Insights into the Genetic Evolution of Duck Hepatitis A Virus in Egypt
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Samples Collection, Virus Detection and Isolation
2.3. Complete Genome Sequencing
2.4. Genetic and Phylogenetic Analyses
2.5. Detection of Putative Recombination and Selective Pressure
2.6. Histopathology
3. Results
3.1. Virus Screening and Isolation
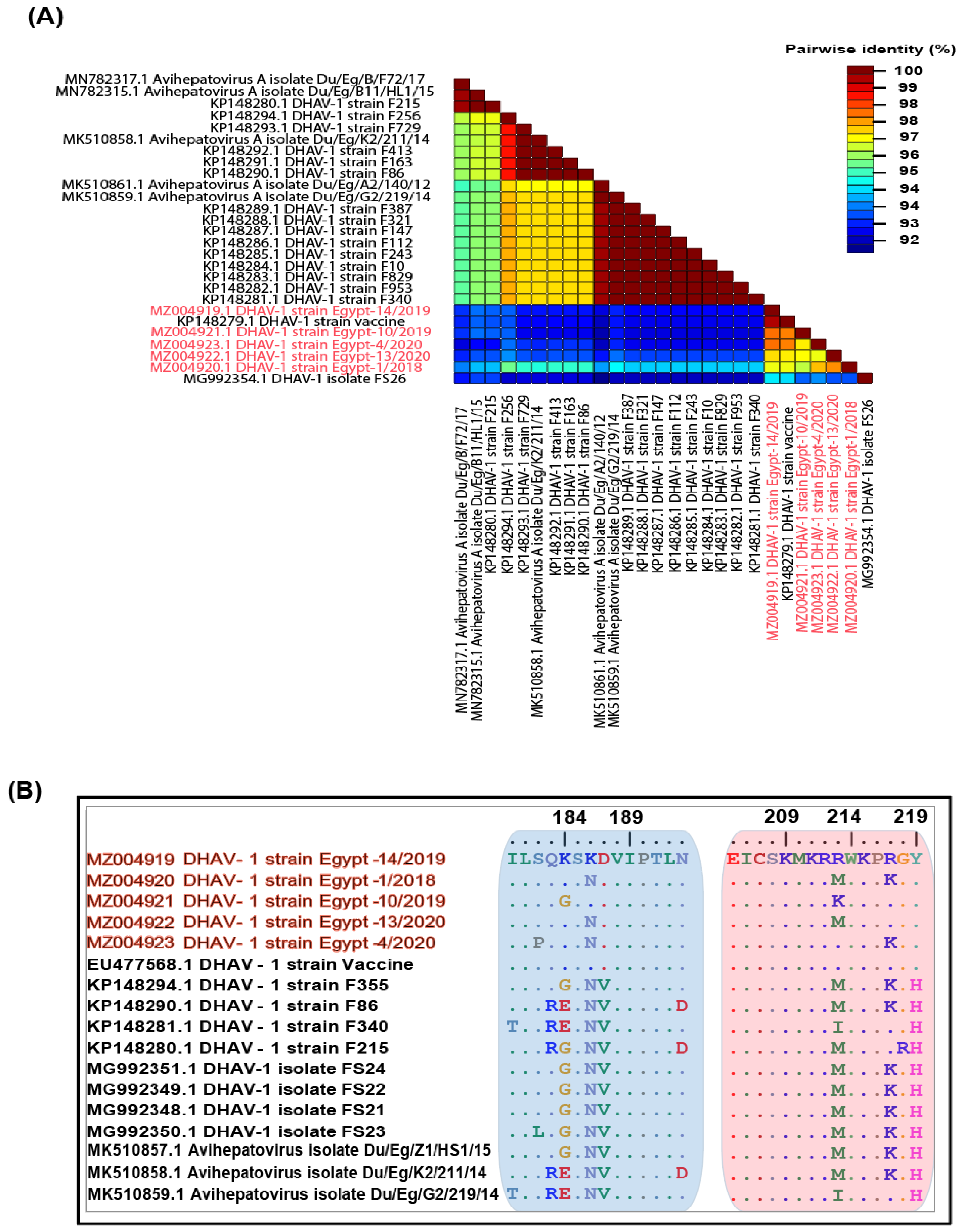
3.2. Genetic Characterisation of Complete Genomes
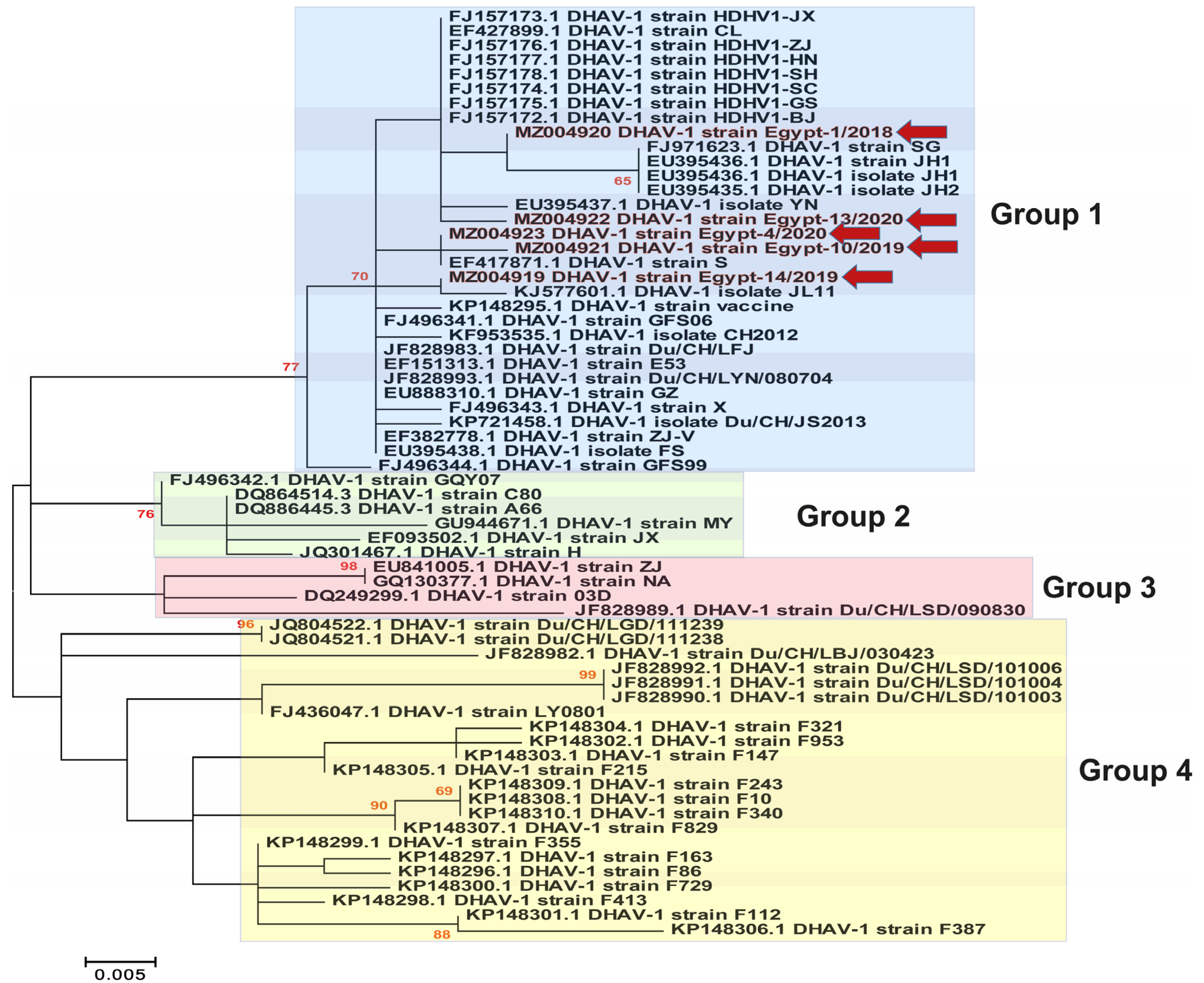
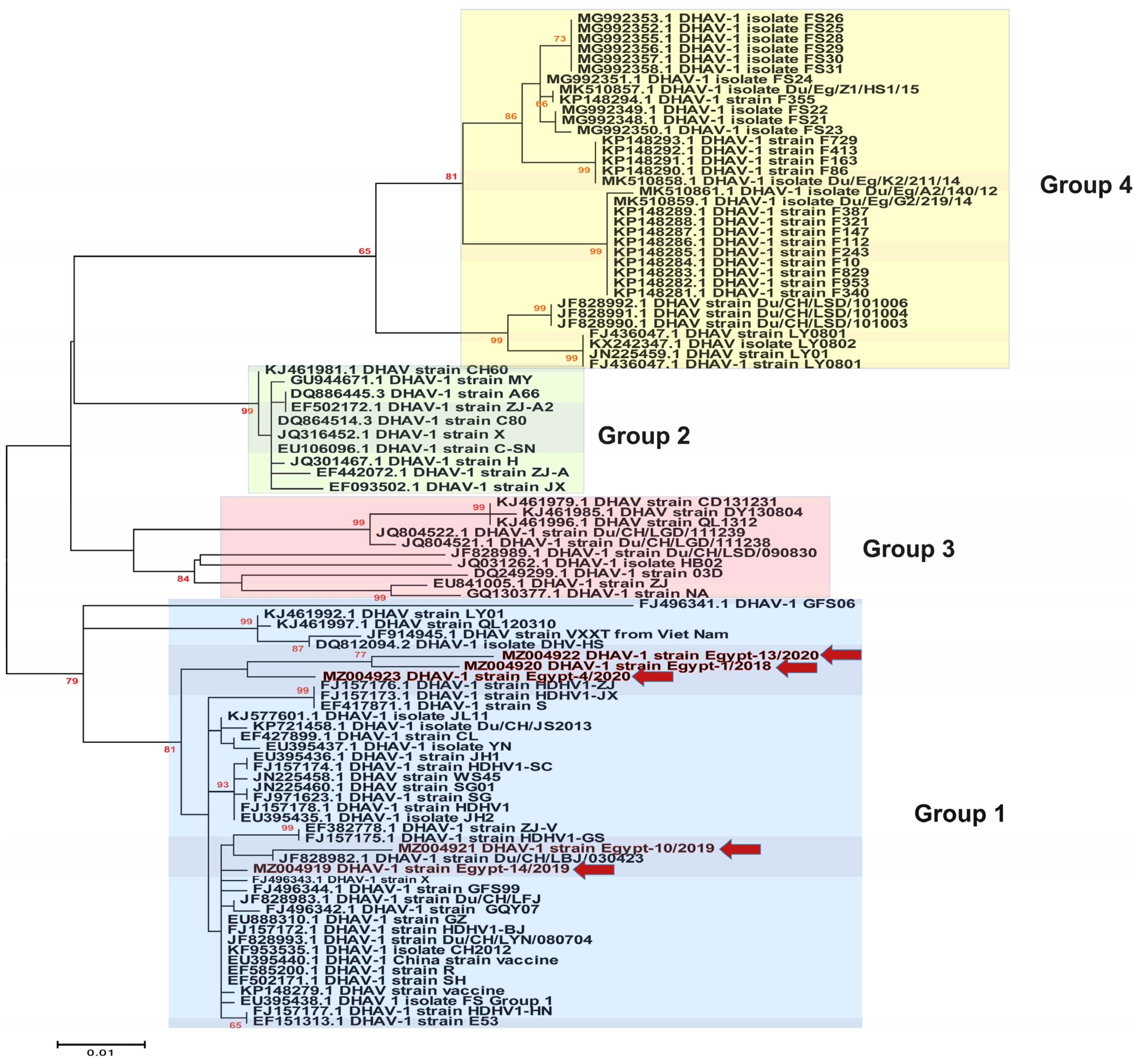
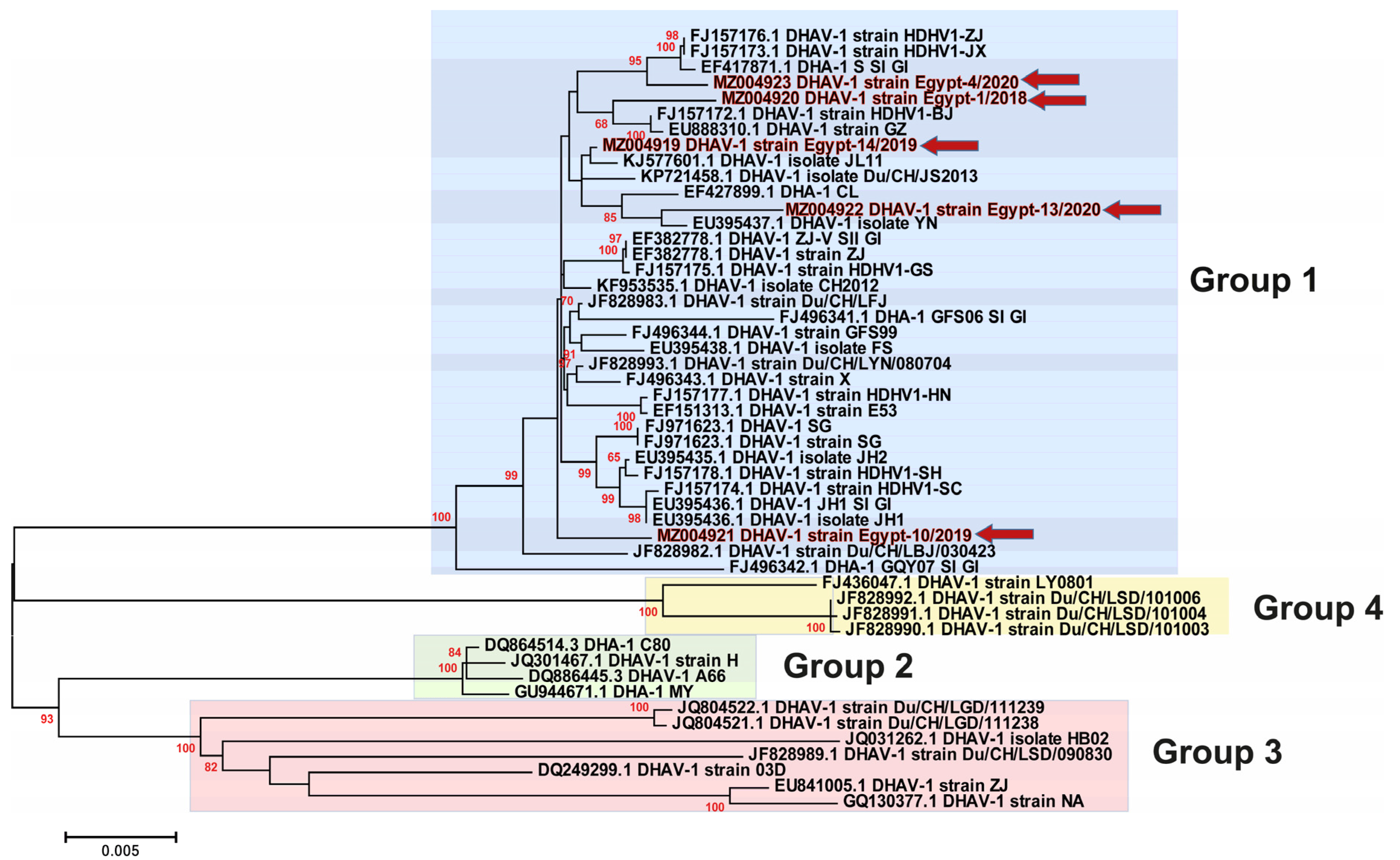
3.3. Phylogenetic Analyses
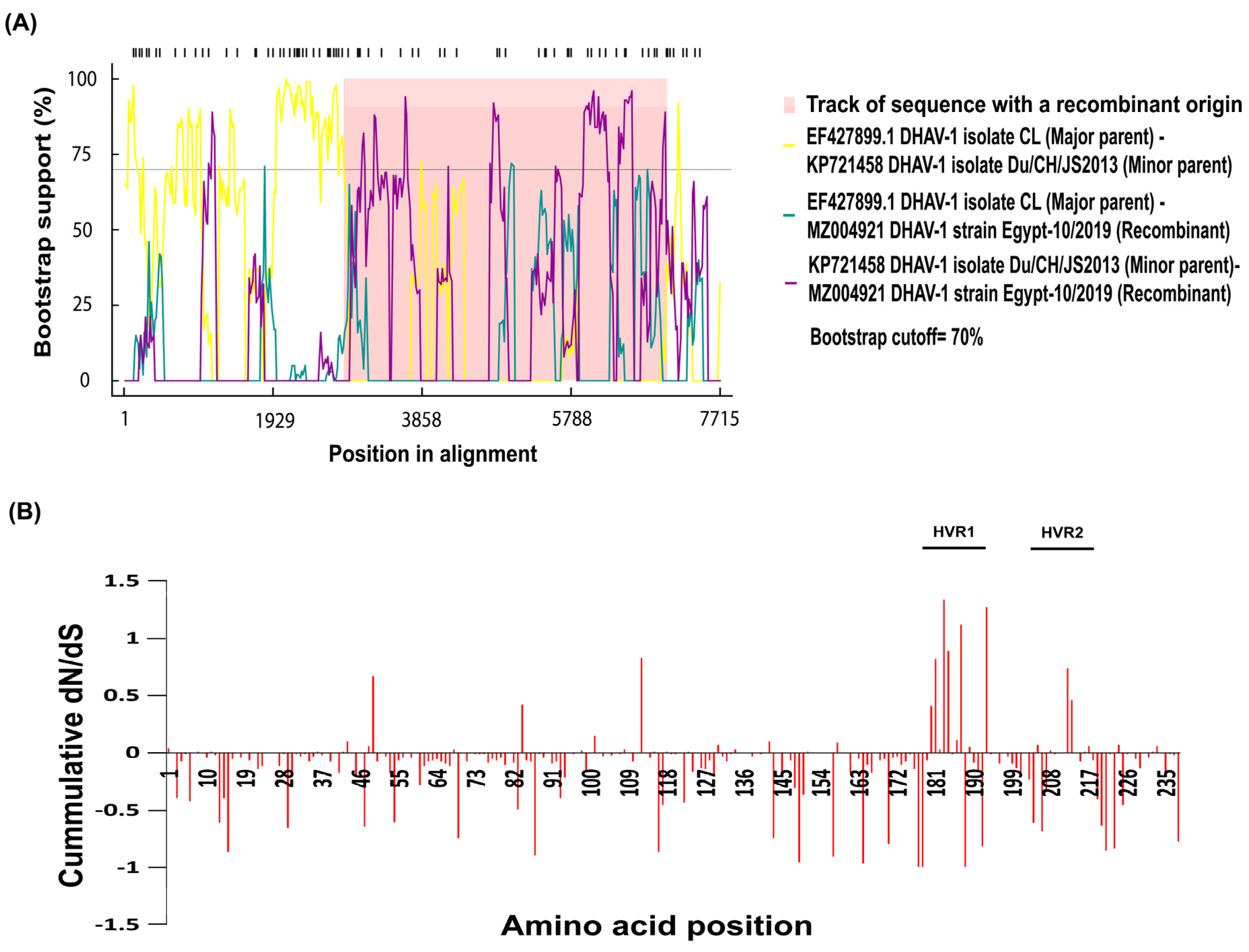
3.4. Recombination and Selective Pressure Analyses
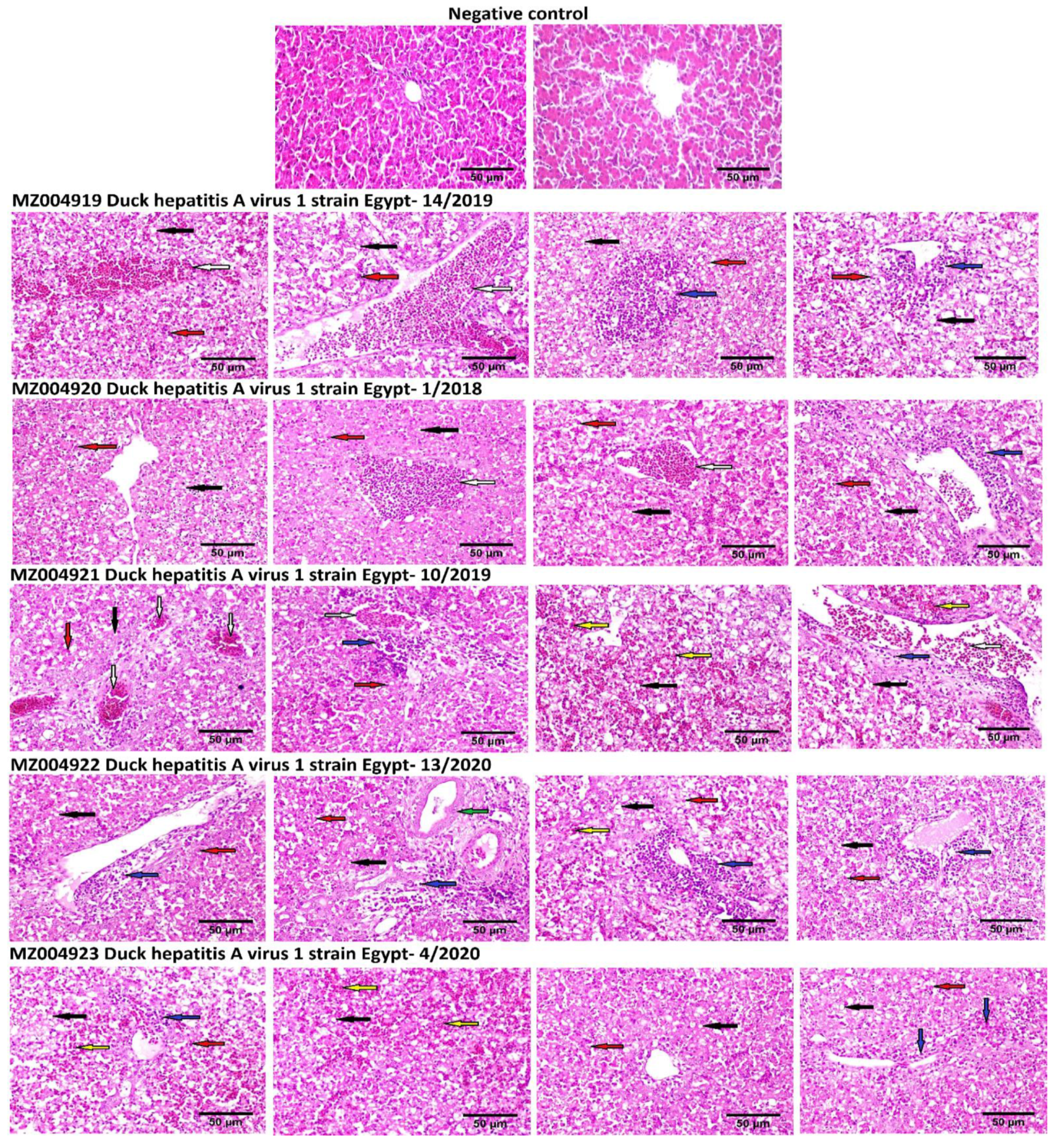
3.5. Histological Examinations
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Woolcock, P.R. Viral infections of waterfowl; duck hepatitis. In Diseases of Poultry, 12th Ed.; Saif, Y.M., Barnes, H.J., Glisson, J.R., Fadly, A.M., McDougald, L.R., Swayne, D.E., Eds.; Wiley: Blackwell, IA, USA, 2008; pp. 373–384. [Google Scholar]
- Kim, M.C.; Kwon, Y.K.; Joh, S.J.; Kim, S.J.; Tolf, C.; Kim, J.H.; Sung, H.W.; Lindberg, A.M.; Kwon, J.H. Recent Korean isolates of duck hepatitis virus reveal the presence of a new geno- and serotype when compared to duck hepatitis virus type 1-type strains. Arch. Virol. 2007, 152, 2059–2072. [Google Scholar] [CrossRef]
- Li, J.; Bi, Y.; Chen, C.; Yang, L.; Ding, C.; Liu, W. Genetic characterization of Duck Hepatitis A Viruses isolated in China. Virus Res. 2013, 178, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.Y.; Su, S.; Huang, Z.; Zhu, W.J.; Chen, J.D.; Zhao, F.R.; Wang, Y.J.; Xie, J.X.; Wang, H.; Zhang, G. Complete genome sequence of a novel duck hepatitis A virus discovered in southern China. J. Virol. 2012, 86, 10247. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Yang, X.; Zhou, L.; Ge, X.; Guo, X.; Liu, J.; Zhang, D.; Yang, H. Duck hepatitis A virus possesses a distinct type IV internal ribosome entry site element of picornavirus. J. Virol. 2012, 86, 1129–1144. [Google Scholar] [CrossRef]
- Wang, L.; Pan, M.; Fu, Y.; Zhang, D. Classification of duck hepatitis virus into three genotypes based on molecular evolutionary analysis. Virus Genes 2008, 37, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Chen, J.; Si, X.; Xie, Z.; Zhu, Y.; Zhang, X.; Wang, S.; Jiang, S. Genetic variation of the VP1 gene of the virulent duck hepatitis A virus type 1, DHAV-1) isolates in Shandong province of China. Virol. Sin. 2012, 27, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Zhang, R.; Chen, L.; Yang, L.; Li, J.; Dou, P.; Wang, H.; Xie, Z.; Wang, Y.; Jiang, S. Complete genome sequence of a duck hepatitis a virus type 3 identified in Eastern China. J. Virol. 2012, 86, 13848. [Google Scholar] [CrossRef][Green Version]
- Liu, G.; Wang, F.; Ni, Z.; Yun, T.; Yu, B.; Huang, J.; Chen, J. Genetic diversity of the VP1 gene of duck hepatitis virus type I, DHV-I) isolates from southeast China is related to isolate attenuation. Virus Res. 2008, 137, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, M.A.; Ayoub, M.N.K.; Reda, I.M. A study on a new isolate of duck hepatitis virus and its relationship to other duck hepatitis virus strains. Vet. Med. J. 1978, 26, 215–221. [Google Scholar]
- Ellakany, H.; El Sebai, A.H.; Sultan, H.; Sami, A.A. Control of experimental DHV infection by amantadine. In Proceedings of the 6th Scientific Veterinary Medical Conference of Zagazig University, Hurghada, Egypt, 7–9 September 2002; pp. 757–775. [Google Scholar]
- Erfan, A.M.; Selim, A.A.; Moursi, M.K.; Nasef, S.A.; Abdelwhab, E.M. Epidemiology and molecular characterisation of duck hepatitis A virus from different duck breeds in Egypt. Vet. Microbiol. 2015, 177, 347–352. [Google Scholar] [CrossRef]
- Hisham, I.; Ellakany, H.F.; Selim, A.A.; Abdalla, M.; Zain El-Abideen, M.A.; Kilany, W.H.; Ali, A.; Elbestawy, A.R. Comparative Pathogenicity of Duck Hepatitis A Virus-1 Isolates in Experimentally Infected Pekin and Muscovy Ducklings. Front. Vet. Sci. 2020, 7, 234. [Google Scholar] [CrossRef]
- Abd-Elhakim, M.A.; Thieme, O.; Schwabenbauer, K.; Ahmed, Z.A. Mapping traditional poultry hatcheries in Egypt. In AHBL—Promoting Strategies for Prevention and Control of HPAI; FAO: Rome, Italy, 2008. [Google Scholar]
- OIE. Duck Virus Hepatitis. OIE Terrestrial Manual 2010. Available online: https://brd.bsvgateway.org/document/1001/ (accessed on 31 December 2016).
- Shi, S.; Chen, H.; Chen, Z.; Fu, G.; Wan, C.; Huang, Y.; Lin, S.; Cheng, L.; Fu, Q.; Lin, J.; et al. Complete genome sequence of a duck hepatitis a virus 1 isolated from a pigeon in china. Genome Announc. 2013, 1, e00451–e00513. [Google Scholar] [CrossRef]
- Edgar, R.C.; Batzoglou, S. Multiple sequence alignment. Curr. Opin. Struct. Biol. 2006, 16, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Muhire, B.M.; Varsani, A.; Martin, D.P. SDT: A virus classification tool based on pairwise sequence alignment and identity calculation. PLoS ONE 2014, 9, e108277. [Google Scholar]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. PhyML 3.0: New Algorithms and Methods to Estimate Maximum-Likelihood Phylogenies: Assessing the Performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef]
- Martin, D.P.; Murrell, B.; Golden, M.; Khoosal, A.; Muhire, B. RDP4: Detection and analysis of recombination patterns in virus genomes. Virus Evol. 2015, 1, vev003. [Google Scholar] [CrossRef] [PubMed]
- Kryazhimskiy, S.; Plotkin, J.B. The population genetics of dN/dS. PLoS Genet. 2008, 4, e1000304. [Google Scholar] [CrossRef]
- Suvarna, S.K.; Layton, C.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques; Churchill Livingstone Elsevier: Oxford, UK, 2018. [Google Scholar]
- Mansour, S.M.G.; Mohamed, F.F.; El Bakrey, R.M.; Eid, A.A.M.; Mor, S.K.; Goyal, S.M. Outbreaks of Duck Hepatitis A Virus in Egyptian Duckling Flocks. Avian Dis. 2019, 63, 68–74. [Google Scholar] [CrossRef]
- Song, C.; Liao, Y.; Gao, W.; Yu, S.; Sun, Y.; Qiu, X. Virulent and attenuated strains of duck hepatitis a virus elicit discordant innate immune responses in vivo. J. Gen. Virol. 2014, 95, 2716–2726. [Google Scholar] [CrossRef]
- Su, Y.; Liu, C.; Liu, L.; Ye, C.H.; Cao, W.Q.; Huang, Y.Q.; Zheng, J.; Cai, D.Y.; Olowofeso, O. Studies on Genetic Variation of Different Chinese Duck Populations with Random Amplified Polymorphic DNA Analysis. Asian Australas. J. Anim. Sci. 2006, 19, 475–481. [Google Scholar]
- Guerin, J.L.; Noutary, V.; Boissieu, C.; Albaric, O.; Wyers, M. Viral pancreatitis and encephalitis of Muscovy ducklings. Vet. Record. 2005, 157, 328. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Qi, B.; Liu, R.; Jiang, X.; Lu, R.; Xiao, L.; Fu, G.; Fu, Q.; Shi, S.; Wan, C.; et al. Comparative pathogenicity of two subtypes, hepatitis-type and pancreatitis-type) of duck hepatitis A virus type 1 in experimentally infected Muscovy ducklings. Avian Pathol. 2019, 48, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Sheng, Z.; Huang, B.; Qi, L.; Li, Y.; Yu, K.; Liu, C.; Qin, Z.; Wang, D.; Song, M.; et al. Molecular Evolution and Genetic Analysis of the Major Capsid Protein VP1 of Duck Hepatitis A Viruses: Implications for Antigenic Stability. PLoS ONE. 2015, 10, e0132982. [Google Scholar]
- Jin, X.; Zhang, W.; Zhang, W.; Gu, C.; Cheng, G.; Hu, X. Identification and molecular analysis of the highly pathogenic duck hepatitis virus type 1 in Hubei province of China. Res. Vet. Sci. 2008, 85, 595–598. [Google Scholar] [CrossRef]

| Sample ID | Breed | Age (Days) | Governorate | Year | Virus Detection (RT-PCR) | Strain |
|---|---|---|---|---|---|---|
| 1 | Pekin | 5 | Giza | 2018 | Yes | DHAV-1 strain Egypt-1/2018 |
| 2 | Pekin | 4 | Monufia | 2018 | - | - |
| 3 | Mallard | 6 | Qalyubia | 2018 | - | - |
| 4 | Mallard | 5 | Monufia | 2020 | Yes | DHAV-1 strain Egypt-4/2020 |
| 5 | Pekin | 7 | Qalyubia | 2019 | - | - |
| 6 | Mallard | 6 | Giza | 2019 | - | - |
| 7 | Mallard | 9 | Faiyum | 2018 | - | - |
| 8 | Pekin | 8 | Giza | 2019 | - | - |
| 9 | Mallard | 10 | Gharbia | 2019 | - | - |
| 10 | Pekin | 4 | Giza | 2019 | Yes | DHAV-1 strain Egypt-10/2019 |
| 11 | Pekin | 3 | Giza | 2020 | - | - |
| 12 | Mallard | 10 | Monufia | 2019 | - | - |
| 13 | Pekin | 4 | Beni Suef | 2020 | Yes | DHAV-1 strain Egypt-13/2020 |
| 14 | Pekin | 4 | Faiyum | 2019 | Yes | DHAV-1 strain Egypt-14/2019 |
| 15 | Mallard | 9 | Beni Suef | 2019 | - | - |
| 16 | Mallard | 3 | Giza | 2020 | - | - |
| 17 | Pekin | 8 | Qalyubia | 2019 | - | - |
| 18 | Mallard | 5 | Faiyum | 2020 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rohaim, M.A.; Naggar, R.F.E.; AbdelSabour, M.A.; Ahmed, B.A.; Hamoud, M.M.; Ahmed, K.A.; Zahran, O.K.; Munir, M. Insights into the Genetic Evolution of Duck Hepatitis A Virus in Egypt. Animals 2021, 11, 2741. https://doi.org/10.3390/ani11092741
Rohaim MA, Naggar RFE, AbdelSabour MA, Ahmed BA, Hamoud MM, Ahmed KA, Zahran OK, Munir M. Insights into the Genetic Evolution of Duck Hepatitis A Virus in Egypt. Animals. 2021; 11(9):2741. https://doi.org/10.3390/ani11092741
Chicago/Turabian StyleRohaim, Mohammed A., Rania F. El Naggar, Mohammed A. AbdelSabour, Basem A. Ahmed, Mohamed M. Hamoud, Kawkab A. Ahmed, Osama K. Zahran, and Muhammad Munir. 2021. "Insights into the Genetic Evolution of Duck Hepatitis A Virus in Egypt" Animals 11, no. 9: 2741. https://doi.org/10.3390/ani11092741
APA StyleRohaim, M. A., Naggar, R. F. E., AbdelSabour, M. A., Ahmed, B. A., Hamoud, M. M., Ahmed, K. A., Zahran, O. K., & Munir, M. (2021). Insights into the Genetic Evolution of Duck Hepatitis A Virus in Egypt. Animals, 11(9), 2741. https://doi.org/10.3390/ani11092741









