Paratuberculosis: The Hidden Killer of Small Ruminants
Abstract
Simple Summary
Abstract
1. Introduction
2. Mycobacterium avium subsp. Paratuberculosis (MAP)
3. Susceptibility to Infection with MAP
4. Transmission of MAP
5. Clinical Signs of PTB
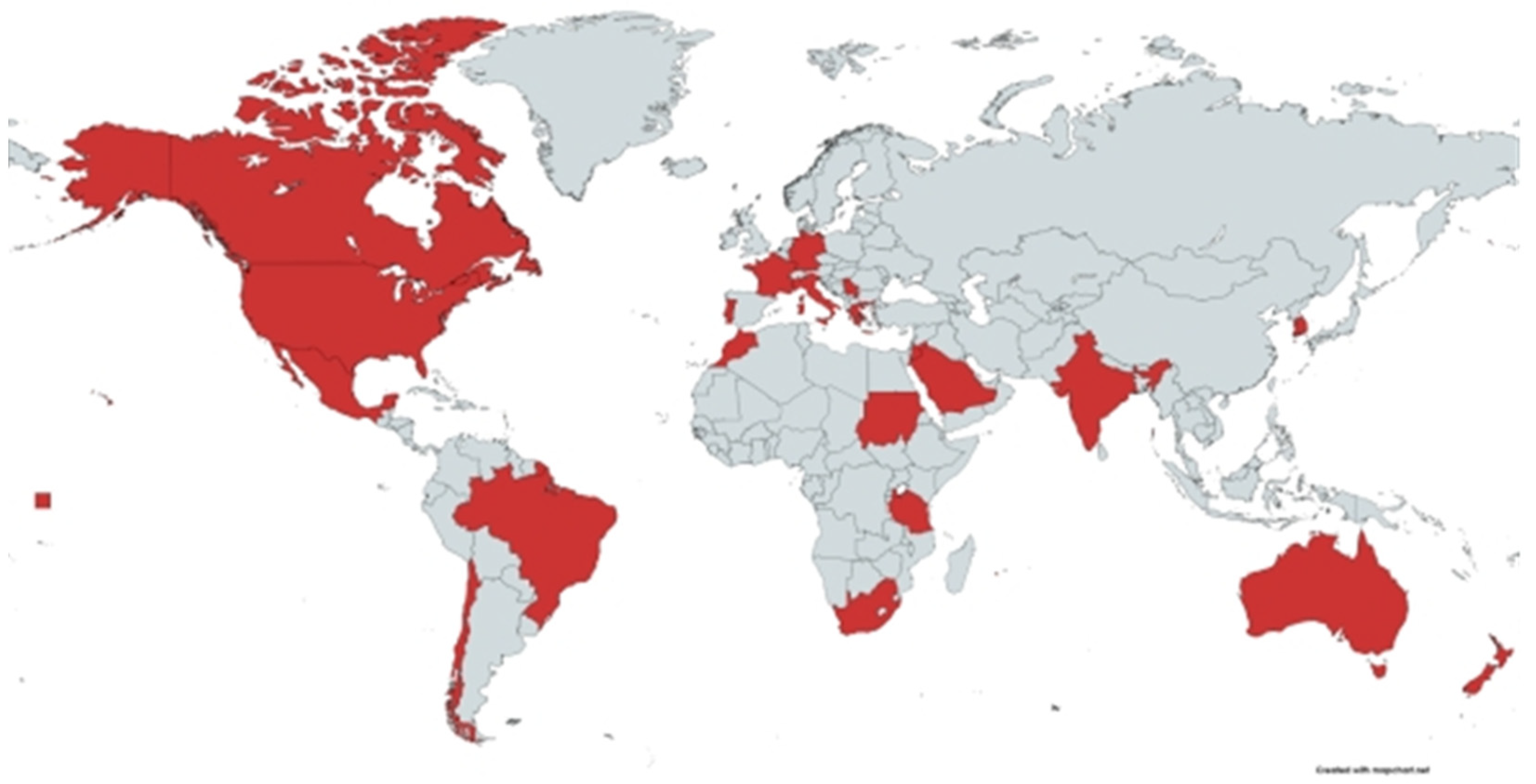
6. Prevalence and Distribution of PTB in Small Ruminants
6.1. Prevalence of PTB in Goats at the Animal Level
6.2. Flock-Level Prevalence of PTB in Goats
6.3. Prevalence of PTB in Sheep at Animal Level
6.4. Flock-Level Prevalence of PTB in Sheep
7. Pathogenesis of PTB
8. Pathologic Changes of PTB
8.1. Pathologic Changes of PTB in Sheep
8.2. Pathologic Changes of PTB in Goats
9. Diagnosis of PTB
9.1. Microscopic Examination
9.2. Culture Methods
9.3. Molecular Assays
9.4. Serologic Tests
9.4.1. Enzyme-Linked Immunosorbent Assays (ELSIA)
9.4.2. Agar Gel Immuno-Diffusion (AGID) Test
9.4.3. The Complement Fixation Test (CFT)
10. Treatment, Control and Prevention
10.1. Changes of Management Practices
10.2. Test-and-Cull
10.3. Vaccination
10.4. Selective Breeding
11. Research Gaps
12. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- West, D.M.; Bruère, A.N.; Ridler, A.L. The Sheep: Health, Disease and Production, 3rd ed.; Veterinary Continuing Education, Massey University: Palmerston North, New Zealnd, 2009. [Google Scholar]
- Cunha, V.M.; Rosalino, L.M.; Leao, C.; Bandeira, V.; Fonseca, C.; Botelho, A.; Reis, A.C. Ecological Drivers of Mycobacterium avium Subsp. Paratuberculosis Detection in Mongoose (Herpestes Ichneumon) Using Is900 as Proxy. Sci. Rep. 2020, 10, 860. [Google Scholar] [CrossRef] [PubMed]
- Curlik, J.; Lazar, P.; Iglodyova, A.; Barbusinova, E.; Smiga, L.; Novotny, J.; Mojzisova, J.; Ondrejkova, A.; Hromada, R.; Konjevic, D.; et al. Detection of Mycobacterium avium Subsp. Paratuberculosis in Slovakian Wildlife. Pol. J. Vet. Sci. 2020, 23, 529–535. [Google Scholar]
- Stanitznig, A.; Khol, J.L.; Lambacher, B.; Franz, S.; Wittek, T.; Kralik, P.; Slana, I.; Vasickova, P. Prevalence of Mycobacterium avium Subspecies Paratuberculosis and Hepatitis E in New World Camelids in Austria. Vet. Rec. 2017, 181, 46. [Google Scholar] [CrossRef] [PubMed]
- Garvey, M. Mycobacterium avium Subspecies Paratuberculosis: A Possible Causative Agent in Human Morbidity and Risk to Public Health Safety. Open Vet. J. 2018, 8, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Bharathy, S.; Gunaseelan, L.; Porteen, K. Exploring the Potential Hazard of Mycobacterium avium Subspecies Paratuberculosis as a Cause for Crohn’s Disease. Vet. World 2017, 10, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Garvey, M. Mycobacterium avium Paratuberculosis: A Disease Burden on the Dairy Industry. Animals 2020, 10, 1773. [Google Scholar] [CrossRef]
- Singh, S.; Dhakal, I.P.; Singh, U.M.; Devkota, B.N. Current Diagnostic Techniques of Mycobacterium avium Subsp. Paratuberculosis in Domestic Ruminants. J. Agric. For. Univ. 2018, 2, 23–34. [Google Scholar]
- Okuni, J.B. Occurence of Paratuberculosis in African Countries: A Review. J. Vet. Adv. 2013, 3, 1–8. [Google Scholar]
- Okuni, J.B.; Hansen, S.; Eltom, K.H.; Eltayeb, E.; Amanzada, A.; Omega, J.A.; Czerny, C.P.; el Wahed, A.A.; Ojok, L. Paratuberculosis: A Potential Zoonosis and a Neglected Disease in Africa. Microorganisms 2020, 8, 1007. [Google Scholar] [CrossRef] [PubMed]
- Omega, J.A.; Musalia, L.M.; Kuria, J.K. Knowledge, Attitude and Practices Towards Paratuberculosis in Cattle and Sheep in Kericho County and Konoin Sub-County, Kenya. Afr. J. Educ. Sci. Technol. 2019, 5, 76–86. [Google Scholar]
- OIE. Terrestrial Animal Health Code, 28th ed.; Volume 1, Disease, Infections and Infestations Listed by the OIE; World Organization for Animal Health: Paris, France, 2019. [Google Scholar]
- Matthews, C.; Cotter, P.D.; O’Mahony, J. Map, Johne’s Disease and the Microbiome; Current Knowledge and Future Considerations. Anim. Microbiome. 2021, 3, 34. [Google Scholar] [CrossRef]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in Gut Microbiota Control Metabolic Endotoxemia-Induced Inflammation in High-Fat Diet-Induced Obesity and Diabetes in Mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef]
- Rieger, A.; Meylan, M.; Hauser, C.; Knubben-Schweizer, G. Meta-Analysis to Estimate the Economic Losses Caused by Reduced Milk Yield and Reproductive Performance Associated with Bovine Paratuberculosis in Switzerland. Schweiz. Arch. Tierheilkd. 2021, 164, 737–751. [Google Scholar] [CrossRef]
- Rasmussen, P.; Barkema, H.W.; Mason, S.; Beaulieu, E.; Hall, D.C. Economic Losses Due to Johne’s Disease (Paratuberculosis) in Dairy Cattle. J. Dairy Sci. 2021, 104, 3123–3143. [Google Scholar] [CrossRef] [PubMed]
- Mendes, S.; Boinas, F.; Albuquerque, T.; Fernandes, L.; Afonso, A.; Amado, A. Epidemiological Studies on Paratuberculosis in Small Ruminants in Portugal. Epidémiol. Santé Anim. 2004, 45, 61–71. [Google Scholar]
- Devendra, C. Small Ruminants: Potential Value and Contribution to Sustainable Development. Outlook Agric. 1994, 23, 97–103. [Google Scholar] [CrossRef]
- Whittington, R.; Donat, K.; Weber, M.F.; Kelton, D.; Nielsen, S.S.; Eisenberg, S.; Arrigoni, N.; Juste, R.; Saez, J.L.; Dhand, N.; et al. Control of Paratuberculosis: Who, Why and How. A Review of 48 Countries. BMC Vet. Res. 2019, 15, 198. [Google Scholar] [CrossRef] [PubMed]
- Bush, R.D.; Windsor, P.A.; Toribio, J.A. Losses of Adult Sheep Due to Ovine Johne’s Disease in 12 Infected Flocks over a 3-Year Period. Aust. Vet. J. 2006, 84, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Windsor, P.A. Managing Control Programs for Ovine Caseous Lymphadenitis and Paratuberculosis in Australia, and the Need for Persistent Vaccination. Vet. Med. 2014, 5, 11–22. [Google Scholar] [CrossRef][Green Version]
- Ashworth, S.; Gunn, G.J. Losses Associated with Paratuberculosis in Sheep. In Assessment of Surveillance and Control of Johne’s Disease in Farm Animals in Gb; Galdow, G., Gunn, G.J., Eds.; SAC Veterinary Division: Edinburgh, UK, 2001; pp. 103–115. [Google Scholar]
- Sardaro, R.; Pieragostini, E.; Rubino, G.; Petazzi, F. Impact of Mycobacterium avium Subspecies Paratuberculosis on Profit Efficiency in Semi-Extensive Dairy Sheep and Goat Farms of Apulia, Southern Italy. Prev. Vet. Med. 2017, 136, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Bryant, J.M.; Thibault, V.C.; Smith, D.G.; McLuckie, J.; Heron, I.; Sevilla, I.A.; Biet, F.; Harris, S.R.; Maskell, D.J.; Bentley, S.D.; et al. Phylogenomic Exploration of the Relationships between Strains of Mycobacterium avium Subspecies Paratuberculosis. BMC Genom. 2016, 17, 79. [Google Scholar] [CrossRef] [PubMed]
- Fawzy, A.; Zschock, M.; Ewers, C.; Eisenberg, T. Genotyping Methods and Molecular Epidemiology of Mycobacterium avium Subsp. Paratuberculosis (Map). Int. J. Vet. Sci. Med. 2018, 6, 258–264. [Google Scholar] [CrossRef] [PubMed]
- de Juan, L.; Mateos, A.; Dominguez, L.; Sharp, J.M.; Stevenson, K. Genetic Diversity of Mycobacterium avium Subspecies Paratuberculosis Isolates from Goats Detected by Pulsed-Field Gel Electrophoresis. Vet. Microbiol. 2005, 106, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.; Hsu, C.; Alyamani, E.J.; Shehata, M.M.; Al-Dubaib, M.A.; Al-Naeem, A.; Hashad, M.; Mahmoud, O.M.; Alharbi, K.B.; Al-Busadah, K.; et al. Genome-Wide Analysis of the Emerging Infection with Mycobacterium avium Subspecies Paratuberculosis in the Arabian Camels (Camelus Dromedarius). PLoS ONE 2012, 7, e31947. [Google Scholar] [CrossRef] [PubMed]
- Mizzi, R.; Timms, V.J.; Price-Carter, M.L.; Gautam, M.; Whittington, R.; Heuer, C.; Biggs, P.J.; Plain, K.M. Comparative Genomics of Mycobacterium avium Subspecies Paratuberculosis Sheep Strains. Front. Vet. Sci. 2021, 8, 637637. [Google Scholar] [CrossRef]
- Sallam, A.M.; Zare, Y.; Alpay, F.; Shook, G.E.; Collins, M.T.; Alsheikh, S.; Sharaby, M.; Kirkpatrick, B.W. An across-Breed Genome Wide Association Analysis of Susceptibility to Paratuberculosis in Dairy Cattle. J. Dairy Res. 2017, 84, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Romdhane, B.R.; Beaunee, G.; Camanes, G.; Guatteo, R.; Fourichon, C.; Ezanno, P. Which Phenotypic Traits of Resistance Should Be Improved in Cattle to Control Paratuberculosis Dynamics in a Dairy Herd: A Modelling Approach. Vet. Res. 2017, 48, 62. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.A.; Hickey, S.M.; Henderson, H.V. The Effect of Johne’s Disease on Production Traits in Romney, Merino and Merino X Romney-Cross Ewes. N. Z. Vet. J. 2006, 54, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.K.; Singh, S.V.; Singh, M.K.; Saxena, V.K.; Horin, P.; Singh, A.V.; Sohal, J.S. Effect of Genetic Variation in the Mhc Class Ii Drb Region on Resistance and Susceptibility to Johne’s Disease in Endangered Indian Jamunapari Goats. Int. J. Immunogenet. 2012, 39, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Begg, D.J.; Purdie, A.C.; de Silva, K.; Dhand, N.K.; Plain, K.M.; Whittington, R.J. Variation in Susceptibility of Different Breeds of Sheep to Mycobacterium avium Subspecies Paratuberculosis Following Experimental Inoculation. Vet. Res. 2017, 48, 36. [Google Scholar] [CrossRef]
- Stewart, D.J.; Vaughan, J.A.; Stiles, P.L.; Noske, P.J.; Tizard, M.L.; Prowse, S.J.; Michalski, W.P.; Butler, K.L.; Jones, S.L. A Long-Term Bacteriological and Immunological Study in Holstein-Friesian Cattle Experimentally Infected with Mycobacterium avium Subsp. Paratuberculosis and Necropsy Culture Results for Holstein-Friesian Cattle, Merino Sheep and Angora Goats. Vet. Microbiol. 2007, 122, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Stabel, J.R.; Bannantine, J.P.; Hostetter, J.M. Comparison of Sheep, Goats, and Calves as Infection Models for Mycobacterium avium Subsp. Paratuberculosis. Vet. Immunol. Immunopathol. 2020, 225, 110060. [Google Scholar] [CrossRef] [PubMed]
- Park, H.T.; Park, H.E.; Cho, Y.I.; Kim, E.H.; Jung, M.; Shin, S.W.; Lee, S.H.; Kim, D.Y.; Yoo, H.S. Potential Biomarkers as an Indicator of Vertical Transmission of Johne’s Disease in a Korean Native Cattle Farm. J. Vet. Sci. 2017, 18, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Barrett, D.J.; Mee, J.F.; Mullowney, P.; Good, M.; McGrath, G.; Clegg, T.; More, S.J. Risk Factors Associated with Johne’s Disease Test Status in Dairy Herds in Ireland. Vet. Rec. 2011, 168, 410. [Google Scholar] [CrossRef] [PubMed]
- Clarke, C.J. The Pathology and Pathogenesis of Paratuberculosis in Ruminants and Other Species. J. Comp. Pathol. 1997, 116, 217–261. [Google Scholar] [CrossRef]
- Whittington, R.J.; Sergeant, E.S. Progress Towards Understanding the Spread, Detection and Control of Mycobacterium avium Subsp Paratuberculosis in Animal Populations. Aust. Vet. J. 2001, 79, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Carrigan, M.J.; Seaman, J.T. The Pathology of Johne’s Disease in Sheep. Aust. Vet. J. 1990, 67, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.G.; Kay, J.M. Serum Biochemistry and the Diagnosis of Johne’s Disease (Paratuberculosis) in Sheep. Vet. Rec. 1996, 139, 498–499. [Google Scholar] [CrossRef] [PubMed]
- Bauman, C.A.; Jones-Bitton, A.; Menzies, P.; Toft, N.; Jansen, J.; Kelton, D. Prevalence Ofparatuberculosis in the Dairy Goat and Dairy Sheep Industries in Ontario. Can. Vet. J. 2016, 57, 169–175. [Google Scholar] [PubMed]
- Kruze, J.; Salgado, M.; Paredes, E.; Mella, A.; Collins, M.T. Goat Paratuberculosis in Chile: First Isolation and Confirmation of Mycobacterium avium Subspecies Paratuberculosis Infection in a Dairy Goat. J. Vet. Diagn. Investig. 2006, 18, 476–479. [Google Scholar] [CrossRef] [PubMed]
- Djønne, B. Paratuberculosis in Goats. In Paratuberculosis: Organism, Disease, Control; Behr, M.A., Collins, D.M., Eds.; CAB International: Oxfordshire, UK, 2010; pp. 169–178. [Google Scholar]
- Stau, A.; Seelig, B.; Walter, D.; Schroeder, C.; Ganter, M. Seroprevalence of Mycobacterium avium Subsp. Paratuberculosis in Small Ruminants in Germany. Small Rumin. Res. 2012, 105, 361–365. [Google Scholar] [CrossRef]
- Mercier, P.; Baudry, C.; Beaudeau, F.; Seegers, H.; Malher, X. Estimated Prevalence of Mycobacterium avium Subspecies Paratuberculosis Infection in Herds of Dairy Goats in France. Vet. Rec. 2010, 167, 412–415. [Google Scholar] [CrossRef] [PubMed]
- Michel, A. Paratuberculosis in Sheep: An Emerging Disease in South Africa. Vet. Microbiol. 2000, 77, 299–307. [Google Scholar] [CrossRef]
- Eamens, G.J.; Marsh, I.M.; Plain, K.M.; Whittington, R.J. Paratuberculosis (Johne’s Disease). In Australian and New Zealand Standard Diagnostic Procedures; Department of Agriculture, Water and the Environment, Australian Government: Canberra, Australia, 2015; pp. 1–68. [Google Scholar]
- Zhao, L.; Wang, Y.; Wang, J.L.; Zhao, W.H.; Cheng, H.X.; Ma, Y.M.; Chai, H.L.; Zhang, Z.S.; Wang, L.F.; Miao, Z.Q.; et al. Serological Investigation and Genotyping of Mycobacterium avium Subsp. Paratuberculosis in Sheep and Goats in Inner Mongolia, China. PLoS ONE 2021, 16, e0256628. [Google Scholar] [CrossRef] [PubMed]
- Pithua, P.; Kollias, N.S. Estimated Prevalence of Caprine Paratuberculosis in Boer Goat Herds in Missouri, USA. Vet. Med. Int. 2012, 2012, 674085. [Google Scholar] [CrossRef] [PubMed]
- Freitas; Diógenes, T.; de Azevedo, S.S.; Silva, M.L.C.R.; Júnior, F.G.; Santos, C.d.S.A.B.; Clementino, I.; Amaral, F.R.-C.; Alves, C.J. Epidemiological Characterization and Risk Factors Associated with Mycobacterium avium Subsp. Paratuberculosis Infection in Dairy Goats in the Brazilian Semiarid Region. Semin. Ciências Agrárias 2015, 36, 267. [Google Scholar] [CrossRef]
- Debien, E.; Hélie, P.; Buczinski, S.; Lebœuf, A.; Bélanger, D.; Drolet, R. Proportional Mortality: A Study of 152 Goats Submitted for Necropsy from 13 Goat Herds in Quebec, with a Special Focus on Caseous Lymphadenitis. Can. Vet. J. 2013, 54, 581–587. [Google Scholar] [PubMed]
- Robbe-Austerman, S. Control of Paratuberculosis in Small Ruminants. Vet. Clin. N. Am. Food Anim. Pract. 2011, 27, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Abbas, B.; Idris, S.E.O.; Burhan, A. Isolation of M. Paratuberculosis from Goats in Sudan. Sudan J. Vet. Sci. Anim. Husb. 1986, 25, 41–42. [Google Scholar]
- Benazzi, S.; el Hamidi, M.; Schliesser, T. Paratuberculosis in Sheep Flocks in Morocco: A Serological, Microscopical and Cultural Survey. Zent. Vet. B 1996, 43, 213–219. [Google Scholar] [CrossRef]
- Elsohaby, I.; Fayez, M.; Alkafafy, M.; Refaat, M.; Al-Marri, T.; Alaql, F.A.; al Amer, A.S.; Abdallah, A.; Elmoslemany, A. Serological and Molecular Characterization of Mycobacterium avium Subsp. Paratuberculosis (Map) from Sheep, Goats, Cattle and Camels in the Eastern Province, Saudi Arabia. Animals 2021, 11, 323. [Google Scholar] [CrossRef] [PubMed]
- Hailat, N.Q.; Hananeh, W.; Metekia, A.S.; Stabel, J.R.; Al-Majali, A.; Lafi, S. Pathology of Subclinical Paratuberculosis (Johne’s Disease) in Awassi Sheep with Reference to Its Occurrence in Jordan. Veterinární Med. 2010, 55, 590–602. [Google Scholar] [CrossRef]
- Selim, A.; Abdelhady, A.; Abdelrahman, A. Ovine Paratuberculosis: Seroprevalence and Comparison of Fecal Culture and Direct Fecal Pcr Assay. Comp. Immunol. Microbiol. Infect. Dis. 2021, 74, 101526. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Silva, J.A.; Correa-Valencia, N.M.; Ramirez, N.F. Systematic Review of the Prevalence of Paratuberculosis in Cattle, Sheep, and Goats in Latin America and the Caribbean. Trop. Anim. Health Prod. 2014, 46, 1321–1340. [Google Scholar] [CrossRef] [PubMed]
- Borujeni, P.M.; Hajikolaei, M.R.H.; Ghorbanpoor, M.; Sahar, H.E.; Bagheri, S.; Roveyshedzadeh, S. Comparison of Mycobacterium avium Subsp. Paratuberculosis Infection in Cattle, Sheep and Goats in the Khuzestan Province of Iran: Results of a Preliminary Survey. Vet. Med. Sci. 2021, 7, 1970–1979. [Google Scholar] [CrossRef] [PubMed]
- Kumthekar, S.; Manning, E.J.; Ghosh, P.; Tiwari, K.; Sharma, R.N.; Hariharan, H. Mycobacterium avium Subspecies Paratuberculosis Confirmed Following Serological Surveillance of Small Ruminants in Grenada, West Indies. J. Vet. Diagn. Investig. 2013, 25, 527–530. [Google Scholar] [CrossRef] [PubMed]
- Salgado, M.; Kruze, J.; Collins, M.T. Diagnosis of Paratuberculosis by Fecal Culture and Elisa on Milk and Serum Samples in Two Types of Chilean Dairy Goat Herds. J. Vet. Diagn. Investig. 2007, 19, 99–102. [Google Scholar] [CrossRef]
- Mpenda, F.N.; Buza, J. Seroprevalence of Paratuberculosis in Goats and Sheep in Arusha, Northern Tanzania. Int. J. Sci. Res. 2014, 3, 2319–7064. [Google Scholar]
- Martínez-Herrera, D.I.; Sarabia-Bueno, C.C.; Peniche-Cardeña, A.; Villagómez-Cortés, J.A.; Magdaleno-Méndez, A.; Ruíz, S.G.H.; Morales-Alvarez, J.F.; Flores-Castro, R. Seroepidemiology of Goat Paratuberculosis in Five Municipalities of Central Veracruz, Mexico. Trop. Subtrop. Agroecosyst. 2012, 15, 82–88. [Google Scholar]
- Callejas-García, S.A. Estudio Epidemiológico De La Paratuberculosis Caprina En La Zona Centro Del Estado De Veracruz; Tesis Médico Veterinario Zootecnista, Universidad Veracruzana: Veracruz, Mexico, 2013. [Google Scholar]
- Singh, K.; Chandel, B.S.; Dadawala, A.I.; Singh, S.V.; Chauhan, H.C.; Singh, B.; Agrawal, N.D.; Gupta, S.; Chaubey, K.K. Incidence of Mycobacterium avium Subspecies Paratuberculosis in Mehsana Breed of Goats from South Gujarat Using Multiple Tests. Adv. Anim. Vet. Sci. 2013, 1, s28–s31. [Google Scholar]
- Kim, J.; Pham, L.; Jang, Y.; Kim, N.; Ryoo, S.; Jang, Y.; Jang, J.; Jung, S. Isolation and Characterization of Mycobacterium avium Subspecies Paratuberculosis in Korean Black Goat (Capra Hircus Aegagrus). Arch. Med. Vet. 2015, 47, 387–390. [Google Scholar] [CrossRef]
- Julie, A.; Girard, C.; Dubreuil, P.; Daignault, D.; Galarneau, J.-R.; Boisclair, J.; Simard, C.; Bélanger, D. Prevalence of and Carcass Condemnation from Maedi–Visna, Paratuberculosis and Caseous Lymphadenitis in Culled Sheep from Quebec, Canada. Prev. Vet. Med. 2003, 59, 67–81. [Google Scholar]
- Khbou, K.M.; Romdhane, R.; Sassi, L.; Amami, A.; Rekik, M.; Benzarti, M. Seroprevalence of Anti-Mycobacterium avium Subsp. Paratuberculosis Antibodies in Female Sheep in Tunisia. Vet. Med. Sci. 2020, 6, 393–398. [Google Scholar] [CrossRef]
- Vidic, B.; Grgic, Z.; Jovicin, M.; Rasic, Z.; Savic, S.; Vidic, V.; Prica, N. Prevalence of Paratuberculosis Infection in Sheep. Vet. Glas. 2014, 68, 165–174. [Google Scholar] [CrossRef]
- Rita, A.A.; Victor, N.N.; Silvia, P.; Luciana, P.; Anastasia, D.; Vincenzo, C. Ovine Paratuberculosis: A Seroprevalence Study in Dairy Flocks Reared in the Marche Region, Italy. Vet. Med. Int. 2011, 2011, 782875. [Google Scholar]
- Coelho, A.C.; Pinto, M.L.; Silva, S.; Coelho, A.M.; Rodrigues, J.; Juste, R.A. Seroprevalence of Ovine Paratuberculosis Infection in the Northeast of Portugal. Small Rumin. Res. 2007, 71, 298–303. [Google Scholar] [CrossRef]
- Sharma, S.; Gautam, A.; Singh, S.V.; Chaubey, K.K.; Mehta, R.; Gupta, S.; Sharma, M.; Rose, M.K.; Jain, V.K. Prevalence of Mycobacterium avium Subspecies Paratuberculosis (Map) Infection in Suspected Diarrhoeic Buffaloes and Cattle Reporting at Veterinary University in India. Comp. Immunol. Microbiol. Infect. Dis. 2020, 73, 101533. [Google Scholar] [CrossRef] [PubMed]
- Lambeth, C.; Reddacliff, L.A.; Windsor, P.; Abbott, K.A.; McGregor, H.; Whittington, R.J. Intrauterine and Transmammary Transmission of Mycobacterium avium Subsp Paratuberculosis in Sheep. Aust. Vet. J. 2004, 82, 504–508. [Google Scholar] [CrossRef]
- Henderson, D.C.; Caldow, G.; Low, C.J. Paratuberculosis in Cattle: Pathology and Clinical Disease. In Assessment of Surveillance and Control of Johne’s Disease in Farm Animals in GB; Scottish Agricultural College, Veterinary Science Division: Edinburgh, UK, 2000; pp. 15–19. [Google Scholar]
- Milner, A.R.; Wendy, N.; Mack, K.J.; Coates, J.H.; Ian, G.; Sheldrick, P. The Sensitivity and Specificity of a Modified Elisa for the Diagnosis of Johne’s Disease from a Field Trial in Cattle. Vet. Microbiol. 1990, 25, 193–198. [Google Scholar]
- Clarke, C.J.; Little, D. The Pathology of Ovine Paratuberculosis: Gross and Histological Changes in the Intestine and Other Tissues. J. Comp. Pathol. 1996, 114, 419–437. [Google Scholar] [CrossRef]
- Corpa, J.M.; Garrido, J.; Marin, J.F.G.; Perez, V. Classification of Lesions Observed in Natural Cases of Paratuberculosis in Goats. J. Comp. Pathol. 2000, 122, 255–265. [Google Scholar] [CrossRef]
- Kreeger, J.M. Ruminant Paratuberculosis—A Century of Progress and Frustration. J. Vet. Diagn. Investig. 1991, 3, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Pérez, V.; Marín, J.F.G.; Badiola, J.J. Description and Classification of Different Types of Lesion Associated with Natural Paratuberculosis Infection in Sheep. J. Comp. Pathol. 1996, 114, 107–122. [Google Scholar] [CrossRef]
- Catton, B.A. Paucibacillary Paratuberculosis in a Goat. Can. Vet. J. 2002, 43, 787–788. [Google Scholar]
- Sikandar, A.; Cheema, A.H.; Adil, M.; Younus, M.; Zaneb, H.; Zaman, M.A.; Masood, S. Ovine Paratuberculosis-a Histopathological Study from Pakistan. J. Anim. Plant Sci. 2013, 23, 749–753. [Google Scholar]
- Chaturvedi, S.; Singh, S.V.; Srivastava, A.K.; Gangwar, N.K.; Kumar, N.; Rawat, K.D.; Dhama, K. Comparative Evaluation of Fat, Is900 Pcr and Microscopy Vis a Vis Histo-Pathology for the Detection of Mycobacterium avium Subsp Paratuberculosis Infection in Tissues of Goats Naturally Died in Herds Endemic for Johne’s Disease. Indian J. Anim. Sci. 2017, 87, 685–693. [Google Scholar]
- Srikanth, M.; Narayanaswamy, H.D.; Satyanarayana, M.L.; Suguna, R.A.O.; Rathnamma, D.; Ranganath, L.; Mukurtal, S.Y.; Sarvesha, K.; Manjunatha, S.S. Pathomorphological Studies on Ovine Paratuberculosis in An Organised Sheep Farm in Karnataka. J. Cell Tissue Res. 2017, 17, 6067–6072. [Google Scholar]
- Hamid, M.A.; Mohammed, G.E.E.; Bakheit, A.O.; Saeed, E.M.A. Histopathological and RT-PCR Detection of Mycobacterium Paratuberculosis in Tissues of Clinically Suspected Small Ruminants. Int. J. Life Sci. Sci. Res. 2018, 4, 2012–2018. [Google Scholar]
- Stehman, S.M. Paratuberculosis in Small Ruminants, Deer, and South American Camelids. Vet. Clin. N. Am. Food Anim. Pract. 1996, 12, 441–455. [Google Scholar] [CrossRef]
- Coelho, A.C.; Coelho, A.M.; GarcÍA-Diez, J.; Pires, M.A.; Pinto, M.L. Detection of Mycobacterium avium Subsp. Paratuberculosis by Several Diagnostics Techniques in Clinical Suspected Sheep. J. Hell. Vet. Med. Soc. 2018, 68, 167. [Google Scholar] [CrossRef][Green Version]
- Shulaw, W.P.; Bech-Nielsen, S.; Rings, D.M.; Getzy, D.M.; Woodruff, T.S. Serodiagnosis of Paratuberculosis in Sheep by Use of Agar Gel Immunodiffusion. Am. J. Vet. Res. 1993, 54, 13–19. [Google Scholar] [PubMed]
- Derakhshandeh, A.; Namazi, F.; Khatamsaz, E.; Eraghi, V.; Hemati, Z. Goat Paratuberculosis in Shiraz: Histopathological and Molecular Approaches. Vet. Res. Forum 2018, 9, 253–257. [Google Scholar] [PubMed]
- Thakur, M.; Madhulina, M.; Shweta, S.; Vipan, K.G. Comparative Evaluation of Different Diagnostic Techniques for Detection of Naturally Occurring Paratuberculosis in Gaddi Goats. Small Rumin. Res. 2019, 174, 92–98. [Google Scholar] [CrossRef]
- Greig, A. Johne’s Disease in Sheep and Goats. In Practice 2000, 22, 146–151. [Google Scholar] [CrossRef]
- Marin, G.J.F.; Chavez, G.; Ajuriz, J.J. Prevalence of Paratuberculosis in Infected Goat Flocks and Comparison of Different Methods of Diagnosis. In Proceedings of the Third International Colloquium on Paratuberculosis, Orlando, FL, USA, 28 September–2 October 1991. [Google Scholar]
- Kawaji, S.; Taylor, D.L.; Mori, Y.; Whittington, R.J. Detection of Mycobacterium avium Subsp. Paratuberculosis in Ovine Faeces by Direct Quantitative Pcr Has Similar or Greater Sensitivity Compared to Radiometric Culture. Vet. Microbiol. 2007, 125, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Chaubey, K.K.; Gupta, R.D.; Gupta, S.; Singh, S.V.; Bhatia, A.K.; Jayaraman, S.; Kumar, N.; Goel, A.; Rathore, A.S.; Sahzad; et al. Trends and Advances in the Diagnosis and Control of Paratuberculosis in Domestic Livestock. Vet. Q. 2016, 36, 203–227. [Google Scholar] [CrossRef]
- Buergelt, C.D.; Ginn, P.E. The Histopathologic Diagnosis of Subclinical Johne’s Disease in N. American Bison (Bison Bison). Vet. Microbiol. 2000, 77, 325–331. [Google Scholar] [CrossRef]
- Whittington, R.J.; Begg, D.J.; de Silva, K.; Purdie, A.C.; Dhand, N.K.; Plain, K.M. Case Definition Terminology for Paratuberculosis (Johne’s Disease). BMC Vet. Res. 2017, 13, 328. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.S.; Toft, N. Ante Mortem Diagnosis of Paratuberculosis: A Review of Accuracies of Elisa, Interferon-Gamma Assay and Faecal Culture Techniques. Vet. Microbiol. 2008, 129, 217–235. [Google Scholar] [CrossRef]
- Hosseiniporgham, S.; Cubeddu, T.; Rocca, S.; Sechi, L.A. Identification of Mycobacterium avium Subsp. Paratuberculosis (Map) in Sheep Milk, a Zoonotic Problem. Microorganisms 2020, 8, 1264. [Google Scholar] [CrossRef]
- OIE. Principles and Methods of Validation of Diagnostic Assays for Infectious. In Manual of Diagnstic Tests and Vaccines for Terrestrial Animals; World Organization for Animal Health: Paris, France, 2018; pp. 72–87. [Google Scholar]
- Manning, E.J.B.; Collins, M.T. Mycobacterium avium Subsp. Paratuberculosis: Pathogen, Pathogenesis and Diagnosis. Rev. Sci. Tech. 2001, 20, 133–150. [Google Scholar] [CrossRef]
- Butot, S.; Ricchi, M.; Sevilla, I.A.; Michot, L.; Molina, E.; Tello, M.; Russo, S.; Arrigoni, N.; Garrido, J.M.; Tomas, D. Estimation of Performance Characteristics of Analytical Methods for Mycobacterium avium Subsp. Paratuberculosis Detection in Dairy Products. Front. Microbiol. 2019, 10, 509. [Google Scholar] [CrossRef]
- Pavlik, I. Parallel Faecal and Organ Mycobacterium avium Subsp. Paratuberculosis Culture of Different Productivity Types of Cattle. Vet. Microbiol. 2000, 77, 309–324. [Google Scholar] [CrossRef]
- Reddacliff, L.A.; Whittington, R.J. Experimental Infection of Weaner Sheep with S Strain Mycobacterium avium Subsp. Paratuberculosis. Vet. Microbiol. 2003, 96, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Whittington, R.J.; Marsh, I.; Turner, M.J.; McAllister, S.; Choy, E.; Eamens, G.J.; Marshall, D.J.; Ottaway, S. Rapid Detection of Mycobacterium Paratuberculosis in Clinical Samples from Ruminants and in Spiked Environmental Samples by Modified Bactec 12b Radiometric Culture and Direct Confirmation by Is900 Pcr. J. Clin. Microbiol. 1998, 36, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Whittington, R.J.; Marsh, I.; McAllister, S.; Turner, M.J.; Marshall, D.J.; Fraser, C.A. Evaluation of Modified Bactec 12b Radiometric Medium and Solid Media for Culture of Mycobacterium avium Subsp. Paratuberculosis from Sheep. J. Clin. Microbiol. 1999, 37, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- de Juan, L.; Alvarez, J.; Aranaz, A.; Rodriguez, A.; Romero, B.; Bezos, J.; Mateos, A.; Dominguez, L. Molecular Epidemiology of Types I/Iii Strains of Mycobacterium avium Subspecies Paratuberculosis Isolated from Goats and Cattle. Vet. Microbiol. 2006, 115, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Sonawane, G.G.; Tripathi, B.N. Comparative Evaluation of Diagnostic Tests for the Detection of Mycobacterium avium Subsp. Paratuberculosis in the Tissues of Sheep Affected with Distinct Pathology of Paratuberculosis. Int. J. Mycobacteriol. 2016, 5, S88–S89. [Google Scholar] [CrossRef]
- Ibrahim, A.; El Sanousi, S.; Aradaib, I. Detection of Mycobacterium avium Subspecies Paratuberculosis Using Nested Polymerase Chain Reaction (Npcr). Veterinarski Arhiv. 2004, 74, 27–35. [Google Scholar]
- Rachlin, J.; Ding, C.; Cantor, C.; Kasif, S. Computational Tradeoffs in Multiplex Pcr Assay Design for Snp Genotyping. BMC Genom. 2005, 6, 102. [Google Scholar] [CrossRef] [PubMed]
- Rajeev, S.; Zhang, Y.; Sreevatsan, S.; Motiwala, A.S.; Byrum, B. Evaluation of Multiple Genomic Targets for Identification and Confirmation of Mycobacterium avium Subsp. Paratuberculosis Isolates Using Real-Time Pcr. Vet. Microbiol. 2005, 105, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Stephan, R.; Schumacher, S.; Tasara, T.; Grant, I.R. Prevalence of Mycobacterium avium Subspecies Paratuberculosis in Swiss Raw Milk Cheeses Collected at the Retail Level. J. Dairy Sci. 2007, 90, 3590–3595. [Google Scholar] [CrossRef] [PubMed]
- Slana, I.; Paolicchi, F.; Janstova, B.; Navratilova, P.; Pavlik, I. Detection Methods for Mycobacterium avium Subsp Paratuberculosis in Milk and Milk Products: A Review. Veterinární Med. 2008, 53, 283–306. [Google Scholar] [CrossRef]
- Sange, M.D.; Becker, A.; Hassan, A.A.; Bulte, M.; Ganter, M.; Siebert, U.; Abdulmawjood, A. Development and Validation of a Loop-Mediated Isothermal Amplification Assay—A Rapid and Sensitive Detection Tool for Mycobacterium avium Subsp. Paratuberculosis in Small Ruminants. J. Appl. Microbiol. 2019, 127, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Sevilla, I.; Singh, S.V.; Garrido, J.M.; Aduriz, G.; Rodriguez, S.; Geijo, M.V.; Whittington, R.J.; Saunders, V.; Whitlock, R.H.; Juste, R.A. Pcr-Rea Genotype of Paratuberculosis Strains Isolated from Different Host Species and Geographical Locations. Rev. Sci. Tech. 2005, 24, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Whittington, R.J.; Eamens, G.J.; Cousins, D.V. Specificity of Absorbed Elisa and Agar Gel Immuno-Diffusion Tests for Paratuberculosis in Goats with Observations About Use of These Tests in Infected Goats. Aust. Vet. J. 2003, 81, 71–75. [Google Scholar] [CrossRef]
- Kumar, V.; Bhatia, A.K.; Singh, S.V. Evaluation of Efficacy of the Species Specific Antigens in the Diagnosis of Ovine and Caprine Paratuberculosis Using Plate Elisa. J. Immunol. Immunopathol. 2006, 8, 48–53. [Google Scholar]
- Singh, S.V.; Singh, A.V.; Singh, P.K.; Sohal, J.S.; Singh, N.P. Evaluation of an Indigenous Elisa for Diagnosis of Johne’s Disease and Its Comparison with Commercial Kits. Indian J. Microbiol. 2007, 47, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Hope, A.F.; Kluver, P.F.; Jones, S.L.; Condron, R.J. Sensitivity and Specificity of Two Serological Tests for the Detection of Ovine Paratuberculosis. Aust. Vet. J. 2000, 78, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Gwozdz, J.M.; Thompson, K.G.; Manktelow, B.W.; Murray, A.; West, D.M. Vaccination against Paratuberculosis of Lambs Already Infected Experimentally with Mycobacterium avium Subspecies Paratuberculosis. Aust. Vet. J. 2000, 78, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Sergeant, E.S.; Marshall, D.J.; Eamens, G.J.; Kearns, C.; Whittington, R.J. Evaluation of an Absorbed Elisa and an Agar-Gel Immuno-Diffusion Test for Ovine Paratuberculosis in Sheep in Australia. Prev. Vet. Med. 2003, 61, 235–248. [Google Scholar] [CrossRef]
- Robbe-Austerman, S.; Gardner, I.A.; Thomsen, B.V.; Morrical, D.G.; Martin, B.M.; Palmer, M.V.; Thoen, C.O.; Ewing, C. Sensitivity and Specificity of the Agar-Gel-Immunodiffusion Test, Elisa and the Skin Test for Detection of Paratuberculosis in United States Midwest Sheep Populations. Vet. Res. 2006, 37, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, I.; Cipolini, F.; Wigdorovitz, A.; Trono, K.; Barrandeguy, M.E. The Efficacy of Elisa Commercial Kits for the Screening of Equine Infectious Anemia Virus Infection. Rev. Argent. Microbiol. 2015, 47, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Kaba, J.; Gerlach, G.F.; Nowicki, M.; Rypuła, K. Agreement between Elisa and Complement Fixation Test Used for Diagnosing of Paratuberculosis in Goats. Pol. J. Vet. Sci. 2008, 11, 209–212. [Google Scholar] [PubMed]
- Maroudam, V.; Subramanian, B.M.; Kumar, P.P.; Raj, G.D. Paratuberculosis: Diagnostic Methods and Their Constraints. J. Vet. Sci. Technol. 2015, 6, 4172. [Google Scholar]
- Sherman, D.M.; Gay, J.M.; Bouley, D.S.; Nelson, G.H. Comparison of the Complement-Fixation and Agar Gel Immunodiffusion Tests for Diagnosis of Subclinical Bovine Paratuberculosis. Am. J. Vet. Res. 1990, 51, 461–465. [Google Scholar]
- Sockett, D.C.; Conrad, T.A.; Thomas, C.B.; Collins, M.T. Evaluation of Four Serological Tests for Bovine Paratuberculosis. J. Clin. Microbiol. 1992, 30, 1134–1139. [Google Scholar] [CrossRef]
- Reichel, M.P.; Kittelberger, R.; Penrose, M.E.; Meynell, R.M.; Cousins, D.; Ellis, T.; Mutharia, L.M.; Sugden, E.A.; Johns, A.H.; de Lisle, G.W. Comparison of Serological Tests and Faecal Culture for the Detection of Mycobacterium avium Subsp. Paratuberculosis Infection in Cattle and Analysis of the Antigens Involved. Vet. Microbiol. 1999, 66, 135–150. [Google Scholar] [CrossRef]
- Singh, S.V.; Singh, A.V.; Singh, R.; Sandhu, K.S.; Gupta, V.K. Survey of Ruminant Population of Northern India for the Incidence of Mycobacterium avium Subsp. Paratuberculosis Infection. In Proceedings of the Eighth International Colloquium on Paratuberculosis, Denmark, Copenhagen, 14–17 August 2005. [Google Scholar]
- Kalis, C.H.; Barkema, H.W.; Hesselink, J.W.; van Maanen, C.; Collins, M.T. Evaluation of Two Absorbed Enzyme-Linked Immunosorbent Assays and a Complement Fixation Test as Replacements for Fecal Culture in the Detection of Cows Shedding Mycobacterium avium Subspecies Paratuberculosis. J. Vet. Diagn. Investig. 2002, 14, 219–224. [Google Scholar] [CrossRef]
- Barad, D.B.; Chandel, B.S.; Dadawala, A.I.; Chauhan, H.C.; Kher, H.S.; Shroff, S.; Bhagat, A.G.; Singh, S.V.; Singh, P.K.; Singh, A.V.; et al. Incidence of Mycobacterium avium Subspecies Paratuberculosis in Mehsani and Surti Goats of Indian Origin Using Multiple Diagnostic Tests. J. Biol. Sci. 2014, 14, 124–133. [Google Scholar] [CrossRef][Green Version]
- Mukartal, S.Y.; Rathnamma, D.; Narayanaswamy, H.D.; Isloor, S.; Singh, S.V.; Chandranaik, B.M.; Shambanna, M.S. Prevalence of Ovine Johne’s Disease in Bannur Breed of Sheep in Organized Farm Using Multiple Diagnostic Tests. Adv. Anim. Vet. Sci. 2016, 4, 506–512. [Google Scholar] [CrossRef]
- Biswal, S.; Rath, A.P.; Singh, S.V.; Sahoo, N. Detection of Mycobacterium avium Subsp. Paratuberculosis. Indian J. Anim. Res. 2020, 54, 709–715. [Google Scholar]
- Bastida, F.; Juste, R.A. Paratuberculosis Control: A Review with a Focus on Vaccination. J. Immune Based Ther. Vaccines 2011, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Singh, S.V.; Singh, M.; Chaubey, K.K.; Karthik, K.; Bhatia, A.K.; Kumar, N.; Dhama, K. Vaccine Approaches for the ’Therapeutic Management’ of Mycobacterium avium Subspecies Paratuberculosis Infection in Domestic Livestock. Vet. Q. 2019, 39, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, R.W. Transmission of Paratuberculosis. Vet. Clin. N. Am. Food Anim. Pract. 1996, 12, 305–312. [Google Scholar] [CrossRef]
- Windsor, P.A.; Whittington, R.J. Evidence for Age Susceptibility of Cattle to Johne’s Disease. Vet. J. 2010, 184, 37–44. [Google Scholar] [CrossRef]
- Windsor, C.; Jääskelä, J.P.; Finlay, R. Housing Wealth Effects: Evidence from an Australian Panel. Economica 2015, 82, 552–577. [Google Scholar] [CrossRef]
- Kudahl, A.B.; Sorensen, J.T.; Nielsen, S.S.; Ostergaard, S. Simulated Economic Effects of Improving the Sensitivity of a Diagnostic Test in Paratuberculosis Control. Prev. Vet. Med. 2007, 78, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Dorshorst, N.C.; Collins, M.T.; Lombard, J.E. Decision Analysis Model for Paratuberculosis Control in Commercial Dairy Herds. Prev. Vet. Med. 2006, 75, 92–122. [Google Scholar] [CrossRef] [PubMed]
- Kirkeby, C.; Graesboll, K.; Nielsen, S.S.; Christiansen, L.E.; Toft, N.; Halasa, T. Adaptive Test Schemes for Control of Paratuberculosis in Dairy Cows. PLoS ONE 2016, 11, e0167219. [Google Scholar] [CrossRef]
- Reddacliff, L.; Eppleston, J.; Windsor, P.; Whittington, R.; Jones, S. Efficacy of a Killed Vaccine for the Control of Paratuberculosis in Australian Sheep Flocks. Vet. Microbiol. 2006, 115, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Fridriksdottir, V. Paratuberculosis in Iceland: Epidemiology and Control Measures, Past and Present. Vet. Microbiol. 2000, 77, 263–267. [Google Scholar] [CrossRef]
- Windsor, P.A. Understanding the Efficacy of Vaccination in Controlling Ovine Paratuberculosis. Small Rumin. Res. 2013, 110, 161–164. [Google Scholar] [CrossRef]
- Serrano, M.; Elguezabal, N.; Sevilla, I.A.; Geijo, M.V.; Molina, E.; Arrazuria, R.; Urkitza, A.; Jones, G.J.; Vordermeier, M.; Garrido, J.M.; et al. Tuberculosis Detection in Paratuberculosis Vaccinated Calves: New Alternatives against Interference. PLoS ONE 2017, 12, e0169735. [Google Scholar]
- Dhand, N.K.; Eppleston, J.; Whittington, R.J.; Windsor, P.A. Changes in Prevalence of Ovine Paratuberculosis Following Vaccination with Gudair(R): Results of a Longitudinal Study Conducted over a Decade. Vaccine 2016, 34, 5107–5113. [Google Scholar] [CrossRef] [PubMed]
- Gautam, M.; Anderson, P.; Ridler, A.; Wilson, P.; Heuer, C. Economic Cost of Ovine Johne’s Disease in Clinically Affected New Zealand Flocks and Benefit-Cost of Vaccination. Vet. Sci. 2018, 5, 16. [Google Scholar] [CrossRef]
- Espinosa, J.; Fernandez, M.; Royo, M.; Grau, A.; Collazos, J.A.; Benavides, J.; Ferreras, M.d.; Minguez, O.; Perez, V. Influence of Vaccination against Paratuberculosis on the Diagnosis of Caprine Tuberculosis During Official Eradication Programmes in Castilla Y Leon (Spain). Transbound. Emerg. Dis. 2021, 68, 692–703. [Google Scholar] [CrossRef]
- Singh, S.V.; Saurabh, G.; Chaubey, K.K.; Saket, B.; Rawat, K.D.; Naveen, K.; Tiwari, H.A.; Vinay, C.; Sohal, J.S.; Kuldeep, D.; et al. ‘Therapeutic Management’ of Incurable Paratuberculosis Using ‘indigenous Vaccine’ in Goatherds, Endemically Infected with Johne’s Disease. Int. J. Pharmacol. 2017, 13, 145–155. [Google Scholar] [CrossRef]
- Luttikholt, S.; Lievaart-Peterson, K.; Gonggrijp, M.; Aalberts, M.; van Schaik, G.; Vellema, P. Mycobacterium avium Subsp. Paratuberculosis Elisa Responses in Milk Samples from Vaccinated and Nonvaccinated Dairy Goat Herds in The Netherlands. Vet. Sci. 2019, 6, 58. [Google Scholar] [CrossRef] [PubMed]
- Windsor, P. Research into Vaccination against Ovine Johne’s Disease in Australia. Small Rumin. Res. 2006, 62, 139–142. [Google Scholar]
- Reddacliff, L.A. Field Evaluation of Ojd Control Using Gudair; Meat and Livestock Australia Ltd.: North Sydney, Australia, 2005. [Google Scholar]
- Koets, A.; Hoek, A.; Langelaar, M.; Overdijk, M.; Santema, W.; Franken, P.; Eden, W.; Rutten, V. Mycobacterial 70 Kd Heat-Shock Protein Is an Effective Subunit Vaccine against Bovine Paratuberculosis. Vaccine 2006, 24, 2550–2559. [Google Scholar] [CrossRef] [PubMed]
- Roupie, V.; Leroy, B.; Rosseels, V.; Piersoel, V.N.-G.I.; Romano, M.; Huygen, K. Immunogenicity and Protective Efficacy of DNA Vaccines Encoding Map0586c and Map4308c of Mycobacterium avium Subsp. Paratuberculosis Secretome. Vaccine 2008, 26, 4783–4794. [Google Scholar] [CrossRef] [PubMed]
- Juste, R.A.; Garrido, J.M.; Elguezabal, N.; Sevilla, I.A. Paratuberculosis Vaccines and Vaccination. In Paratuberculosis: Organism, Disease, Control; Marcel, A., Behr, M.A., Stevenson, K., Kapur, K., Eds.; CABI International: Wallingford, UK, 2020; pp. 365–379. [Google Scholar]
- Pant, S.D.; Schenkel, F.S.; Verschoor, C.P.; You, Q.; Kelton, D.F.; Moore, S.S.; Karrow, N.A. A Principal Component Regression Based Genome Wide Analysis Approach Reveals the Presence of a Novel Qtl on Bta7 for Map Resistance in Holstein Cattle. Genomics 2010, 95, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, B.W.; Shook, G.E. Genetic Susceptibility to Paratuberculosis. Vet. Clin. N. Am. Food Anim. Pract. 2011, 27, 559–571. [Google Scholar] [CrossRef] [PubMed]
| No. of Studies | Total No. of Animals or Samples | Smears from Faeces or Tissue/Inclusion Criteria | PCR/Inclusion Criteria | Real-Time PCR | ELISA | AGID/Inclusion Criteria | Culture | Histopathology | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 479 sheep 260 goats | 4/5 AGID +ve | 11 (2.3%) sheep 1 (0%) goat | 5/12 ELISA +ve | 2 sheep +ve in 3 tests | [61] | |||
| 2 | 219 goats | 9.2% (7/76) | 12.5% (5/40) | 43.3% (95/219) | 10% (24/219) | [130] | |||
| 3 | 200 sera from goats | 14/50(28.0%) (strong reactors in ELISA) | 1/14 (7.14%) +ve faecal smears and 6/50 (12.0%) strong ELISA +ve. | 63.5% | [66] | ||||
| 4 | 30 sheep | 4 (13.3%) faeces, 19 (63.3%) tissues, 7 (23.3%) blood | 3 (10.0%) | 2 (6.7%) faeces, 6 (20.0%) tissues | 21 (70.0%) | [87] | |||
| 5 | 66 slaughtered goats | 9 (13.63%) tissue | 9 (13.63%) | [89] | |||||
| 6 | 130 (8.7%) suspected small ruminants | 62 (47.7%) faeces | 25 (65.8%)/38 +ve faecal smears | [85] | |||||
| 7 | 192 goats | 21 (10.9%; 7.3–16.1%) | [63] | ||||||
| 8 | 168 sheep (farm 1), 112 sheep (farm 2) | 30 (60.0%), 5 (10.0%) | 24 (35.2%) 6 (50.0%) | 38 (76.0%) 7 (14.0%) | [131] | ||||
| 9 | 121 serum samples 16 pooled faecal samples | 2/16 faecal samples | 11/23 (9%) ELISA strong +ve | 23/121 (19.01%)/strong +ve 85/121 (70.25%) +ve | [132] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Idris, S.M.; Eltom, K.H.; Okuni, J.B.; Ojok, L.; Elmagzoub, W.A.; El Wahed, A.A.; Eltayeb, E.; Gameel, A.A. Paratuberculosis: The Hidden Killer of Small Ruminants. Animals 2022, 12, 12. https://doi.org/10.3390/ani12010012
Idris SM, Eltom KH, Okuni JB, Ojok L, Elmagzoub WA, El Wahed AA, Eltayeb E, Gameel AA. Paratuberculosis: The Hidden Killer of Small Ruminants. Animals. 2022; 12(1):12. https://doi.org/10.3390/ani12010012
Chicago/Turabian StyleIdris, Sanaa M., Kamal H. Eltom, Julius B. Okuni, Lonzy Ojok, Wisal A. Elmagzoub, Ahmed Abd El Wahed, ElSagad Eltayeb, and Ahmed A. Gameel. 2022. "Paratuberculosis: The Hidden Killer of Small Ruminants" Animals 12, no. 1: 12. https://doi.org/10.3390/ani12010012
APA StyleIdris, S. M., Eltom, K. H., Okuni, J. B., Ojok, L., Elmagzoub, W. A., El Wahed, A. A., Eltayeb, E., & Gameel, A. A. (2022). Paratuberculosis: The Hidden Killer of Small Ruminants. Animals, 12(1), 12. https://doi.org/10.3390/ani12010012







