatic degradation of the envelope or structural polysaccharides, including exopolysaccharides which are the main component of the bacterial biofilm. During the last phase of the cycle, lysines are produced, which are responsible for the lysis of bacteria and the release of progeny viruses [48,50].
Lytic and lysogenic infections are most frequently mentioned in the literature. However, these are only two of the many possibilities. Not all infections necessarily result in the death of the host cell, and the replication of bacteriophage particles does not always occur. Each bacteriophage can follow several different infection pathways depending on the environmental conditions and the genetic and physiological characteristics of the host.
3.1.1. Lytic Cycle
While they are technically not living organisms, bacteriophages are certainly dynamic entities. During the lytic replication cycle, the bacteriophage attaches to a sensitive host cell. It then introduces its genome into the bacterial cytoplasm and uses its ribosomes to produce proteins. Bacterial resources are rapidly transformed in the capsid protein and the virus genome, which consists of multiple copies of the original bacteriophage. By multiplying in a bacterial cell, it destroys it. When the host cell dies, it most often releases new bacteriophages with the participation of lysines, which then infect another bacterial cell [51,52]. Such phages are termed virulent or lytic.
3.1.2. Lysogenic Cycle
In the lysogenic replication cycle, the bacteriophage also attaches to a susceptible bacterial cell and introduces its genome into the bacterial cytoplasm. The bacteriophage genome is then integrated into the chromosome of the bacterial cell or remains unbound. In both cases, it is replicated and transferred to progeny bacterial cells without lysing them. The integrated bacteriophage genomes are called prophages. Prophages can return to the lytic replication cycle leading to the lysis of their host, most often in response to changing environmental conditions [51,52,53].
3.1.3. Pseudolysogenic Cycle
During pseudolysogeny, the bacteriophage enters the cell, but does not replicate in the cell and does not integrate stably with the host genome. It seems that pseudolysogeny plays an important role in the survival of bacterial viruses, enabling the preservation of its genome when the host cell encounters unfavorable growth conditions, such as the lack of a sufficient amount of nutrients [54]. Pseudolysogeny is not a permanent state. After changing the conditions causing it, the bacteriophage often enters the lytic or lysogenic pathway. Sometimes the term “carrier” is used instead of the term pseudolysogeny [42,51].
3.1.4. Chronic Infection
In the case of chronic infection, new bacteriophage particles are produced continuously over a long period. However, lysis of the host cell does not occur. Virions are released or are exported out of the cell by protein complexes. It is associated with high energy expenditure and may negatively affect the ability of bacteria to compete for an ecological niche. Examples of bacteriophages capable of causing a chronic infection are some archaeal viruses, filamentous phages (ssDNA phages), and mycoplasma-infecting plasmaviruses [42].
3.1.5. Abortive Infection
Faced with frequent exposure to bacteriophages, bacteria have developed numerous mechanisms to counteract infection, including abortion infection, also called bacteriophage exclusion. The bacteriophage genome enters the host cell, but the infected cell self-destructs before the bacteriophage completes its replication cycle. This reduces the number of progeny particles and limits their spread to other cells, allowing the bacterial population to survive [55]. The abortive infection manifests itself in a wide variety of bacterial defense systems. An example is the toxin/antitoxin system of the genus Lactococcus. In uninfected bacteria, the action of both components of the system is balanced. After infection by the virus, bacterial death occurs due to an increase in the toxin–antitoxin ratio [55,56].
3.2. Bacteriophage Therapy
For targeted therapy, strictly lytic bacteriophages are preferred, characterized by rapid multiplication leading to the lysis of the bacterial cell, and at the same time by an exponential increase in their number. Lysogenic bacteriophages are avoided because of their inherent ability to mediate gene transfer between bacteria, which can increase bacterial virulence, for example by promoting antibiotic resistance. Currently, advances in sequencing technologies and synthetic biology are creating new possibilities for using these bacteriophages in the treatment of bacterial infections as well. In addition to the ability to lyse a bacterial cell, several important features determine the antimicrobial efficacy of bacteriophages. One is the bacteriophage generation time, which includes effective adhesion, the latent period, and the release of progeny particles. The second aspect is the growth rate of the bacteriophage population, which is the number of bacteriophage particles formed during one life cycle. High adsorption rate to specific bacteria, large burst size, and short generation time are determinants of strong antibacterial efficacy [48].
3.2.1. Methods of Administering Bacteriophages
The effectiveness of the application of bacteriophages depends on their ability to reach the host, which is not always possible. Depending on the location of the infection in the body, and due to the effectiveness of penetration and the ability to maintain the highest bacteriophage titer, preparations containing bacteriophages can be administered in various forms. Bacteriophages used to combat bacterial diseases in aquaculture can be administered orally with feed [57,58,59,60], parenterally (intramuscularly, subcutaneously, intraperitoneal) [58,59,61,62,63], topically to the skin and lesions [64], in a bath [60,65,66] or can be directly released in the water system [67,68,69,70].
In aquaculture, methods that reduce the need to perform additional activities are preferred, and thus limit exposure to manipulation stress; therefore, the most frequently chosen method of administering drugs is in water or orally, e.g., with feed. The oral administration of bacteriophages has been proven to be effective in treating gastrointestinal infections. The absorption of orally administered bacteriophages into the systemic circulation in a process similar to bacterial translocation has also been demonstrated, which allows this route of administration to also be used in systemic infections. The passage of bacteriophages is determined by several factors, including their concentration, the presence of specific sequences within the capsid proteins that interact with enterocyte receptors, and the interaction of bacteriophages with intestinal immune cells [71]. Application in water is the most common method of applying different substances to aquaculture animals. It is used in several ways, from high drug concentration/short exposure time (bath) to low drug concentration/long exposure time (immersion) [72]. The application of bacteriophage preparations with these methods is very popular, not only because of the ease of administration resulting in bacteriophages entering internal organs directly from the water through the gills of fish, but due to the additional benefits of cleaning the environment [73]. However, the use of this route of administration can be problematic in commercial-scale aquaculture, where the volume of water requiring bacteriophage treatment can be impractically large.
Unfortunately, there is no universal application. The appropriate method of bacteriophage administration depends on many factors and situations should be considered on a case-by-case basis. It is not practical to perform injections on very small fish or crustaceans. Similarly, performing bathing with a high bacteriophage titer is difficult in large bodies of water, and the immersion method may depend on the environment, the nature of the infection, or the bacteriophage properties [74]. Each method of administration has its strengths and weaknesses, and the choice of method largely depends on the nature of the bacterial pathogen, the species of animal, and its size.
There are two forms of therapy—active and passive. In active therapy, bacteriophages are administered at a dose that is capable of reducing the host population through multiple cycles of reproduction. In passive therapy, such reproduction is not needed to ensure an effective therapy since the number of bacteriophages administered is so large that the entire host population is lysed without the need for one or more cycles of bacteriophage reproduction. Unfortunately, the passive method is much more expensive but can bypass bacterial defense mechanisms such as abortive infection [73,75].
Various approaches to bacteriophage therapy have been tested. Monophage therapy refers to the use of one type of bacteriophage. It is used primarily for the development of experimental models of bacteriophage therapy, as a confirmation of the concept when testing preparations. Unfortunately, it requires the precise matching of the pathogen and the bacteriophage.
In aquaculture, the actual situation is often much more complicated than “one pathogen-one disease”. Fish can suffer simultaneously from infections caused by multiple strains or species of bacteria that can affect the outcome of the disease which creates additional challenges for phage therapy [76]. Polyphage therapy with a bacteriophage cocktail uses a combination of several phages. Unlike monophage therapy, it targets many strains of one bacterial species or many bacterial species. The use of bacteriophage cocktails containing two or more bacteriophages is increasingly being tested in aquaculture. One of the benefits of using multiple bacteriophages is that they allow for a more thorough treatment of infection as they can attack a wide range of pathogenic bacterial strains, with better bacterial titer reduction and faster-acting effects [77]. In addition, the use of bacteriophage cocktails targeting different receptors of the same bacterium may help reduce the rate of resistance development [73].
3.2.2. In Vivo Use of Bacteriophages
In the human and animal health sectors, bacteriophage therapy has been practiced in regions of Eastern Europe for over 60 years [78]. Between 1930 and 1940, the discovery of antibiotics led to the abandonment of bacteriophage therapy in Western countries; meanwhile, due to the isolation of many Eastern European countries from the advances in the production of antibiotics, the region continued to develop and improve bacteriophage therapies at that time.
The first use of bacteriophages as a therapy in aquaculture was described by Wu et al. in 1981 [79]. Since then, interest in bacteriophage therapy in various species of aquatic animals has attracted a lot of attention, including in the control of diseases caused by Aeromonas spp., Pseudomonas spp., Yersinia ruckeri, Flavobacterium psychrophilum, and many others. In recent years, several in vivo experiments have been performed to assess the potential of bacteriophages to combat bacterial infections in aquaculture. Their effectiveness was tested on various animal models, including many species of fish, crustaceans, and mollusks, showing promising results and revealing the ability of certain bacteriophages to significantly reduce pathogen concentrations and increase the survival rate of aquaculture animals. The main achievements of in vivo studies of bacteriophages specific for pathogenic bacteria in aquaculture animals and their potential uses are presented in Table 2.

Table 2.
Outcomes of in vivo bacteriophage application in aquaculture.
Most of the published articles discuss monophage therapy and describe the discovery of new potentially useful bacteriophages, while few describe cocktails or other associations. The presented in vivo experiments demonstrate the effectiveness of bacteriophage therapy in controlling aquaculture-related diseases; however, the results for different combinations of bacteriophages and bacteria varied. They range from 100% bacterial removal and lack of mortality [57,91] to no therapeutic effect [60]. In some cases, disease progression was delayed; however, the final mortality did not differ statistically from the group where bacteriophage therapy was not used [90,103].
The ratio of bacteriophage to bacterial concentration required for effective active bacteriophage therapy varies widely, depending on the pathogen, fish species, and bacteriophage. Different doses were used in both the experimental and field studies. Le et al. (2018) [83], using different amounts of bacteriophage in catfish therapy, obtained survival rates that varied by up to 68%, indicating that determining the correct bacteriophage dose is very important for successful bacteriophage therapy.
The time of administration was also found to be a very important factor. Mortality typically increased significantly when treatment was delayed from time of infection [91,97,99]. The best choice seems to be the prophylactic administration of bacteriophages before infection and its continuation. Jun et al. (2018) [121] achieved a 75% higher survival rate for white shrimp with the prophylactic use of bacteriophages in immersion, and a 50% survival rate with prophylaxis in feed. After the administration of the bacteriophage preparation a day before the infection, Schulz et al. [86,87] achieved a 16% higher survival of European eel and 6% higher survival of rainbow trout, compared to the group treated 24 h after infection. This may be related to the time needed for bacteriophages to multiply to a sufficient concentration to cause the host population to collapse. Further research into the effect of timing and bacteriophage number on the success of bacteriophage therapy may yield interesting results, as a more concentrated administration may compensate for delayed treatment [73].
The most important aspect is to compare the effectiveness of therapy with bacteriophages to that of antibiotic therapy. Zhang et al. [106] found no statistical differences in the survival rate of the sea cucumber infected with V. alginolyticus after treatment with a bacteriophage cocktail compared to the group treated with antibiotics. Karunasagar and co-authors [113] achieved a 20% higher survival rate of shrimps treated with bacteriophages compared to survival with the use of antibiotics, while Vinod et al. [114] achieved 46% higher survival compared to antibiotic treatment after natural V. harveyi infection of shrimp. This suggests that the effectiveness of bacteriophage therapy may not only match that of antibiotics but may even be more effective. However, further studies are needed to both compare the efficacy of antibiotics and bacteriophages, and to study the extent and rate of bacteriophage resistance.
4. Conclusions
The production of various species of fish, crustaceans, and mollusks has made the aquaculture industry an important economic factor in many countries. Despite advances in good management practices, non-specific immunoprophylaxis, and vaccine production, bacterial infections remain a serious problem both in hatcheries and during rearing, often resulting in a high level of mortality. An additional problem is that more and more of them have been characterized by multi-drug resistance [47]. In the absence of an adequate strategy to combat bacterial pathogens, alternative, environmentally friendly disease control strategies should be developed that should reduce the risk of the development and spread of microbial resistance. In line with this idea, the use of bacteriophage therapy in aquaculture seems very promising.
Therapy with bacteriophages has a few advantages over traditional antibiotic therapy. Phage isolation is relatively rapid, simple, and inexpensive. Bacteriophage resistance develops about ten times slower than antibiotic resistance because bacteriophages can evolve, creating new genotypes capable of re-infecting a given bacterial strain [124]. Bacteriophages remain infectious under very harsh environmental conditions and tend to continue to replicate until the host bacterial population density is significantly reduced. These features indicate that bacteriophage therapy—unlike traditional therapies—may require fewer administrations and at the same time work as well or better than conventional treatments [125].
Several key issues must be considered in practical application to achieve the desired outcome, reduction, or elimination of mortality due to bacterial infection. First, careful planning of the timing and frequency of bacteriophage administration should be performed, keeping in mind the virulence characteristics of the bacterial pathogen. Second, the optimal route of bacteriophage administration for each bacterial infection should be determined, considering that pathogenic bacteria have different routes of infection. Third, the appropriate bacteriophage dose should be determined, which will depend on the expected number of target bacteria [76]. Some very pertinent issues have yet to be addressed before the widespread use of bacteriophage treatment is made possible, including the presence of bacteriophage-resistant bacteria, the high specificity of bacteriophages and the transfer of virulence genes. In some cases, the reproduction of the lytic bacteriophage leads to undesirable consequences, which should also be considered. The rapid release of cellular toxins or the breakdown of the outer membrane of Gram-negative bacteria in the short term can result in a systemic inflammatory response and severe side effects [126]. Bacteriophage treatment also requires a consideration of factors such as cost-effectiveness, environmental impact, and more importantly, it must be standardized and specified to geographic regions and species [74].
Most studies with pathogens from aquaculture have been conducted under controlled laboratory conditions. Future studies conducting experiments in conditions resembling the real-life rearing environment are important to develop a better understanding of the efficiency of bacteriophage treatments. It is also necessary to promote knowledge about bacteriophages for consumer acceptance of bacteriophage-based products. Given the renewed interest and enthusiasm in the field of bacteriophage therapy, there is reason to believe that these challenges can be overcome in the years to come.
Author Contributions
Conceptualization, P.S. and A.K.S.; investigation, P.S. and J.P.-C.; writing—original draft preparation, P.S. and J.P.-C. All authors have read and agreed to the published version of the manuscript.
Funding
Project financially supported by the Minister of Education and Science under the program entitled “Regional Initiative of Excellence” for the years 2019–2022, Project No. 010/RID/2018/19, amount of funding 12.000.000 PLN.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- FAO. The State of World Fisheries and Aquaculture 2020; Sustainability in Action; FAO: Rome, Italy, 2020. [Google Scholar]
- Santos, L.; Ramos, F. Antimicrobial resistance in aquaculture: Current knowledge and alternatives to tackle the problem. Int. J. Antimicrob. Agents 2018, 52, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Sieiro, C.; Areal-Hermida, L.; Pichardo-Gallardo, Á.; Almuiña-González, R.; de Miguel, T.; Sánchez, S.; Sánchez-Perez, A.; Villa, T.G. A Hundred Years of Bacteriophages: Can Phages Replace Antibiotics in Agriculture and Aquaculture? Antibiotics 2020, 9, 493. [Google Scholar] [CrossRef] [PubMed]
- Crumlish, M. Bacterial diagnosis and control in fish and shellfish. In Diagnosis and Control of Diseases of Fish and Shellfish; Wiley: Hoboken, NJ, USA, 2017; pp. 5–18. [Google Scholar]
- Haenen, O. Major bacterial diseases affecting aquaculture. In Proceedings of the Aquatic AMR Workshop 1, Mangalore, India, 10–11 April 2017. [Google Scholar]
- Gui, L.; Zhang, Q.-Y. Disease Prevention and Control. In Aquaculture in China: Success Stories and Modern Trends; Gui, J.-F., Tang, Q., Li, Z., Liu, J., De Silva, S.S., Eds.; Wiley Blackwell: Hoboken, NJ, USA, 2018; Section 7; pp. 577–598. [Google Scholar]
- European Medicines Agency. Sales of Veterinary Antimicrobial Agents in 31 European Countries in 2018; Trends from 2010 to 2018. Tenth ESVAC Report; European Medicines Agency: Amsterdam, The Netherlands, 2020; p. 21. [Google Scholar]
- US Food and Drug Administration. 2019 Summary Report on Antimicrobials Sold or Distributed for Use in Food-Producing Animals; FDA Report; US Food and Drug Administration: Silver Spring, MD, USA, 2020.
- Cabello, F.C. Heavy use of prophylactic antibiotics in aquaculture: A growing problem for human and animal health and for the environment. Environ. Microbiol. 2006, 8, 1137–1144. [Google Scholar] [CrossRef]
- Cabello, F.C.; Godfrey, H.P.; Tomova, A.; Ivanova, L.; Dölz, H.; Millanao, A.; Buschmann, A.H. Antimicrobial use in aquaculture re-examined: Its relevance to antimicrobial resistance and to animal and human health. Environ. Microbiol. 2013, 15, 1917–1942. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Carlson, K. Evolution of antibiotic occurrence in a river through pristine, urban and agricultural landscapes. Water Res. 2003, 37, 4645–4656. [Google Scholar] [CrossRef]
- Batt, A.L.; Snow, D.D.; Aga, D.S. Occurrence of sulfonamide antimicrobials in private water wells in Washington County, Idaho, USA. Chemosphere 2006, 64, 1963–1971. [Google Scholar] [CrossRef] [PubMed]
- Lulijwa, R.; Rupia, E.J.; Alfaro, A.C. Antibiotic use in aquaculture, policies and regulation, health and environmental risks: A review of the top 15 major producers. Rev. Aquac. 2020, 12, 640–663. [Google Scholar] [CrossRef]
- Sapkota, A.; Sapkota, A.R.; Kucharski, M.; Burke, J.; McKenzie, S.; Walker, P.; Lawrence, R. Aquaculture practices and potential human health risks: Current knowledge and future priorities. Environ. Int. 2008, 34, 1215–1226. [Google Scholar] [CrossRef]
- MARD. List of veterinary drugs banned from use. In Promulgated Together with Circular No. 2016/TT-BNN dated 2016 of the Minister of Agriculture and Rural Development; Department of Animal Health Board: Hanoi, Vietnam, 2016. [Google Scholar]
- Liu, X.; Steele, J.C.; Meng, X.Z. Usage, residue, and human health risk of antibiotics in Chinese aquaculture: A review. Environ. Pollut. 2017, 223, 161–169. [Google Scholar] [CrossRef]
- Śnieszko, S.F.; Bullock, G.L. Treatment of sulfonamide resistant furunculosis in trout and determination of drug sensitivity. Fish. Bull. 1957, 125, 555–564. [Google Scholar]
- Watts, J.E.; Schreier, H.J.; Lanska, L.; Hale, M.S. The rising tide of antimicrobial resistance in aquaculture: Sources, sinks and solutions. Mar. Drugs 2017, 15, 158. [Google Scholar] [CrossRef] [PubMed]
- Preena, P.G.; Swaminathan, T.R.; Kumar, V.J.R.; Singh, I.S.B. Antimicrobial resistance in aquaculture: A crisis for concern. Biologia 2020, 75, 1497–1517. [Google Scholar] [CrossRef]
- Yano, Y.; Hamano, K.; Tsutsui, I.; Aue-Umneoy, D.; Ban, M.; Satomi, M. Occurrence, molecular characterization, and antimicrobial susceptibility of Aeromonas spp. in marine species of shrimps cultured at inland low salinity ponds. Food Microbiol. 2015, 47, 21–27. [Google Scholar] [CrossRef]
- McPhearson, R.M.; DePaola, A.; Zywno, S.R.; Motes, M.L.; Guarino, A.M. Antibiotic resistance in Gram-negative bacteria from cultured catfish and aquaculture ponds. Aquaculture 1991, 99, 203–211. [Google Scholar] [CrossRef]
- Dixon, B.A.; Issvoran, G. Antibacterial drug resistance in Aeromonas spp. isolated from domestic goldfish and koi from California. J. World Aquac. Soc. 1993, 24, 102–104. [Google Scholar] [CrossRef]
- Schmidt, A.S.; Bruun, M.S.; Dalsgaard, I.; Pedersen, K.; Larsen, J.L. Occurrence of antimicrobial resistance in fish-pathogenic and environmental bacteria associated with four Danish rainbow trout farms. Appl. Environ. Microb. 2000, 66, 4908–4915. [Google Scholar] [CrossRef]
- Akinbowale, A.L.; Peng, H.; Grant, P.; Barton, M.D. Antibiotic and heavy metal resistance in motile aeromonads and pseudomonads from rainbow trout (Oncorhynchus mykiss) farms in Australia. Int. J. Antimicrob. Agents 2007, 30, 177–182. [Google Scholar] [CrossRef]
- Vega-Sánchez, V.; Latif-Eugenín, F.; Soriano-Vargas, E.; Beaz-Hidalgo, R.; Figueras, M.J.; Aguilera-Arreola, M.G.; Castro-Escarpulli, G. Re-identification of Aeromonas isolates from rainbow trout and incidence of class 1 integron and β-lactamase genes. Vet. Microbiol. 2014, 172, 528–533. [Google Scholar] [CrossRef]
- John, N.; Hatha, A.A.M. Prevalence, distribution and drug resistance of motile aeromonads in freshwater ornamental fishes. Indian J. Fish. 2012, 59, 161–164. [Google Scholar]
- Chenia, H.Y. Prevalence and characterization of plasmid-mediated quinolone resistance genes in Aeromonas spp. isolated from South African freshwater fish. Int. J. Food Microbiol. 2016, 231, 26–32. [Google Scholar] [CrossRef]
- Dobiasova, H.; Kutilova, I.; Piackova, V.; Vesely, T.; Cizek, A.; Dolejska, M. Ornamental fish as a source of plasmid-mediated quinolone resistance genes and antibiotic resistance plasmids. Vet. Microbiol. 2014, 171, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.E.; Cho, M.Y.; Kim, J.W.; Kang, H.Y. Large antibiotic-resistance plasmid of Edwardsiella tarda contributes to virulence in fish. Microb. Pathog. 2012, 52, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Wang, Q.; Liu, Q.; Wang, X.; Liu, H.; Zhang, Y. Isolation and identification of fish pathogen Edwardsiella tarda from mariculture in China. Aquac. Res. 2008, 40, 13–17. [Google Scholar] [CrossRef]
- Sousa, M.; Torres, C.; Barros, J.; Somalo, S.; Igrejas, G.; Poeta, P. Gilthead seabream (Sparus aurata) as carriers of SHV-12 and TEM-52 extended-spectrum beta-lactamases-containing Escherichia coli isolates. Foodborne Pathog. Dis. 2011, 8, 1139–1141. [Google Scholar] [CrossRef] [PubMed]
- Ruzauskas, M.; Klimiene, I.; Armalyte, J.; Bartkiene, E.; Siugzdiniene, R.; Skerniskyte, J.; Krasauskas, R.; Suziedeliene, E. Composition and antimicrobial resistance profile of Gram-negative microbiota prevalent in aquacultured fish. J. Food Saf. 2018, 38, e12447. [Google Scholar] [CrossRef]
- Shah, S.Q.; Nilsen, H.; Bottolfsen, K.; Colquhoun, D.J.; Sørum, H. DNA gyrase and topoisomerase IV mutations in quinolone-resistant Flavobacterium psychrophilum isolated from diseased salmonids in Norway. Microb. Drug Resist. 2012, 18, 207–214. [Google Scholar] [CrossRef]
- Kim, M.J.; Hirono, I.; Kurokawa, K.; Maki, T.; Hawke, J.; Kondo, H.; Santos, M.D.; Aoki, T. Complete DNA sequence and analysis of the transferable multiple-drug resistance plasmids (R plasmids) from Photobacterium damselae subsp. piscicida isolates collected in Japan and the United States. Antimicrob. Agents Chemother. 2008, 52, 606–611. [Google Scholar] [CrossRef]
- Lamari, F.; Chakroun, I.; Rtimi, S. Assessment of the correlation among antibiotic resistance, adherence to abiotic and biotic surfaces, invasion and cytotoxicity of Pseudomonas aeruginosa isolated from diseased gilthead sea bream. Colloid Surf. B 2017, 158, 229–236. [Google Scholar] [CrossRef]
- Nguyen, T.T.T.; Nguyen, H.T.; Tsai, M.A.; Byadgi, O.; Wang, P.C.; Yoshida, T.; Chen, S.C. Genetic diversity, virulence genes, and antimicrobial resistance of Streptococcus dysgalactiae isolates from different aquatic animal sources. Aquaculture 2017, 479, 256–264. [Google Scholar] [CrossRef]
- Abraham, T.J. Pathogenicity and antibiotic sensitivity of luminous Vibrio harveyi isolated from diseased penaeid shrimp. J. Aquac. Trop. 1997, 12, 1–8. [Google Scholar]
- Tendencia, E.A.; de la Peña, L.D. Antibiotic resistance of bacteria from shrimp ponds. Aquaculture 2001, 195, 193–204. [Google Scholar] [CrossRef]
- Nonaka, L.; Suzuki, S. New Mg2+-dependent oxytetracycline resistance determinant Tet 34 in Vibrio isolates from marine fish intestinal contents. Antimicrob. Agents Chemother. 2002, 46, 1550–1552. [Google Scholar] [CrossRef] [PubMed]
- WHO. Anitimicrobial Resistance. In Global Report on Surveillance; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- WHO. No Time to Wait: Securing the Future from Drug-Resistant Infections; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Clokie, M.R.; Millard, A.D.; Letarov, A.V.; Heaphy, S. Phages in nature. Bacteriophage 2011, 1, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Muniesa Perez, M.T.; Navarro Risueño, F. Bacteriophages in clinical samples can interfere with microbiological diagnostic tools. Sci. Rep. 2016, 6, 33000. [Google Scholar]
- Twort, F.W. An investigation on the nature of ultra-microscopic viruses. Lancet 1915, 186, 1241–1243. [Google Scholar] [CrossRef]
- d’Herelle, F. Sur un microbe invisible antagoniste des bacilles dysentériques. CR Acad. Sci. Paris 1917, 165, 373–375. [Google Scholar]
- Simmonds, P.; Aiewsakun, P. Virus classification–where do you draw the line? Arch. Virol. 2018, 163, 2037–2046. [Google Scholar] [CrossRef]
- Pal, S. Phage Therapy an alternate disease control in Aquaculture: A review on recent advancements. IOSR J. Agric. Vet. Sci. 2015, 8, 68–81. [Google Scholar]
- Drulis-Kawa, Z.; Majkowska-Skrobek, G.; Maciejewska, B. Bacteriophages and phage-derived proteins–application approaches. Curr. Med. Chem. 2015, 22, 1757–1773. [Google Scholar] [CrossRef]
- Seed, K.D. Battling phages: How bacteria defend against viral attack. PLoS Pathog. 2015, 11, e1004847. [Google Scholar] [CrossRef]
- Maszewska, A. Phage associated polysaccharide depolymerases—Characteristics and application. Postepy Hig. Med. Dosw. 2015, 69, 690–702. [Google Scholar] [CrossRef] [PubMed]
- Cenens, W.; Makumi, A.; Mebrhatu, M.T.; Lavigne, R.; Aertsen, A. Phage–host interactions during pseudolysogeny: Lessons from the Pid/dgo interaction. Bacteriophage 2013, 3, e1003269. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, Z.; Abedon, S.T. Diversity of phage infection types and associated terminology: The problem with ‘Lytic or lysogenic’. FEMS Microbiol. Lett. 2016, 363, fnw047. [Google Scholar] [CrossRef] [PubMed]
- Kasman, L.M.; Porter, L.D. Bacteriophages. StatPearls [Internet]—NCBI Bookshelf. Available online: https://www.ncbi.nlm.nih.gov/books/NBK493185/?msclkid=41765f02b40411ec87dc1f40ad8c6220 (accessed on 20 March 2020).
- Łoś, M.; Węgrzyn, G. Pseudolysogeny. Adv. Virus Res. 2012, 82, 339–349. [Google Scholar] [CrossRef]
- Chopin, M.C.; Chopin, A.; Bidnenko, E. Phage abortive infection in lactococci: Variations on a theme. Curr. Opin. Microbiol. 2005, 8, 473–479. [Google Scholar] [CrossRef]
- Lopatina, A.; Tal, N.; Sorek, R. Abortive infection: Bacterial suicide as an antiviral immune strategy. Annu. Rev. Virol. 2020, 7, 371–384. [Google Scholar] [CrossRef]
- Prasad, Y.; Kumar, D.; Sharma, A.K. Lytic bacteriophages specific to Flavobacterium columnare rescue catfish, Clarias batrachus (Linn) from columnaris disease. J. Environ. Biol. 2011, 32, 161–168. [Google Scholar]
- Jun, J.W.; Kim, J.H.; Shin, S.P.; Han, J.E.; Chai, J.Y.; Park, S.C. Protective effects of the Aeromonas phages pAh1-C and pAh6-C against mass mortality of the cyprinid loach (Misgurnus anguillicaudatus) caused by Aeromonas hydrophila. Aquaculture 2013, 416, 289–295. [Google Scholar] [CrossRef]
- Cao, Y.; Li, S.; Han, S.; Wang, D.; Zhao, J.; Xu, L.; Liu, H.; Lu, T. Characterization and application of a novel Aeromonas bacteriophage as treatment for pathogenic Aeromonas hydrophila infection in rainbow trout. Aquaculture 2020, 523, 735193. [Google Scholar] [CrossRef]
- Donati, V.L.; Dalsgaard, I.; Sundell, K.; Castillo, D.; Er-Rafik, M.; Clark, J.; Wilkund, T.; Middelboe, M.; Madsen, L. Phage-Mediated Control of Flavobacterium psychrophilum in Aquaculture: In vivo Experiments to Compare Delivery Methods. Front. Microbiol. 2021, 12, 628309. [Google Scholar] [CrossRef]
- Kim, J.H.; Choresca, C.H.; Shin, S.P.; Han, J.E.; Jun, J.W.; Park, S.C. Biological Control of Aeromonas salmonicida subsp. salmonicida Infection in Rainbow Trout (Oncorhynchus mykiss) Using Aeromonas Phage PAS-1. Transbound. Emerg. Dis. 2015, 62, 81–86. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, J.; Li, X.; Wang, X.; Cao, Z.; Wang, L.; Xu, Y. Efficiency of a bacteriophage in controlling vibrio infection in the juvenile sea cucumber Apostichopus japonicus. Aquaculture 2016, 451, 345–352. [Google Scholar] [CrossRef]
- Luo, X.; Liao, G.; Liu, C.; Jiang, X.; Lin, M.; Zhao, C.; Tao, J.; Huang, Z. Characterization of bacteriophage HN48 and its protective effects in Nile tilapia Oreochromis niloticus against Streptococcus agalactiae infections. J. Fish Dis. 2018, 41, 1477–1484. [Google Scholar] [CrossRef] [PubMed]
- Khairnar, K.; Raut, M.P.; Chandekar, R.H.; Sanmukh, S.G.; Paunikar, W.N. Novel bacteriophage therapy for controlling metallo-beta-lactamase producing Pseudomonas aeruginosa infection in catfish. BMC Vet. Res. 2013, 9, 264. [Google Scholar] [CrossRef] [PubMed]
- Laanto, E.; Bamford, J.K.; Ravantti, J.J.; Sundberg, L.R. The use of phage FCL-2 as an alternative to chemotherapy against columnaris disease in aquaculture. Front. Microbiol. 2015, 6, 829. [Google Scholar] [CrossRef] [PubMed]
- Akmal, M.; Rahimi-Midani, A.; Hafeez-ur-Rehman, M.; Hussain, A.; Choi, T.J. Isolation, characterization, and application of a bacteriophage infecting the fish pathogen Aeromonas hydrophila. Pathogens 2020, 9, 215. [Google Scholar] [CrossRef]
- Onarinde, B.A.; Dixon, R.A. Prospects for Biocontrol of Vibrio parahaemolyticus Contamination in Blue Mussels (Mytilus edulus)—A Year-Long Study. Front. Microbiol. 2018, 9, 1043. [Google Scholar] [CrossRef]
- Makarov, R.; Lomelí-Ortega, C.O.; Zermeño-Cervantes, L.A.; García-Álvarez, E.; Gutiérrez-Rivera, J.N.; Cardona-Félix, C.S.; Martínez-Díaz, S.F. Evaluation of a cocktail of phages for the control of presumptive Vibrio parahaemolyticus strains associated to acute hepatopancreatic necrosis disease. Aquac. Res. 2019, 50, 3107–3116. [Google Scholar] [CrossRef]
- Le, T.S.; Southgate, P.C.; O’Connor, W.; Vu, S.V.; Kurtböke, D.İ. Application of bacteriophages to control Vibrio alginolyticus contamination in oyster (Saccostrea glomerata) larvae. Antibiotics 2020, 9, 415. [Google Scholar] [CrossRef]
- Veyrand-Quirós, B.; Gómez-Gil, B.; Lomeli-Ortega, C.O.; Escobedo-Fregoso, C.; Millard, A.D.; Tovar-Ramírez, D.; Balcazar, J.L.; Quiroz-Guzmán, E. Use of bacteriophage vB_Pd_PDCC-1 as biological control agent of Photobacterium damselae subsp. damselae during hatching of longfin yellowtail (Seriola rivoliana) eggs. J. Appl. Microbiol. 2020, 129, 1497–1510. [Google Scholar] [CrossRef]
- Górski, A.; Ważna, E.; Dąbrowska, B.W.; Dąbrowska, K.; Świtała-Jeleń, K.; Międzybrodzki, R. Bacteriophage translocation. FEMS Immunol. Med. Mic. 2006, 46, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Noga, E.J. Fish Disease: Diagnosis and Treatment, 2nd ed.; Wiley Blackwell: Ames, IA, USA, 2010. [Google Scholar]
- Culot, A.; Grosset, N.; Gautier, M. Overcoming the challenges of phage therapy for industrial aquaculture: A review. Aquaculture 2019, 513, 734423. [Google Scholar] [CrossRef]
- Choudhury, T.G.; Nagaraju, V.T.; Gita, S.; Paria, A.; Parhi, J. Advances in bacteriophage research for bacterial disease control in aquaculture. Rev. Fish. Sci. Aquac. 2017, 25, 113–125. [Google Scholar] [CrossRef]
- Cairns, B.J.; Payne, R.J. Bacteriophage therapy and the mutant selection window. Antimicrob. Agents Chemother. 2008, 52, 4344–4350. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kunttu, H.M.; Runtuvuori-Salmela, A.; Middelboe, M.; Clark, J.; Sundberg, L.R. Comparison of Delivery Methods in Phage Therapy against Flavobacterium columnare Infections in Rainbow Trout. Antibiotics 2021, 10, 914. [Google Scholar] [CrossRef]
- Romero, J.; Feijoó, C.G.; Navarrete, P. Antibiotics in aquaculture—Use, abuse and alternatives. In Health and Environment in Aquaculture; Carvalho, E.D., David, G.S., DaSilva, R.J., Eds.; IntechOpen: London, UK, 2012; pp. 159–198. [Google Scholar] [CrossRef]
- Kutter, E.; De Vos, D.; Gvasalia, G.; Alavidze, Z.; Gogokhia, L.; Kuhl, S.; Abedon, S.T. Phage therapy in clinical practice: Treatment of human infections. Curr. Pharm. Biotechnol. 2010, 11, 69–86. [Google Scholar] [CrossRef]
- Wu, J.L.; Lin, H.M.; Jan, L.; Hsu, Y.L.; Chang, L.H. Biological control of fish bacterial pathogen, Aeromonas hydrophila, by bacteriophage AH 1. Fish Pathol. 1981, 15, 271–276. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, L.; Feng, C.; Chi, T.; Qi, Y.; Abbas Raza, S.H.; Gao, N.; Jia, K.; Zhang, Y.; Fan, R.; et al. A phage cocktail in controlling phage resistance development in multidrug resistant Aeromonas hydrophila with great therapeutic potential. Microb. Pathog. 2022, 162, 105374. [Google Scholar] [CrossRef]
- El-Araby, D.A.; El-Didamony, G.; Megahed, M. New approach to use phage therapy against Aeromonas hydrophila induced motile Aeromonas septicemia in Nile tilapia. J. Mar. Sci. Res. Dev. 2016, 6, 3. [Google Scholar] [CrossRef]
- Dien, L.T.; Ky, L.B.; Huy, B.T.; Mursalim, M.F.; Kayansamruaj, P.; Senapin, S.; Rodkhum, C.; Dong, H.T. Characterization and protective effects of lytic bacteriophage pAh6.2TG against a pathogenic multidrug-resistant Aeromonas hydrophila in Nile tilapia (Oreochromis niloticus). Transbound. Emerg. Dis. 2021. [Google Scholar] [CrossRef]
- Le, T.S.; Nguyen, T.H.; Vo, H.P.; Doan, V.C.; Nguyen, H.L.; Tran, M.T.; Tran, T.T.; Southgate, P.C.; Kurtböke, D.İ. Protective Effects of Bacteriophages against Aeromonas hydrophila Species Causing Motile Aeromonas Septicemia (MAS) in Striped Catfish. Antibiotics 2018, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Dang, T.H.O.; Xuan, T.T.; Duyen, L.T.; Le, N.P.; Hoang, H.A. Protective efficacy of phage PVN02 against haemorrhagic septicaemia in striped catfish Pangasianodon hypophthalmus via oral administration. J. Fish Dis. 2021, 44, 1255–1263. [Google Scholar] [CrossRef] [PubMed]
- Easwaran, M.; Dananjaya, S.; Park, S.C.; Lee, J.; Shin, H.; De Zoysa, M. Characterization of Bacteriophage PAh-1 and Its Protective Effects on Experimental Infection of Aeromonas hydrophila in Zebrafish (Danio rerio). J. Fish Dis. 2017, 40, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Schulz, P.; Robak, S.; Dastych, J.; Siwicki, A.K. Influence of bacteriophages cocktail on European eel (Anguilla anguilla) immunity and survival after experimental challenge. Fish Shellfish Immunol. 2019, 84, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Schulz, P.; Pajdak-Czaus, J.; Robak, S.; Dastych, J.; Siwicki, A.K. Bacteriophage-based cocktail modulates selected immunological parameters and post-challenge survival of rainbow trout (Oncorhynchus mykiss). J. Fish Dis. 2019, 42, 1151–1160. [Google Scholar] [CrossRef]
- Imbeault, S.; Parent, S.; Lagacé, M.; Uhland, C.F.; Blais, J.F. Using bacteriophages to prevent furunculosis caused by Aeromonas salmonicida in farmed brook trout. J. Aquat. Anim. Health 2006, 18, 203–214. [Google Scholar] [CrossRef]
- Silva, Y.J.; Moreirinha, C.; Pereira, C.; Costa, L.; Rocha, R.J.; Cunha, Â.; Gomez, N.C.M.; Calado, R.; Almeida, A. Biological control of Aeromonas salmonicida infection in juvenile Senegalese sole (Solea senegalensis) with Phage AS-A. Aquaculture 2016, 450, 225–233. [Google Scholar] [CrossRef]
- Verner–Jeffreys, D.W.; Algoet, M.; Pond, M.J.; Virdee, H.K.; Bagwell, N.J.; Roberts, E.G. Furunculosis in Atlantic salmon (Salmo salar L.) is not readily controllable by bacteriophage therapy. Aquaculture 2007, 270, 475–484. [Google Scholar] [CrossRef]
- Jia, K.; Yang, N.; Zhang, X.; Cai, R.; Zhang, Y.; Tian, J.; Raza, S.H.A.; Kang, Y.; Qian, A.; Li, Y.; et al. Genomic, Morphological and Functional Characterization of Virulent Bacteriophage IME-JL8 Targeting Citrobacter freundii. Front. Microbiol. 2020, 11, 585261. [Google Scholar] [CrossRef]
- Royam, M.M.; Nachimuthu, R. Isolation, characterization, and efficacy of bacteriophages isolated against Citrobacter spp. an in vivo approach in a zebrafish model (Danio rerio). Res. Microbiol. 2020, 171, 341–350. [Google Scholar] [CrossRef]
- Cui, H.; Xu, Y.; Cong, C.; Li, C.; Li, X.; Li, S.; Li, J.; Wang, L. Evaluation of the preventive effect of phage cocktails on turbot ascites and its influence on main physiological indicators. Aquaculture 2022, 547, 737539. [Google Scholar] [CrossRef]
- Nikapitiya, C.; Chandrarathna, H.P.S.U.; Dananjaya, S.H.S.; De Zoysa, M.; Lee, J. Isolation and characterization of phage (ETP-1) specific to multidrug resistant pathogenic Edwardsiella tarda and its in vivo biocontrol efficacy in zebrafish (Danio rerio). Biologicals 2020, 63, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Castillo, D.; Higuera, G.; Villa, M.; Middelboe, M.; Dalsgaard, I.; Madsen, L.; Espejo, R.T. Diversity of Flavobacterium psychrophilum and the potential use of its phages for protection against bacterial cold water disease in salmonids. J. Fish Dis. 2012, 35, 193–201. [Google Scholar] [CrossRef]
- Sundell, K.; Landor, L.; Castillo, D.; Middelboe, M.; Wiklund, T. Bacteriophages as Biocontrol Agents for Flavobacterium psychrophilum Biofilms and Rainbow Trout Infections. Phage 2020, 1, 198–204. [Google Scholar] [CrossRef]
- Nakai, T.; Sugimoto, R.; Park, K.H.; Matsuoka, S.; Mori, K.I.; Nishioka, T.; Maruyama, K. Protective effects of bacteriophage on experimental Lactococcus garvieae infection in yellowtail. Dis. Aquat. Org. 1999, 37, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, S.M.; Bouzari, M.; Emtiazi, G. Preliminary characterization of Lactococcus garvieae bacteriophage isolated from wastewater as a potential agent for biological control of lactococcosis in aquaculture. Aquac. Int. 2014, 22, 1469–1480. [Google Scholar] [CrossRef]
- Park, S.C.; Shimamura, I.; Fukunaga, M.; Mori, K.I.; Nakai, T. Isolation of bacteriophages specific to a fish pathogen, Pseudomonas plecoglossicida, as a candidate for disease control. Appl. Environ. Microb. 2000, 66, 1416–1422. [Google Scholar] [CrossRef]
- Park, S.C.; Nakai, T. Bacteriophage control of Pseudomonas plecoglossicida infection in ayu Plecoglossus altivelis. Dis. Aquat. Organ. 2003, 53, 33–39. [Google Scholar] [CrossRef]
- Matsuoka, S.; Hashizume, T.; Kanzaki, H.; Iwamoto, E.; Park, S.C.; Yoshida, T.; Nakai, T. Phage therapy against beta-hemolytic streptococcicosis of Japanese flounder Paralichthys olivaceus. Fish Pathol. 2007, 42, 181–189. [Google Scholar] [CrossRef]
- Kwon, A.S.; Kang, B.J.; Jun, S.Y.; Yoon, S.J.; Lee, J.H.; Kang, S.H. Evaluating the effectiveness of Streptococcus parauberis bacteriophage Str-PAP-1 as an environmentally friendly alternative to antibiotics for aquaculture. Aquaculture 2017, 468, 464–470. [Google Scholar] [CrossRef]
- Rørbo, N.; Rønneseth, A.; Kalatzis, P.G.; Rasmussen, B.B.; Engell-Sørensen, K.; Kleppen, H.P.; Wergeland, H.I.; Gram, L.; Middelboe, M. Exploring the effect of phage therapy in preventing Vibrio anguillarum infections in cod and turbot larvae. Antibiotics 2018, 7, 42. [Google Scholar] [CrossRef]
- Higuera, G.; Bastías, R.; Tsertsvadze, G.; Romero, J.; Espejo, R.T. Recently discovered Vibrio anguillarum phages can protect against experimentally induced vibriosis in Atlantic salmon, Salmo salar. Aquaculture 2013, 392, 128–133. [Google Scholar] [CrossRef]
- Kalatzis, P.G.; Bastías, R.; Kokkari, C.; Katharios, P. Isolation and Characterization of Two Lytic Bacteriophages, ΦSt2 and ΦGrn1; Phage Therapy Application for Biological Control of Vibrio alginolyticus in Aquaculture Live Feeds. PLoS ONE 2016, 11, e0151101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cao, Z.; Li, Z.; Wang, L.; Li, H.; Wu, F.; Jin, L.; Li, X.; Li, S.; Xu, Y. Effect of bacteriophages on Vibrio alginolyticus infection in the sea cucumber, Apostichopus japonicus (Selenka). J. World Aquac. Soc. 2015, 46, 149–158. [Google Scholar] [CrossRef]
- Silva, Y.J.; Costa, L.; Pereira, C.; Mateus, C.; Cunha, A.; Calado, R.; Gomez, N.C.M.; Pardo, M.A.; Hernandez, I.; Almeida, A. Phage therapy as an approach to prevent Vibrio anguillarum infections in fish larvae production. PLoS ONE 2014, 9, e114197. [Google Scholar] [CrossRef] [PubMed]
- Lomelí-Ortega, C.O.; Martínez-Sández, A.; Barajas-Sandoval, D.R.; Reyes, A.G.; Magallón-Barajas, F.; Veyrand-Quíros, B.; Gannon, L.; Harrison, C.; Michniewski, S.; Millard, A. Isolation and Characterization of Vibriophage VB_Vc_SrVc9: An Effective Agent in Preventing Vibrio campbellii Infections in Brine Shrimp Nauplii (Artemia franciscana). J. Appl. Microbiol. 2021, 131, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Jun, J.W.; Giri, S.S.; Chi, C.; Yun, S.; Kim, S.G.; Kim, S.W.; Kang, J.W.; Han, S.J.; Kwon, J.; et al. Application of the bacteriophage pVco-14 to prevent Vibrio coralliilyticus infection in Pacific oyster (Crassostrea gigas) larvae. J. Invertebr. Pathol. 2019, 167, 107244. [Google Scholar] [CrossRef]
- Quiroz-Guzmán, E.; Peña-Rodriguez, A.; Vázquez-Juárez, R.; Barajas-Sandoval, D.R.; Balcázar, J.L.; Martínez-Díaz, S.F. Bacteriophage cocktails as an environmentally-friendly approach to prevent Vibrio parahaemolyticus and Vibrio harveyi infections in brine shrimp (Artemia franciscana) production. Aquaculture 2018, 492, 273–279. [Google Scholar] [CrossRef]
- Misol, G.N.; Kokkari, C.; Katharios, P. Biological and Genomic Characterization of a Novel Jumbo Bacteriophage, vB_VhaM_pir03 with Broad Host Lytic Activity against Vibrio harveyi. Pathogens 2020, 9, 1051. [Google Scholar] [CrossRef]
- Stalin, N.; Srinivasan, P. Efficacy of potential phage cocktails against Vibrio harveyi and closely related Vibrio species isolated from shrimp aquaculture environment in the south east coast of India. Vet. Microbiol. 2017, 207, 83–96. [Google Scholar] [CrossRef]
- Karunasagar, I.; Shivu, M.M.; Girisha, S.K.; Krohne, G.; Karunasagar, I. Biocontrol of pathogens in shrimp hatcheries using bacteriophages. Aquaculture 2007, 268, 288–292. [Google Scholar] [CrossRef]
- Vinod, M.G.; Shivu, M.M.; Umesha, K.R.; Rajeeva, B.C.; Krohne, G.; Karunasagar, I.; Karunasagar, I. Isolation of Vibrio harveyi bacteriophage with a potential for biocontrol of luminous vibriosis in hatchery environments. Aquaculture 2006, 255, 117–124. [Google Scholar] [CrossRef]
- Patil, J.R.; Desai, S.N.; Roy, P.; Durgaiah, M.; Saravanan, R.S.; Vipra, A. Simulated Hatchery System to Assess Bacteriophage Efficacy against Vibrio harveyi. Dis. Aquat. Org. 2014, 112, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Barton, M.; Elliott, L.; Li, X.; Abraham, S.; O’Dea, M.; Munro, J. Bacteriophage therapy for the control of Vibrio harveyi in greenlip abalone (Haliotis laevigata). Aquaculture 2017, 473, 251–258. [Google Scholar] [CrossRef]
- Cui, H.; Cong, C.; Wang, L.; Li, X.; Li, J.; Yang, H.; Li, S.; Xu, Y. Protective effectiveness of feeding phage cocktails in controlling Vibrio harveyi infection of turbot Scophthalmus maximus. Aquaculture 2021, 535, 736390. [Google Scholar] [CrossRef]
- Wu, L.; Tian, Y.; Pang, M.; Yang, Z.; Bao, H.; Zhou, Y.; Sun, L.; Wang, R.; Zhang, H. A novel vibriophage vB_VhaS_PcB-1G capable of inhibiting virulent Vibrio harveyi pathogen. Aquaculture 2021, 542, 736854. [Google Scholar] [CrossRef]
- Alagappan, K.; Karuppiah, V.; Deivasigamani, B. Protective effect of phages on experimental V. parahaemolyticus infection and immune response in shrimp (Fabricius, 1798). Aquaculture 2016, 453, 86–92. [Google Scholar] [CrossRef]
- Lomelí-Ortega, C.O.; Martínez-Díaz, S.F. Phage therapy against Vibrio parahaemolyticus infection in the whiteleg shrimp (Litopenaeus vannamei) larvae. Aquaculture 2014, 434, 208–211. [Google Scholar] [CrossRef]
- Jun, J.W.; Han, J.E.; Giri, S.S.; Tang, K.F.; Zhou, X.; Aranguren, L.F.; Kim, H.J.; Saekil, Y.; Kim, S.G.; Park, S.C. Phage application for the protection from acute hepatopancreatic necrosis disease (AHPND) in Penaeus vannamei. Indian J. Microbiol. 2018, 58, 114–117. [Google Scholar] [CrossRef]
- Ding, T.; Sun, H.; Pan, Q.; Zhao, F.; Zhang, Z.; Ren, H. Isolation and characterization of Vibrio parahaemolyticus bacteriophage vB_VpaS_PG07. Virus Res. 2020, 286, 198080. [Google Scholar] [CrossRef]
- Li, Z.; Li, X.; Zhang, J.; Wang, X.; Wang, L.; Cao, Z.; Xu, Y. Use of phages to control Vibrio splendidus infection in the juvenile sea cucumber Apostichopus japonicus. Fish Shellfish Immunol. 2016, 54, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Parasion, S.; Kwiatek, M.; Gryko, R.; Mizak, L.; Malm, A. Bacteriophages as an alternative strategy for fighting biofilm development. Pol. J. Microbiol. 2014, 63, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Schmelcher, M.; Loessner, M.J. Application of bacteriophages for detection of foodborne pathogens. Bacteriophage 2014, 4, e28137. [Google Scholar] [CrossRef] [PubMed]
- Goodridge, L.D. Designing phage therapeutics. Curr. Pharm. Biotechnol. 2010, 11, 15–27. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).