Antimicrobial Resistance of Staphylococcus sp. Isolated from Cheeses
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. General Microbiological Analysis
2.2. Isolation of Strains
2.3. Identification of Staphylococcal Isolates
2.4. Detection of Antimicrobial Resistance
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oyetunji, O.A.; Adebisi, K.A. Assessment of the functional quality and safety of yoghurts produced with starter cultures obtained from selected commercially sold yoghurts. Potravin. Slovak J. Food Sci. 2018, 12, 587–599. [Google Scholar] [CrossRef][Green Version]
- Franke, G.; Cwiková, O. Biogenic amines in smear ripened cheeses. Potravin. Slovak J. Food Sci. 2019, 13, 378–384. [Google Scholar] [CrossRef]
- Štefániková, J.; Nagyová, V.; Hynšt, M.; Vietoris, V.; Martišová, P. Nagyová, Ľudmila Application of electronic nose for determination of Slovak cheese authentication based on aroma profile. Potravin. Slovak J. Food Sci. 2019, 13, 262–267. [Google Scholar] [CrossRef][Green Version]
- Montel, M.-C.; Buchin, S.; Mallet, A.; Delbes-Paus, C.; Vuitton, D.A.; Desmasures, N.; Berthier, F. Traditional cheeses: Rich and diverse microbiota with associated benefits. Int. J. Food Microbiol. 2014, 177, 136–154. [Google Scholar] [CrossRef] [PubMed]
- Bergonier, D.; Rupp, R.; Lagriffoul, G.; Berthelot, X. Mastitis of dairy small ruminants. Vet. Res. 2003, 34, 689–716. [Google Scholar] [CrossRef]
- Contreras, A.; Sierra, D.; Sánchez, A.; Corrales, J.; Marco, J.; Paape, M.; Gonzalo, C. Mastitis in small ruminants. Small Rumin. Res. 2007, 68, 145–153. [Google Scholar] [CrossRef]
- Fagundes, H.; Barchesi, L.; Filho, A.N.; Ferreira, L.M.; Oliveira, C.A.F. Occurrence of Staphylococcus aureus in raw milk produced in dairy farms in São Paulo state, Brazil. Braz. J. Microbiol. 2010, 41, 376–380. [Google Scholar] [CrossRef]
- Vanderhaeghen, W.; Piepers, S.; Leroy, F.; Van Coillie, E.; Haesebrouck, F.; De Vliegher, S. Identification, typing, ecology and epidemiology of coagulase negative staphylococci associated with ruminants. Vet. J. 2015, 203, 44–51. [Google Scholar] [CrossRef]
- Regecová, I.; Pipová, M.; Jevinová, P.; Kmet, V.; Výrostková, J.; Sopková, D. Antimicrobial Resistance of Coagulase-negative Species of Staphylococci Isolated from the Meat of Wild Pheasants (Phasianus colchicus). Ital. J. Anim. Sci. 2014, 13, 3476. [Google Scholar] [CrossRef]
- Ribeiro, L.F.; Sato, R.A.; de Souza Pollo, A.; Rossi, G.A.M.; do Amaral, L.A. Occurrence of Methicillin-Resistant Staphylococcus spp. on Brazilian Dairy Farms that Produce Unpasteurized Cheese. Toxins 2020, 12, 779. [Google Scholar] [CrossRef]
- Bencúrová, E.; Bhide, M.; Dolinská, S.; Hreško, S.; Mlynárčik, P.; Mucha, R.; Pulzová, L. Nové trendy vo využívaní bioinformatických analýz v genomike a proteomike. In New Trends in the Use of Bioinformatics Analysis in Genomics and Proteomics, 1st ed.; University of Veterinary Medicine and Pharmacy: Košice, Slovakia, 2013; p. 172. ISBN 978-80-8077-321-2. [Google Scholar]
- Croxatto, A.; Prod’hom, G.; Greub, G. Applications of MALDI-TOF Mass Spectrometry in Clinical Diagnostic Microbiology. FEMS Microbiol. Rev. 2012, 36, 380–407. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.E.; Kaleta, E.J.; Arora, A.; Wolk, D.M. Matrix-Assisted Laser Desorption Ionization—Time of Flight Mass Spectrometry: A Fundamental Shift in the Routine Practice of Clinical Microbiology. Clin. Microbiol. Rev. 2013, 26, 547–603. [Google Scholar] [CrossRef] [PubMed]
- Vrabec, M.; Lovayová, V.; Dudriková, K.; Gallo, J.; Dudriková, E. Antibiotic Resistance and Prevalence of Enterococcus Spp. And Escherichia Coli Isolated from Bryndza Cheese. Ital. J. Anim. Sci. 2015, 14, 3968. [Google Scholar] [CrossRef]
- Microbiology of food and animal feeding stuffs. In Preparation of Test Samples, Initial Suspension and Decimal Dilutions for Microbiological Examination. Part 5: Specific Rules for the Preparation of Milk and Milk Products; ISO 6887-5; Slovak Standards Institute: Bratislava, Slovakia, 2010; Available online: https://www.iso.org/obp/ui/#iso:std:iso:6887:-5:ed-1:v1:en (accessed on 18 November 2021).
- Microbiology of food and animal feeding stuffs. In Horizontal Method for the Enumeration of Coagu-Lase-Positive Staphylococci (Staphylococcus Aureus and Other Species). Part 1: Technique Using Baird-Parker Agar Medium; ISO 6888-1; Slovak Standards Institute: Slovakia, Bratislava, 1999; Available online: https://standards.iteh.ai/catalog/standards/cen/74482f1e-5f94-4c3e-a057-7be3d8180ef5/en-iso-6888-1-1999 (accessed on 18 November 2021).
- Hein, I.; Jørgensen, H.J.; Loncarevic, S.; Wagner, M. Quantification of Staphylococcus aureus in unpasteurised bovine and caprine milk by real-time PCR. Res. Microbiol. 2005, 156, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Strommenger, B.; Kettlitz, C.; Werner, G.; Witte, W. Multiplex PCR Assay for Simultaneous Detection of Nine Clinically Relevant Antibiotic Resistance Genes in Staphylococcus aureus. J. Clin. Microbiol. 2003, 41, 4089–4094. [Google Scholar] [CrossRef]
- Bruker Daltonics. Software for Microorganism Identification and Classification User Manual; MALDI Biotyper 2.0; Bruker Scientific LLC: Billerica, MA, USA, 2008. [Google Scholar]
- Clinical and Laboratory Standards Institute. CLSI document M100—S30. In Performance Standards for Antimicrobial Susceptibility Testing; Thirtieth Informational Supplement; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2020. [Google Scholar]
- Poulsen, A.B.; Skov, R.; Pallesen, L.V. Detection of methicillin resistance in coagulase-negative staphylococci and in staphylococci directly from simulated blood cultures using the EVIGENE MRSA Detection Kit. J. Antimicrob. Chemother. 2003, 51, 419–421. [Google Scholar] [CrossRef]
- Doležalová, M.; Stratilová Jermářová, M.; Holko, I. Mikrobiologická nezávadnost sýrů zrajících pod mrazem. Mlékařské Listy-Zprav. 2013, 24, 1–4. [Google Scholar]
- Commission Regulation (EC) No 2073/2005 of 15 November 2005; Microbiological Criteria for Foodstuffs; The Commission of the European Communities: Brussels, Belgium, 2005; Available online: https://www.google.com.hk/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&cad=rja&uact=8&ved=2ahUKEwi5s8y76fv0AhVOHKYKHZrwDDgQFnoECAkQAQ&url=https%3A%2F%2Feur-lex.europa.eu%2Flegal-content%2FEN%2FTXT%2FPDF%2F%3Furi%3DCELEX%3A02005R2073-20140601%26from%3DDA&usg=AOvVaw12u4kr_GmI0e6IzagyF8EL (accessed on 18 November 2021).
- The Commission of the European Communities. Regulation of the Government of the Slovak Republic no. 312/2003 Coll; Health Requirements for the Production and Placing on the Market of Raw Milk, Heat-Treated Milk and Milk-Based Products; The Commission of the European Communities: Brussels, Belgium, 2003. [Google Scholar]
- Carrascosa, C.; Millán, R.; Saavedra, P.; Jaber, J.R.; Raposo, A.; Sanjuán, E. Identification of the risk factors associated with cheese production to implement the hazard analysis and critical control points (HACCP) system on cheese farms. J. Dairy Sci. 2016, 99, 2606–2616. [Google Scholar] [CrossRef]
- Bockelmann, W.; Willems, K.; Neve, H.; Heller, K. Cultures for the ripening of smear cheeses. Int. Dairy J. 2005, 15, 719–732. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Interpretative Criteria for Identification of Bacteria and Fungi by DNA Target Sequencing; Approved Guideline; CLSI Document MM18-A; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Chen, P.-L.; Lee, T.-F.; Wu, C.-J.; Teng, S.-H.; Teng, L.-J.; Ko, W.-C.; Hsueh, P.-R. Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry Can Accurately Differentiate Aeromonas dhakensis from A. hydrophila, A. caviae, and A. veronii. J. Clin. Microbiol. 2014, 52, 2625–2628. [Google Scholar] [CrossRef]
- Deng, J.; Fu, L.; Wang, R.; Yu, N.; Ding, X.; Jiang, L.; Fang, Y.; Jiang, C.; Lin, L.; Wang, Y.; et al. Comparison of MALDI-TOF MS, gene sequencing and the Vitek 2 for identification of seventy-three clinical isolates of enteropathogens. J. Thorac. Dis. 2014, 6, 539–544. [Google Scholar] [CrossRef]
- Cheng, W.-C.; Jan, I.-S.; Chen, J.-M.; Teng, S.-H.; Teng, L.-J.; Sheng, W.-H.; Ko, W.-C.; Hsueh, P.-R. Evaluation of the Bruker Biotyper Matrix-Assisted Laser Desorption Ionization–Time of Flight Mass Spectrometry System for Identification of Blood Isolates of Vibrio Species. J. Clin. Microbiol. 2015, 53, 1741–1744. [Google Scholar] [CrossRef] [PubMed]
- Prod’Hom, G.; Bizzini, A.; Durussel, C.; Bille, J.; Greub, G. Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry for Direct Bacterial Identification from Positive Blood Culture Pellets. J. Clin. Microbiol. 2010, 48, 1481–1483. [Google Scholar] [CrossRef] [PubMed]
- Christner, M.; Rohde, H.; Wolters, M.; Sobottka, I.; Wegscheider, K.; Aepfelbacher, M. Rapid Identification of Bacteria from Positive Blood Culture Bottles by Use of Matrix-Assisted Laser Desorption-Ionization Time of Flight Mass Spectrometry Fingerprinting. J. Clin. Microbiol. 2010, 48, 1584–1591. [Google Scholar] [CrossRef] [PubMed]
- Ferroni, A.; Suarez, S.; Beretti, J.-L.; Dauphin, B.; Bille, E.; Meyer, J.; Bougnoux, M.-E.; Alanio, A.; Berche, P.; Nassif, X. Real-Time Identification of Bacteria and Candida Species in Positive Blood Culture Broths by Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry. J. Clin. Microbiol. 2010, 48, 1542–1548. [Google Scholar] [CrossRef]
- Stevenson, L.G.; Drake, S.K.; Murray, P.R. Rapid Identification of Bacteria in Positive Blood Culture Broths by Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry. J. Clin. Microbiol. 2010, 48, 444–447. [Google Scholar] [CrossRef]
- Clerc, O.; Prod’Hom, G.; Senn, L.; Jaton, K.; Zanetti, G.; Calandra, T.; Greub, G. Matrix-assisted laser desorption ionization time-of-flight mass spectrometry and PCR-based rapid diagnosis of Staphylococcus aureus bacteraemia. Clin. Microbiol. Infect. 2014, 20, 355–360. [Google Scholar] [CrossRef]
- Jamali, H.; Paydar, M.; Radmehr, B.; Ismail, S.; Dadrasnia, A. Prevalence and antimicrobial resistance of Staphylococcus aureus isolated from raw milk and dairy products. Food Control 2015, 54, 383–388. [Google Scholar] [CrossRef]
- Kraemer, J.G.; Pires, J.; Kueffer, M.; Semaani, E.; Endimiani, A.; Hilty, M.; Oppliger, A. Prevalence of extended-spectrum β-lactamase-producing Enterobacteriaceae and Methicillin-Resistant Staphylococcus aureus in pig farms in Switzerland. Sci. Total Environ. 2017, 603–604, 401–405. [Google Scholar] [CrossRef]
- Sampimon, O.C. Coagulase-Negative Staphylococci Mastitis in Dutch Dairy Herds. Ph.D. Thesis, Dutch Animal Health Service (GD), Deventer, The Netherlands, 2009. [Google Scholar]
- Sawant, A.A.; Gillespie, B.E.; Oliver, S.P. Antimicrobial susceptibility of coagulase-negative Staphylococcus species isolated from bovine milk. Vet. Microbiol. 2009, 134, 73–81. [Google Scholar] [CrossRef]
- Waller, K.P.; Aspán, A.; Nyman, A.; Persson, Y.; Andersson, U.G. CNS species and antimicrobial resistance in clinical and subclinical bovine mastitis. Vet. Microbiol. 2011, 152, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Lüthje, P.; Schwarz, S. Antimicrobial resistance of coagulase-negative staphylococci from bovine subclinical mastitis with particular reference to macrolide–lincosamide resistance phenotypes and genotypes. J. Antimicrob. Chemother. 2006, 57, 966–969. [Google Scholar] [CrossRef] [PubMed]
- Chajęcka-Wierzchowska, W.; Zadernowska, A.; Nalepa, B.; Sierpińska, M.; Łaniewska-Trokenheim, Ł. Coagulase-negative staphylococci (CoNS) isolated from ready-to-eat food of animal origin—Phenotypic and genotypic antibiotic resistance. Food Microbiol. 2015, 46, 222–226. [Google Scholar] [CrossRef]
- Vasiľ, M.; Farkašová, Z.; Elečko, J.; Zigo, F. Occurrence of resistance to antibiotics therapy in coagulase-positive and coagulase-negative Staphylococci isolated from sheep’s milk in holding in Slovakia. Potravin. Slovak J. Food Sci. 2020, 14, 781–787. [Google Scholar] [CrossRef]
- Rajala-Schultz, P.J.; Torres, A.H.; DeGraves, F.J.; Gebreyes, W.A.; Patchanee, P. Antimicrobial resistance and genotypic characterization of coagulase-negative staphylococci over the dry period. Vet. Microbiol. 2009, 134, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jeong, J.-M.; Park, Y.-H.; Choi, S.-S.; Kim, Y.-H.; Chae, J.-S.; Moon, J.-S.; Park, H.; Kim, S.; Eo, S.-K. Evaluation of the Methicillin-Resistant Staphylococcus aureus (MRSA)-Screen Latex Agglutination Test for Detection of MRSA of Animal Origin. J. Clin. Microbiol. 2004, 42, 2780–2782. [Google Scholar] [CrossRef]
- Oliver, S.P.; Boor, K.; Murphy, S.C.; Murinda, S.E. Food Safety Hazards Associated with Consumption of Raw Milk. Foodborne Pathog. Dis. 2009, 6, 793–806. [Google Scholar] [CrossRef]
- Vanderhaeghen, W.; Cerpentier, T.; Adriaensen, C.; Vicca, J.; Hermans, K.; Butaye, P. Methicillin-resistant Staphylococcus aureus (MRSA) ST398 associated with clinical and subclinical mastitis in Belgian cows. Vet. Microbiol. 2010, 144, 166–171. [Google Scholar] [CrossRef]
- Aras, Z.; Aydin, I.; Kav, K. Isolation of methicillin-resistant Staphylococcus aureus from caprine mastitis cases. Small Rumin. Res. 2012, 102, 68–73. [Google Scholar] [CrossRef]
- Bogdanovičová, K.; Skočková, A.; Šťástková, Z.; Karpiskova, R. Occurrence and antimicrobial resistance of Staphylococcus aureus in bulk tank milk and milk filters. Potravin. Slovak J. Food Sci. 2014, 8, 97–101. [Google Scholar] [CrossRef]
- Nunes, R.S.C.; Del Aguila, E.M.; Paschoalin, V. Safety Evaluation of the Coagulase-Negative Staphylococci Microbiota of Salami: Superantigenic Toxin Production and Antimicrobial Resistance. BioMed Res. Int. 2015, 2015, 483548. [Google Scholar] [CrossRef]
- Thomas, D.Y.; Jarraud, S.; Lemercier, B.; Cozon, G.; Echasserieau, K.; Etienne, J.; Gougeon, M.-L.; Lina, G.; Vandenesch, F. Staphylococcal Enterotoxin-Like Toxins U2 and V, Two New Staphylococcal Superantigens Arising from Recombination within the Enterotoxin Gene Cluster. Infect. Immun. 2006, 74, 4724–4734. [Google Scholar] [CrossRef] [PubMed]
- Seng, P.; Boushab, B.M.; Romain, F.; Gouriet, F.; Bruder, N.; Martin, C.; Paganelli, F.; Bernit, E.; Le Treut, Y.P.; Thomas, P.; et al. Emerging role of Raoultella ornithinolytica in human infections: A series of cases and review of the literature. Int. J. Infect. Dis. 2016, 45, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Hleba, L.; Petrová, J.; Kántor, A.; Čuboň, J.; Kačániová, M. Antibiotic resistance in Enterobacteriaceae strains isolated from chicken and milk samples. J. Microbiol. Biotechnol. Food Sci. 2015, 04, 19–22. [Google Scholar] [CrossRef]
- Chrobak, D.; Kizerwetter-Świda, M.; Rzewuska, M.; Binek, M. Antibiotic resistance of canine Staphylococcus intermedius group (SIG)—Practical implications. Pol. J. Vet. Sci. 2011, 14, 213–218. [Google Scholar] [CrossRef] [PubMed][Green Version]
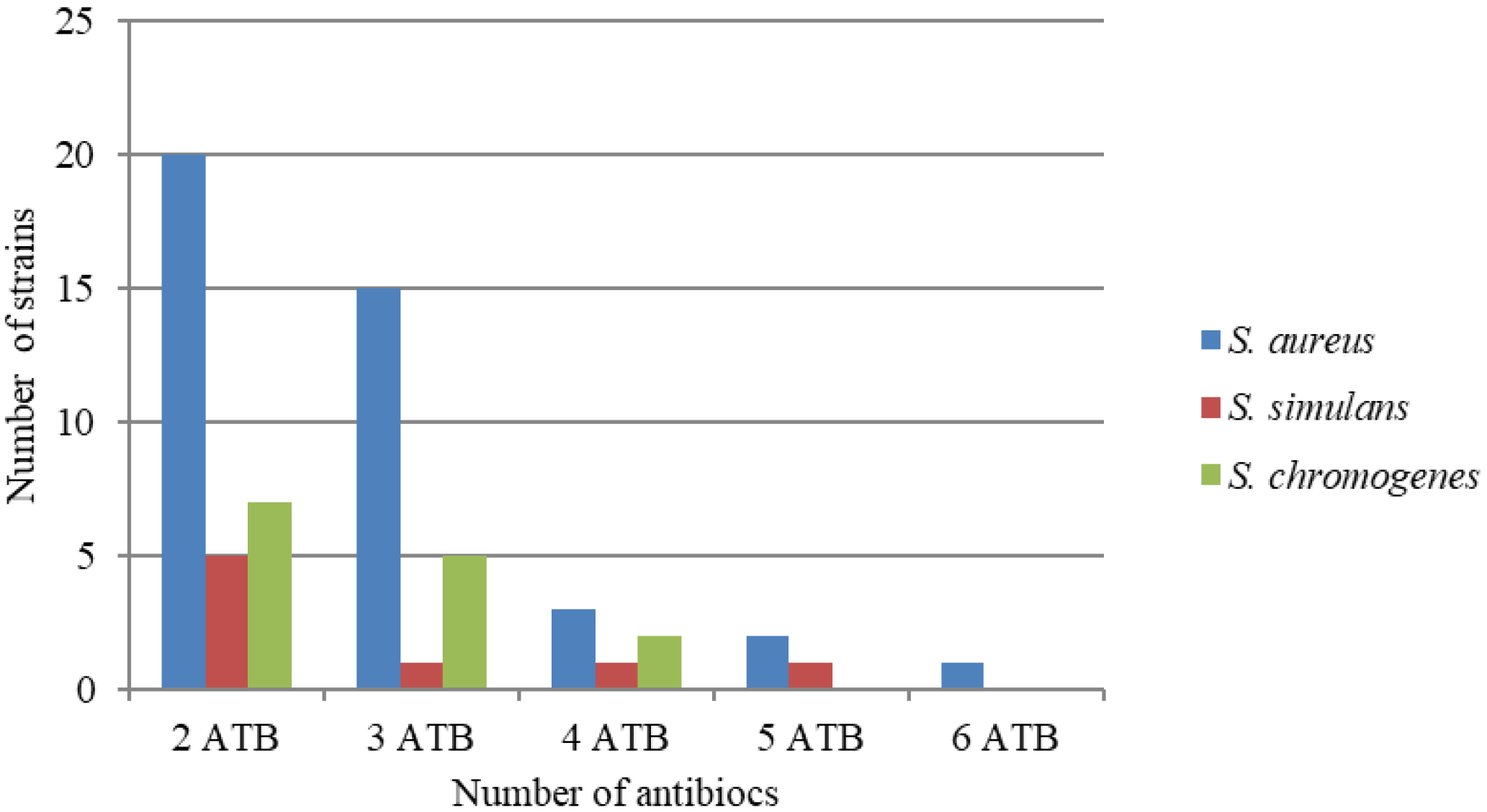
| Type of Samples | Statistical Value | Total Viable Count | Staphylococcus sp. |
|---|---|---|---|
| Goat’s cheese (n = 10) | Minimum | 6.8 | 3.2 |
| Maximum | 8.3 | 4.2 | |
| Mean ± SD | 7.9 ± 0.4 | 3.8 ± 0.3 | |
| Sheep’s cheese (n = 10) | Minimum | 6.9 | 3.3 |
| Maximum | 8.1 | 3.9 | |
| Mean ± SD | 7.7 ± 0.4 | 3.6 ± 0.2 |
| Number of Isolates | 16S rRNA Sequencing Result | Accession Numbers in GenBank | Sequence Similarity % | MALDI-TOF MS | Score Value |
|---|---|---|---|---|---|
| 14 | S. aureus | OK285211.1 | 100 | S. aureus | 2.097–2.268 |
| 10 | S. aureus | CP084892.1 | 100 | S. aureus | 2.215–2.268 |
| 9 | S. aureus | OL344097.1 | 100 | S. aureus | 2.096–2.198 |
| 5 | S. aureus | AP025177.1 | 99 | S. aureus | 2.098–2.215 |
| 4 | S. aureus | CP021178.1 | 100 | S. aureus | 2.126–2.214 |
| 4 | S. aureus | CP084107.1 | 100 | S. aureus | 2.115–2.229 |
| 3 | S. aureus | OL336429.1 | 100 | S. aureus | 2.098–2.183 |
| 2 | S. aureus | CP084878.1 | 100 | S. aureus | 2.176–2.204 |
| 2 | S. aureus | AP025176.1 | 99 | S. aureus | 2.096–2.145 |
| 2 | S. aureus | OK576712.1 | 99 | S. aureus | 2.115–2.178 |
| 1 | S. aureus | OL345568.1 | 100 | S. aureus | 2.099 |
| 3 | S. simulans | MK015778.1 | 100 | S. simulans | 2.100–2.224 |
| 3 | S. simulans | NR_036906.1 | 100 | S. simulans | 2.891–2.189 |
| 1 | S. simulans | MF678910.1 | 99 | S. simulans | 2.145 |
| 1 | S. simulans | FN646077.1 | 99 | S. simulans | 2.058 |
| 1 | S. simulans | LC437030.1 | 99 | S. simulans | 2.002 |
| 1 | S. simulans | KC849411.1 | 100 | S. simulans | 2.220 |
| 5 | S. chromogenes | MT913000.1 | 99 | S. chromogenes | 2.076–2.098 |
| 4 | S. chromogenes | CP031471.1 | 100 | S. chromogenes | 2.097–2.105 |
| 2 | S. chromogenes | CP031274.1 | 100 | S. chromogenes | 2.102 |
| 2 | S. chromogenes | CP046028.1 | 100 | S. chromogenes | 2.088–2.100 |
| 2 | S. chromogenes | CP031470.1 | 100 | S. chromogenes | 2.085–2.101 |
| 1 | S. chromogenes | JN426805.1 | 100 | S. chromogenes | 2.099 |
| Strains | Antibiotics | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PEN | OX | KF | TEC | GN | E | TE | OFX | |||
| S. aureus | (n = 56) | S | 2 | 3 | 40 | 42 | 22 | 6 | 44 | 48 |
| IS | 0 | 0 | 10 | 10 | 24 | 10 | 2 | 2 | ||
| R | 54 | 53 | 6 | 4 | 10 | 40 | 10 | 6 | ||
| S. simulans | (n = 10) | S | 0 | 1 | 4 | 10 | 4 | 0 | 10 | 10 |
| IS | 0 | 0 | 2 | 0 | 4 | 0 | 0 | 0 | ||
| R | 10 | 10 | 4 | 0 | 2 | 10 | 0 | 0 | ||
| S. chromogenes | (n = 16) | S | 0 | 1 | 8 | 6 | 10 | 2 | 4 | 10 |
| IS | 0 | 0 | 4 | 10 | 4 | 4 | 0 | 2 | ||
| R | 16 | 15 | 4 | 0 | 2 | 10 | 12 | 4 | ||
| Chi-quadrate test | G | 25.42 1 | 22.32 1 | 4.708 | 0.964 | 2.862 | 19.22 1 | 6.42 1 | 4.632 | |
| n | PEN | OX | KF | TEC | GN | E | TE | OFX | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Goat cheese | S. aureus | 29 | 28 | 27 | 2 | 2 | 3 | 19 | 5 | 4 |
| S. simulans | 6 | 6 | 6 | 1 | 0 | 1 | 6 | 0 | 0 | |
| S. chromogenes | 7 | 7 | 6 | 2 | 0 | 2 | 5 | 5 | 1 | |
| Sheep cheese | S. aureus | 27 | 26 | 26 | 4 | 2 | 7 | 21 | 5 | 2 |
| S. simulans | 4 | 4 | 4 | 3 | 0 | 1 | 4 | 0 | 0 | |
| S. chromogenes | 9 | 9 | 9 | 2 | 0 | 0 | 5 | 7 | 3 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Výrostková, J.; Regecová, I.; Zigo, F.; Semjon, B.; Gregová, G. Antimicrobial Resistance of Staphylococcus sp. Isolated from Cheeses. Animals 2022, 12, 36. https://doi.org/10.3390/ani12010036
Výrostková J, Regecová I, Zigo F, Semjon B, Gregová G. Antimicrobial Resistance of Staphylococcus sp. Isolated from Cheeses. Animals. 2022; 12(1):36. https://doi.org/10.3390/ani12010036
Chicago/Turabian StyleVýrostková, Jana, Ivana Regecová, František Zigo, Boris Semjon, and Gabriela Gregová. 2022. "Antimicrobial Resistance of Staphylococcus sp. Isolated from Cheeses" Animals 12, no. 1: 36. https://doi.org/10.3390/ani12010036
APA StyleVýrostková, J., Regecová, I., Zigo, F., Semjon, B., & Gregová, G. (2022). Antimicrobial Resistance of Staphylococcus sp. Isolated from Cheeses. Animals, 12(1), 36. https://doi.org/10.3390/ani12010036








