The Resurrection of Mabrokan: Production of Multiple Cloned Offspring from Decade-Old Vitrified Tissue Collected from a Deceased Champion Show Camel
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Media
2.2. Oocyte Collection from Slaughterhouse Ovaries
2.3. Care and Management of Camel
2.4. Collection and Cryopreservation of Mabrokan Tissue
2.5. Establishment of Skin Fibroblast Cell Line
2.6. Somatic Cell Nuclear Transfer (SCNT)
2.7. Embryo Culture and Transfer to the Recipient
2.8. Pregnancy Diagnosis
2.9. Microsatellite Analysis
2.10. Statistical Analysis
3. Results
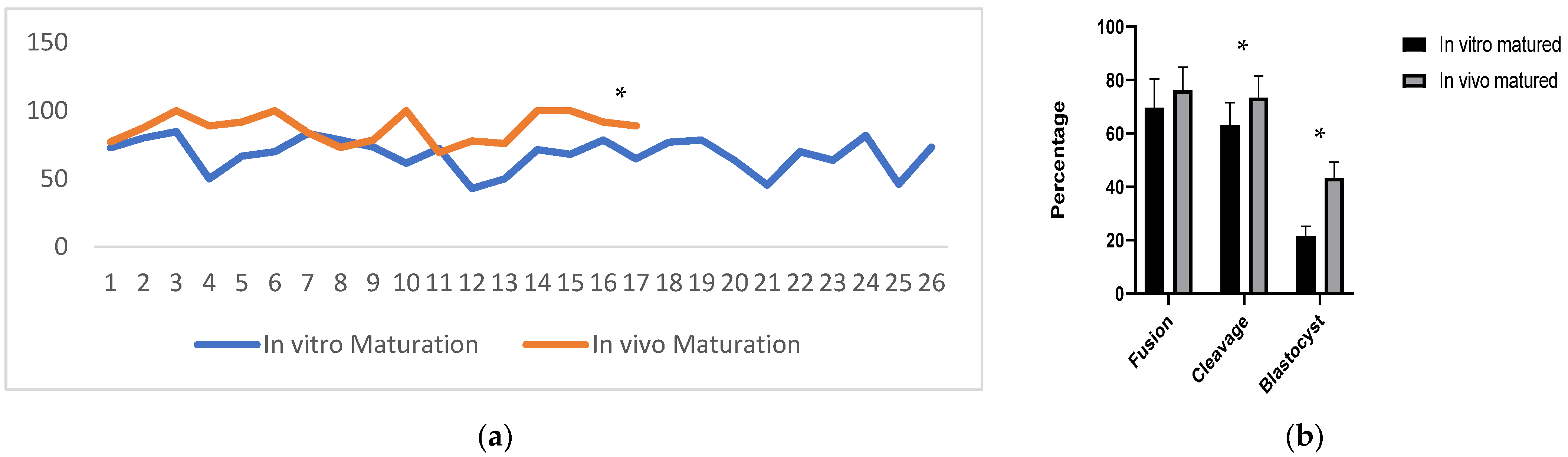
3.1. Effect of the Source of Oocytes on Oocyte Maturation
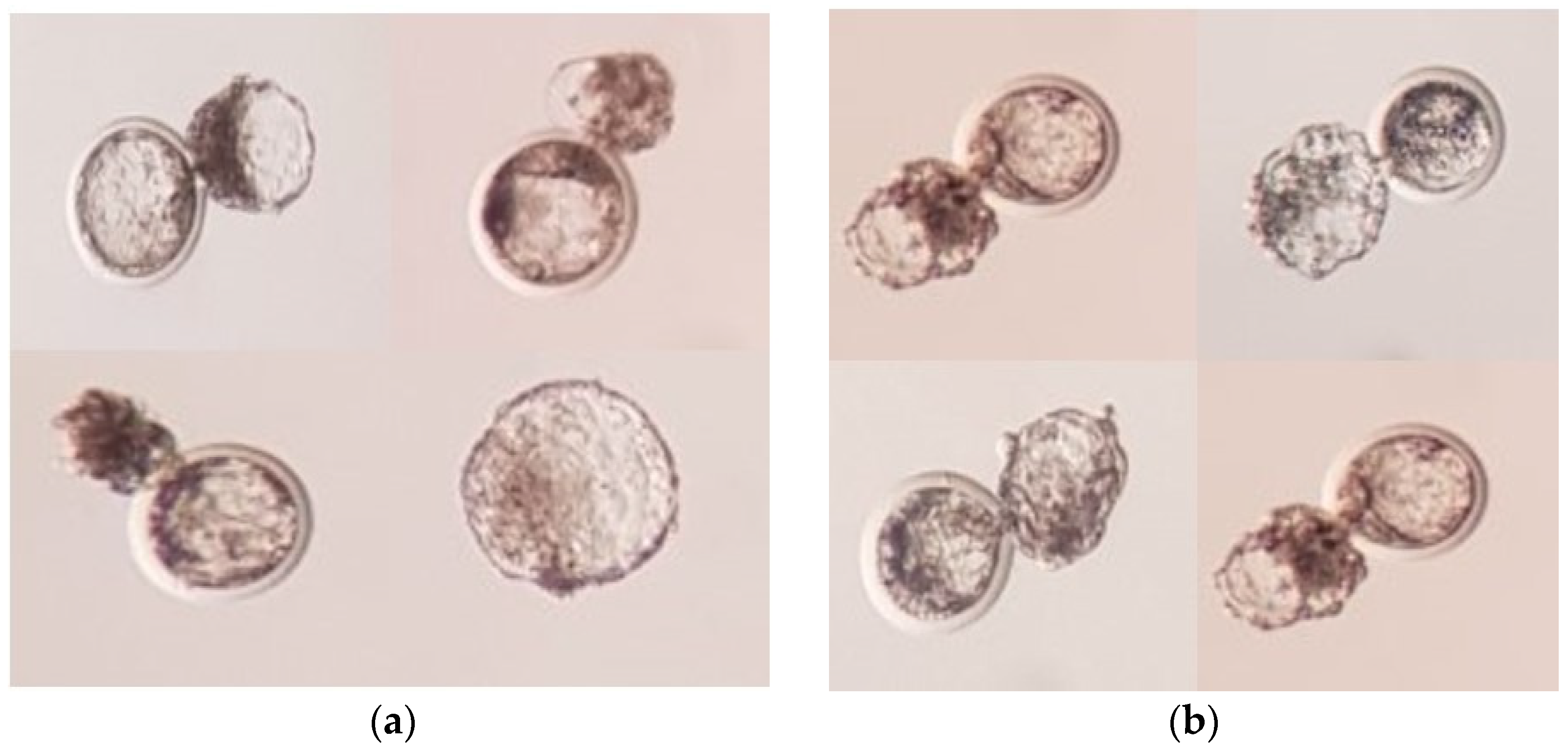
3.2. Developmental Competence of SCNT-Derived Embryos
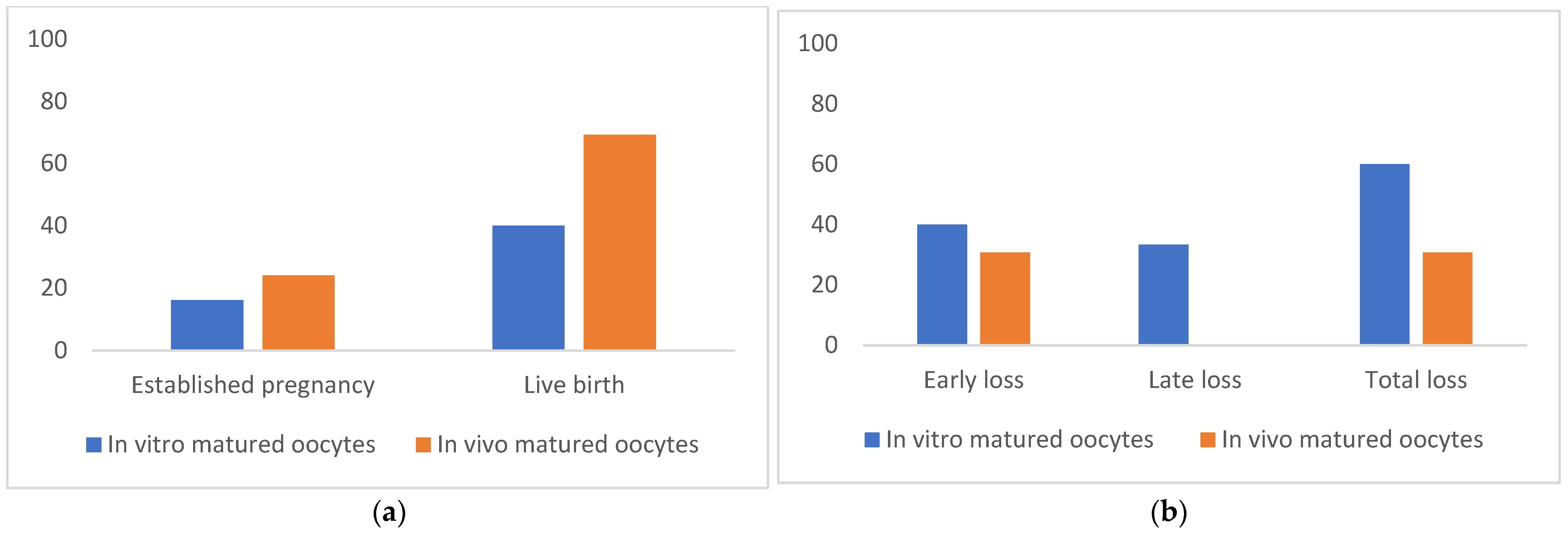
3.3. Efficiency of Pregnancy Rate and Parental Analysis of Cloned Camel
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Meaning |
| 6-DMAP | 6-dimethylaminopurine |
| BSA | Bovine serum albumin |
| CL | Corpus leutium |
| COC | Cumulus oocytes complex |
| DMEM | Dulbecco’s modified Eagle’s medium |
| DPBS | Dulbecco’s phosphate-buffered saline |
| EG | Ethylene glycol |
| FBS | Fetal bovine serum |
| NDC | Nuclear donor cell |
| SCNT | Somatic cell nuclear transfer |
References
- Wani, N.A.; Hong, S.; Vettical, B.S. Cytoplast source influences development of somatic cell nuclear transfer (SCNT) embryos in vitro but not their development to term after transfer to synchronized recipients in dromedary camels (Camelus dromedarius). Theriogenology 2018, 118, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Samiec, M.; Skrzyszowska, M. Preimplantation developmental capability of cloned pig embryos derived from different types of nuclear donor somatic cells. Ann. Anim. Sci. 2010, 10, 385–398. [Google Scholar]
- Son, Y.B.; Jeong, Y.I.; Jeong, Y.W.; Olsson, P.O.; Hossein, M.S.; Cai, L.; Kim, S.; Choi, E.J.; Sakaguchi, K.; Tinson, A.; et al. Development and pregnancy rates of Camelus dromedarius-cloned embryos derived from in vivo- and in vitro-matured oocytes. Anim. Biosci. 2021, in press. [Google Scholar] [CrossRef]
- Skrzyszowska, M.; Samiec, M. Generating Cloned Goats by Somatic Cell Nuclear Transfer-Molecular Determinants and Application to Transgenics and Biomedicine. Int. J. Mol. Sci. 2021, 22, 7490. [Google Scholar] [CrossRef] [PubMed]
- Moulavi, F.; Asadi-Moghadam, B.; Omidi, M.; Yarmohammadi, M.; Ozegovic, M.; Rastegar, A.; Hosseini, S.M. Pregnancy and calving rates of cloned dromedary camels produced by conventional and handmade cloning techniques and in vitro and in vivo matured oocytes. Mol. Biotechnol. 2020, 62, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Samiec, M.; Skrzyszowska, M.; Opiela, J. Creation of cloned pig embryos using contact-inhibited or serum-starved fibroblast cells analysed intra vitam for apoptosis occurrence. Ann. Anim. Sci. 2013, 13, 275–293. [Google Scholar] [CrossRef]
- Khatir, H.; Anouassi, A. Preliminary assessment of somatic cell nuclear transfer in the dromedary (Camelus dromedarius). Theriogenology 2008, 70, 1471–1477. [Google Scholar] [CrossRef] [PubMed]
- Samiec, M.; Skrzyszowska, M. Assessment of in vitro developmental capacity of porcine nuclear-transferred embryos reconstituted with cumulus oophorus cells undergoing vital diagnostics for apoptosis detection. Ann. Anim. Sci. 2013, 13, 513–529. [Google Scholar] [CrossRef]
- Wani, N.A.; Hong, S.B. Source, treatment and type of nuclear donor cells influences in vitro and in vivo development of embryos cloned by somatic cell nuclear transfer in camel (Camelus dromedarius). Theriogenology 2018, 106, 186–191. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, S.H.; Yoon, J.T. Identifying biomarkers of autophagy and apoptosis in transfected nuclear donor cells and transgenic cloned pig embryos. Ann. Anim. Sci. 2019, 19, 127–146. [Google Scholar] [CrossRef]
- Xu, L.; Song, S.H.; Idrees, M.; Mesalam, A.; Joo, M.D.; Sidrat, T.; Wei, Y.; Lee, K.L.; Lu, W.; Kong, I.K. Effects of Donor Cell Types on the Development of Bovine Embryos Using Cytoplasm Injection Cloning Technology. Int. J. Mol. Sci. 2021, 22, 5841. [Google Scholar] [CrossRef]
- Samiec, M.; Skrzyszowska, M.; Bochenek, M. In vitro development of porcine nuclear-transferred embryos derived from fibroblast cells analysed cytometrically for apoptosis incidence and accuracy of cell cycle synchronization at the G0/G1 stages. Ann. Anim. Sci. 2013, 13, 735–752. [Google Scholar] [CrossRef]
- Qu, J.; Sun, M.; Wang, X.; Song, X.; He, H.; Huan, Y. Melatonin Enhances the Development of Porcine Cloned Embryos by Improving DNA Methylation Reprogramming. Cell. Reprogram. 2020, 22, 156–166. [Google Scholar] [CrossRef]
- Opiela, J.; Samiec, M.; Bochenek, M.; Lipiński, D.; Romanek, J.; Wilczek, P. DNA aneuploidy in porcine bone marrow-derived mesenchymal stem cells undergoing osteogenic and adipogenic in vitro differentiation. Cell Reprogram. 2013, 15, 425–434. [Google Scholar] [CrossRef]
- Moulavi, F.; Hosseini, S.M. Development of a modified method of handmade cloning in dromedary camel. PLoS ONE 2019, 14, e0213737. [Google Scholar]
- Samiec, M.; Romanek, J.; Lipiński, D.; Opiela, J. Expression of pluripotency-related genes is highly dependent on trichostatin A-assisted epigenomic modulation of porcine mesenchymal stem cells analysed for apoptosis and subsequently used for generating cloned embryos. Anim. Sci. J. 2019, 90, 1127–1141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gao, S.; Liu, X. Advance in the Role of Epigenetic Reprogramming in Somatic Cell Nuclear Transfer-Mediated Embryonic Development. Stem Cells Int. 2021, 2021, 6681337. [Google Scholar] [CrossRef]
- Samiec, M.; Skrzyszowska, M. Can reprogramming of overall epigenetic memory and specific parental genomic imprinting memory within donor cell-inherited nuclear genome be a major hindrance for the somatic cell cloning of mammals?—A review. Ann. Anim. Sci. 2018, 18, 623–638. [Google Scholar] [CrossRef]
- Zhang, J.; Hao, L.; Wei, Q.; Zhang, S.; Cheng, H.; Zhai, Y.; Jiang, Y.; An, X.; Li, Z.; Zhang, X.; et al. TET3 overexpression facilitates DNA reprogramming and early development of bovine SCNT embryos. Reproduction 2020, 160, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Samiec, M. The role of mitochondrial genome (mtDNA) in somatic and embryo cloning of mammals. A review. J. Anim. Feed Sci. 2005, 14, 213–233. [Google Scholar] [CrossRef]
- Samiec, M. The effect of mitochondrial genome on architectural remodeling and epigenetic reprogramming of donor cell nuclei in mammalian nuclear transfer-derived embryos. J. Anim. Feed Sci. 2005, 14, 393–422. [Google Scholar] [CrossRef]
- Takeda, K. Functional consequences of mitochondrial mismatch in reconstituted embryos and offspring. J. Reprod. Dev. 2019, 65, 485–489. [Google Scholar] [CrossRef]
- Samiec, M.; Skrzyszowska, M. Extranuclear Inheritance of Mitochondrial Genome and Epigenetic Reprogrammability of Chromosomal Telomeres in Somatic Cell Cloning of Mammals. Int. J. Mol. Sci. 2021, 22, 3099. [Google Scholar] [CrossRef]
- Bebbere, D.; Ulbrich, S.E.; Giller, K.; Zakhartchenko, V.; Reichenbach, H.D.; Reichenbach, M.; Verma, P.J.; Wolf, E.; Ledda, S.; Hiendleder, S. Mitochondrial DNA Depletion in Granulosa Cell Derived Nuclear Transfer Tissues. Front. Cell Dev. Biol. 2021, 9, 664099. [Google Scholar] [CrossRef]
- Wani, N.A.; Wernery, U.; Skidmore, J. Production of first cloned camel by somatic cell nuclear transfer. Biol. Reprod. 2010, 82, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Olsson, P.O.; Tinson, A.H.; Al Shamsi, N.; Kuhad, K.S.; Singh, R.; Son, Y.B.; Jeong, Y.; Jeong, Y.W.; Cai, L.; Sakaguchi, K.; et al. Blastocyst formation, embryo transfer and breed comparison in the first reported large scale cloning of camels. Sci. Rep. 2021, 11, 14288. [Google Scholar] [CrossRef]
- Wani, N.A.; Vettical, B.S.; Hong, S.B. First cloned Bactrian camel (Camelus bactrianus) calf produced by interspecies somatic cell nuclear transfer: A step towards preserving the critically endangered wild Bactrian camels. PLoS ONE 2017, 12, e0177800. [Google Scholar] [CrossRef]
- Powell, A.M.; Talbot, N.C.; Wells, K.D.; Kerr, D.E.; Pursel, V.G.; Wall, R.J. Cell Donor Influences Success of Producing Cattle by Somatic Cell Nuclear Transfer. Biol. Reprod. 2004, 71, 210–216. [Google Scholar] [CrossRef]
- Wakayama, S.; Ohta, H.; Hikichi, T.; Mizutani, E.; Iwaki, T.; Kanagawa, O.; Wakayama, T. Production of healthy cloned mice from bodies frozen at -20 degrees C for 16 years. Proc. Natl. Acad. Sci. USA 2008, 105, 17318–17322. [Google Scholar] [CrossRef]
- Jeong, Y.; Olson, O.P.; Lian, C.; Lee, E.S.; Jeong, Y.W.; Hwang, W.S. Dog cloning from post-mortem tissue frozen without cryoprotectant. Cryobiology 2020, 97, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, Y.; Hayashi, N.; Taniguchi, S.; Kobayashi, N.; Sakai, K.; Otani, T.; Iritani, A.; Saeki, K. Resurrection of a bull by cloning from organs frozen without cryoprotectant in a −80 °C freezer for a decade. PLoS ONE 2009, 4, e4142. [Google Scholar] [CrossRef] [PubMed]
- Moulavi, F.; Hosseini, S.M.; Tanhaie-Vash, N.; Ostadhosseini, S.; Hosseini, S.H.; Hajinasrollah, M.; Asghari, M.H.; Gourabi, H.; Shahverdi, A.; Vosough, A.D.; et al. Interspecies somatic cell nuclear transfer in Asiatic cheetah using nuclei derived from post-mortem frozen tissue in absence of cryo-protectant and in vitro matured domestic cat oocytes. Theriogenology 2017, 90, 197–203. [Google Scholar] [CrossRef]
- Santos, R.R.; Tharasanit, T.; Van Haeften, T.; Figueiredo, J.R.; Silva, J.R.; Van den Hurk, R. Vitrification of goat preantral follicles enclosed in ovarian tissue by using conventional and solid-surface vitrification methods. Cell Tissue Res. 2007, 327, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Aguila, L.; Treulen, F.; Therrien, J.; Felmer, R.; Valdivia, M.; Smith, L.C. Oocyte selection for in vitro embryo production in bovine Species: Noninvasive approaches for new challenges of oocyte competence. Animals 2020, 10, 2196. [Google Scholar] [CrossRef] [PubMed]
- Brad, A.M.; Bormann, C.L.; Swain, J.E.; Durkin, R.E.; Johnson, A.E.; Clifford, A.L.; Krisher, R.L. Glutathione and adenosine triphosphate content of in vivo and in vitro matured porcine oocytes. Mol. Reprod. Dev. 2003, 64, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Hossein, M.S.; Oh, H.J.; Fibrianto, H.Y.; Jang, G.; Kim, H.J.; Hong, S.G.; Park, J.E.; Kang, S.K.; Lee, B.C. Glutathione content of in vivo and in vitro matured canine oocytes collected from different reproductive stages. J. Vet. Med. Sci. 2007, 69, 627–632. [Google Scholar] [CrossRef][Green Version]
- Akagi, S.; Kaneyama, K.; Adachi, N.; Tsuneishi, B.; Matsukawa, K.; Watanabe, S.; Kubo, M.; Takahashi, S. Bovine nuclear transfer using fresh cumulus cell nuclei and in vivo- or in vitro-matured cytoplasts. Cloning Stem Cells 2008, 10, 173–180. [Google Scholar] [CrossRef]
- Watson, A.J. Oocyte cytoplasmic maturation: A key mediator of oocyte and embryo developmental competence. J. Anim. Sci. 2007, 85, E1–E3. [Google Scholar] [CrossRef]
- Son, Y.B.; Jeong, Y.I.; Jeong, Y.W.; Hossein, M.S.; Olsson, P.O.; Tinson, A.; Singh, K.K.; Lee, S.Y.; Hwang, W.S. Cell source-dependent in vitro chondrogenic differentiation potential of mesenchymal stem cell established from bone marrow and synovial fluid of Camelus dromedarius. Animals 2021, 11, 1918. [Google Scholar] [CrossRef]
- Anouassi, A.; Tibary, A. Development of a large commercial camel embryo transfer program: 20 years of scientific research. Anim. Reprod. Sci. 2013, 136, 211–221. [Google Scholar] [CrossRef]
- Vettical, B.S.; Hong, S.B.; Umer, M.A.; Wani, N.A. Comparison of pregnancy rates with transfer of in vivo produced embryos derived using multiple ovulation and embryo transfer (MOET) with in vitro produced embryos by somatic cell nuclear transfer (SCNT) in the dromedary camel (Camelus dromedaries). Anim. Reprod. Sci. 2019, 209, 106132. [Google Scholar] [CrossRef] [PubMed]
- Mostafal, T.H.; Abd El-Salaam1, A.M.; Abdel-Khalek, A.E. Study on Some Physiological Markers for Early Embryonic death in Pregnant She-camels Under Egyptian Conditions. IOSR-JAVS 2017, 10, 45–59. [Google Scholar] [CrossRef]

| Markers | Allele Range | doi |
|---|---|---|
| VOLP10 | 240–269 | https://doi.org/10.1046/j.1365-2052.1999.00526-19.x |
| VOLP67 | 145–208 | https://doi.org/10.1046/j.1365-2052.1999.00526-19.x |
| LCA63 | 198–232 | https://doi.org/10.1046/j.1365-2052.1999.00382-8.x |
| LCA66 | 224–242 | https://doi.org/10.1046/j.1365-2052.1999.00382-8.x |
| LCA90 | 234–246 | https://doi.org/10.1046/j.1365-2052.1999.00526-21.x |
| CVRL01 | 188–253 | https://doi.org/10.1046/j.1365-2052.2002.00896_6.x |
| CVRL05 | 155–185 | https://doi.org/10.1046/j.1365-2052.2002.00896_6.x |
| CVRL07 | 270–230 | https://doi.org/10.1046/j.1365-2052.2002.00896_6.x |
| LGU49 | 224–260 | https://doi.org/10.1046/j.1365-294x.2000.01077-3.x |
| LGU75 | 184–230 | https://doi.org/10.1046/j.1365-294x.2000.01077-3.x |
| YWLL44 | 86–120 | https://doi.org/10.1111/j.1365-2052.1996.tb00502.x |
| P149 | 256–284 | https://doi.org/10.1016/j.smallrumres.2009.07.012 |
| PCTD17 | 172–204 | https://doi.org/10.1016/j.smallrumres.2009.07.012 |
| Markers | Donor Cells | Cloned Offspring | Surrogates |
|---|---|---|---|
| VOLP10 | 259/265 | 259/265 | 249/259, 251/259, 249/259, 249/249, 249/259, 249/251, 251/259, 251/251, 249/259, 249/251, 249/259 |
| VOLP67 | 147/147 | 147/147 | 178/178, 155/186, 176/190, 176/186, 153/192, 153/153, 178/188, 153/155, 155/188, 153/178, 153/155 |
| LCA63 | 212/220 | 212/220 | 216/220, 214/216, 216/218, 216/220, 214/220, 212/214, 218/220, 216/220, 214/220, 214/220, 214/220 |
| LCA66 | 240/240 | 240/240 | 238/240, 238/240, 238/240, 238/238, 234/234, 234/240, 234/238, 238/240, 238/238, 234/238, 234/238 |
| LCA90 | 238/240 | 238/240 | 240/242, 240/240, 238/238, 240/240, 240/240, 240/240, 240/240, 238/240, 240/242, 238/240, 240/240 |
| CVRL01 | 228/234 | 228/234 | 204/234, 226/234, 212/214, 202/214, 212/214, 218/224, 226/246, 234/234, 234/234, 228/228, 214/214 |
| CVRL05 | 163/171 | 163/171 | 159/169, 171/179, 159/171, 159/159159/169, 159/159, 159/171, 171/171, 159/159, 159/171, 159/169 |
| CVRL07 | 295/295 | 295/295 | 285/285, 281/285, 285/285, 273/277, 273/277, 273/281, 295/295, 281/285, 281/281, 273/273, 277/277 |
| LGU49 | 223/235 | 223/235 | 223/239, 239/242, 239/242, 221/231, 225/225, 223/229, 223/225, 225/225, 223/225, 225/229, 225/229 |
| LGU75 | 188/226 | 188/226 | 194/204, 204/228, 188/204, 192/204, 192/204, 204/224, 188/230, 208/208, 188/230, 188/204, 192/224 |
| YWLL44 | 148/167 | 148/167 | 135/179, 163/169, 135/163, 148/176, 135/135, 135/163, 135/163, 135/135, 135/167, 135/167, 148/163 |
| P149 | 268/284 | 268/284 | 260/284, 260/284, 268/268, 260/260, 260/268, 260/284, 260/268, 260/284, 260/284, 260/260, 260/268 |
| PCTD17 | 184/184 | 184/184 | 184/192, 188/192, 188/192, 192/192, 184/192, 188/192, 184/188, 188/188, 184/188, 184/188, 192/192 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hossein, M.S.; Yu, X.; Son, Y.-B.; Jeong, Y.-I.; Jeong, Y.-W.; Choi, E.-J.; Tinson, A.H.; Singh, K.K.; Singh, R.; Noura, A.S.; et al. The Resurrection of Mabrokan: Production of Multiple Cloned Offspring from Decade-Old Vitrified Tissue Collected from a Deceased Champion Show Camel. Animals 2021, 11, 2691. https://doi.org/10.3390/ani11092691
Hossein MS, Yu X, Son Y-B, Jeong Y-I, Jeong Y-W, Choi E-J, Tinson AH, Singh KK, Singh R, Noura AS, et al. The Resurrection of Mabrokan: Production of Multiple Cloned Offspring from Decade-Old Vitrified Tissue Collected from a Deceased Champion Show Camel. Animals. 2021; 11(9):2691. https://doi.org/10.3390/ani11092691
Chicago/Turabian StyleHossein, Mohammad Shamim, Xianfeng Yu, Young-Bum Son, Yeon-Ik Jeong, Yeon-Woo Jeong, Eun-Ji Choi, Alex H. Tinson, Kuhad Kuldip Singh, Rajesh Singh, Al Shamsi Noura, and et al. 2021. "The Resurrection of Mabrokan: Production of Multiple Cloned Offspring from Decade-Old Vitrified Tissue Collected from a Deceased Champion Show Camel" Animals 11, no. 9: 2691. https://doi.org/10.3390/ani11092691
APA StyleHossein, M. S., Yu, X., Son, Y.-B., Jeong, Y.-I., Jeong, Y.-W., Choi, E.-J., Tinson, A. H., Singh, K. K., Singh, R., Noura, A. S., & Hwang, W.-S. (2021). The Resurrection of Mabrokan: Production of Multiple Cloned Offspring from Decade-Old Vitrified Tissue Collected from a Deceased Champion Show Camel. Animals, 11(9), 2691. https://doi.org/10.3390/ani11092691







